Abstract
Standardization is an important measurement for ensuring the quality control of herbal drugs. It has become essential to develop reliable, specific and sensitive quality control methods. Ashwagandhadi lehyam is an important Ayurvedic formulation containing Withania somnifera L., as one of the prime ingredient of formulation. The present study was undertaken to develop standardization parameters for Ashwagandhadi lehyam. Evaluation of various standardization parameters like organoleptic characters, Physico-chemical evaluation, HPTLC finger print profiling along with dominant bioactive markers and estimation of bioactive markers Withaferin-A by HPLC. The Rf value of Withaferin-A 0.35 and Withanolide-A 0.45 is in formulation and reference standards were found comparable under UV light at 254 nm and 540 nm. The HPLC chromatogram of Ashwagandhadi lehyam and standard Withaferin-A showed at Rt of 5.015 and 5.050 min. The percentage of Withaferin-A was 0.092% present in Ashwagandhadi lehyam formulation. Bioactive markers are characteristic to the ingredients or botanicals to identify the presence of ingredients in formulation easily. The presence of bioactive markers is possible and its verification through the HPTLC fingerprint profile and quantification of biomarker by HPLC are the best ways to identify evaluate the quality of the finished formulation in the course of development of a standardization protocol for quality control of Ayurvedic formulation.
Keywords: Lehyam, Withaferin- A, Standardization, Ayurvedic, Bioactive markers and HPLC
Lehyam; Withaferin- A; Standardization; Ayurvedic; Bioactive markers and HPLC.
1. Introduction
Avleha or Lehyam is a semi-solid preparation of drugs, prepared with addition of jaggery, sugar or sugar-candy and boiled with prescribed juices or decoction. Ashwagandhadi lehyam formulation is an important classical Ayurvedic formulation in Ayurvedic Formulary of India (AFI Part-I, 3:2) [1]. It is a semisolid formulation, prepared with various ingredients composition like Sugar, Withania somnifera (Roots), Hemidesmus indicus (Roots), Cuminum cyminum (Fruits), Smilax china or Smilax glabra (Official Substitute) (Root tuber), Vitis vinifera (Dried fruits), Elettaria cardamomum (Seeds), Clarified butter from cow milk, honey and potable water [2]. Withania somnifera (L.) Dunal. (Ashwagandha) is the main ingredient of the formulation. Withania somnifera roots are used for preparation of Ashwagandhadi lehyam formulation. It is one of the most valuable herbs in Ayurvedic medicine and is commonly used in various Indian traditional health care systems like Ayurveda, Siddha and Unani as a tonic, hypnotic, sedative and diuretic. Withania somnifera contains group of biologically active constituents known as Withanolides and Withaferin-A. Withaferin-A is an important bioactive constituent present in Withania somnifera, an important ingredient in Ayurvedic formulations. The dominant Withaferin-A and Withanolide is therapeutically active compound reported to be present in Withania somnifera. Anti-inflammatory, anticonvulsive, anticancer activity, antitumor and antioxidant activities are reported for Withaferin-A [3, 4, 5, 6, 7] Ashwagandhadi lehyam formulation is therapeutically used for Raktavikara (Disorders of blood), Krsatva (Cachexia), Arsa (Piles), Unamada (Psychosis) and used as Balya (Tonic), Rasayana (Rejuvenating agents), Vajikarana (Aphrodisiasis) [1].
Demand of Ayurvedic formulations is raise more in recent years in global market because these drugs are safe, cost effective with fewer side effects [8]. According to demand and popularity of Ayurvedic drugs it is mandatory to develop standardization protocol for safety, efficacy and quality control of drugs. Standardization protocol provides the more security and increase the trust of consumers on Ayurvedic formulations. It is not easy to establish the quality control parameters for herbal formulations due to the complex nature and inherent variability of the bioactive chemical constituents of the polyherbal formulations [9].
Present study aimed to develop a standardization protocols for Ashwagandhadi lehyam by analysing organoleptic characters, Physico-chemical evaluation, HPTLC finger print profiling along with dominant biomarkers and estimation of bioactive markers Withaferin-A by HPLC.
2. Materials and methods
Collection of formulation Sample
Ashwagandhadi lehyam formulation was procured from the local market of Chennai.
Reagents and standards
All chemicals and solvents were used analytical grade or HPLC grade and obtained from E-Merck and other renowned companies. Bioactive Markers reference standards were procured from Natural remedies, Bengaluru, India.
Organoleptic characteristics
The organoleptic characteristics like colour, odour, taste and texture were performed using sensory organs [2].
Evaluation of physicochemical parameters
Evaluation of physicochemical parameters such as pH, Total ash, Acid insoluble ash, Loss on drying at 105 °C, Alcohol soluble extractive value, Water soluble extractive value, Total sugar, Reducing sugar, Non-reducing sugar and Fat content were carried out as per the standard Pharmacopoeia methods and WHO guidelines for Ayurvedic formulations [2, 10, 11, 12, 13].
2.1. HPTLC and HPLC analysis
Instrumentation
HPTLC analysis was performed on a CAMAG HPTLC system (Muttenz, Switzerland) equipped with an Automatic TLC sampler IV, twin trough development chamber, TLC Scanner 3 linked with WINCATS software version 1.4.4 [14, 15, 16, 17].
Standard preparation
Stock solutions of Withaferin-A and Withanolide–A (0.17 mg/ml) were separately prepared by dissolving 1.7 mg accurately weighed standards in small amounts of methanol and made up the volume to 10 ml in a standard volumetric flask. The stock solutions were further diluted as per requirement for the preparation of working solutions.
Test sample preparation
5 gm of formulation was taken in 250 ml round bottom flask, 75 ml of methanol (25 ml x 3) was added and reflux on a water bath for 30 min. Combined all the solutions and concentrated up to 25ml, finally filtered the solution through 0.22 μ membrane filter. This filtrate was used for HPTLC and HPLC analysis. From the test sample, 1 ml was pipetted out and diluted up to 10 ml and used for HPLC analysis. Various concentration of test solutions were used for quantification of Withaferin-A.
HPTLC fingerprinting profile
The 10μl of test samples and standards were applied on Tracks 1–4 on E. Merck Aluminium plate pre-coated with Silica gel 60F254 of 0.2 mm thickness using CAMAG Automatic sample applicator IV. The plate was developed in the solvent system of Toluene: Ethyl acetate: Formic acid (6:4:1) (v/v/v) saturated in TLC chamber. The developed plate was dried and observed through CAMAG TLC Visualizer under UV at 254 and 366 nm and photos were documented. Finally the plate was dipped in vanillin sulphuric acid reagent and heated in hot air oven at 105 °C until the colour of the spots appeared and photo was documented under white light. Before derivatization the plate was scanned using CAMAG TLC Scanner with WINCATS software at a wavelength of UV 254 and 366 nm using deuterium lamps and after derivatization plate was scanned at 540 nm using tungsten lamp.
2.2. HPLC analysis
HPLC instrumentation & chromatographic conditions
HPLC analysis was performed on an Agilent 1200 series High Performance Liquid Chromatographic system equipped with quaternary pump, manual sample injector using Chemstation HPLC software. All samples and standards were filtered through 0.22 μm (Millipore) filters. Separation was achieved on C18 Eclipse, XDB, 4.6 mm × 150 mm, 5 μm particle size Agilent Column. The mobile phase was consisted of Acetonitrile: Buffer (35:65) (v/v) in an isocratic elution with flow rate of 1.8 ml/min 10 μl of the test sample (triplicate) was injected to HPLC system. The column temperature was kept 32 °C. The detection of analytes at 227 nm was carried out by using Diode Array Detector (DAD) [17, 18, 19, 20].
Solution A: Acetonitrile.
Solution B: (Buffer) About 0.136 g of anhydrous Potassium dihydrogen orthophosphate (KH2PO4) was dissolved in 900 ml of HPLC grade water, to that 0.5ml of Orthophosphoric acid was added and made up to 1000 ml with HPLC grade water, filtered through 0.45 μm membrane and degased for 3 min using a sonicator (0.1 M KH2PO4 containing 0.5 ml of Orthophosphoric acid).
Good separations and suitable retention time of Withaferin-A was obtained in isocratic elution using following optimized chromatographic conditions:
Column: C18 Eclipse, XDB, 4.6 mm × 150 mm, 5μm particle size.
Detection: 227 nm wavelength.
Detector: Diode Array Detector (DAD).
Column temperature: 32 °C.
Mobile phase: Acetonitrile:Buffer (35:65) (v/v).
Flow rate: 1.8 ml/min.
Injection volume: 10 μl.
Mode of Operation: Isocratic elution.
Retention Time (Std.): 5.050.
Retention Time (Test): 5.015.
Run time: 10 min.
3. Results and discussion
The Ashwagandhadi lehyam was evaluated for its physical characters including colour, odour, taste, texture, physicochemical parameters, HPTLC fingerprint profiling and estimation of Withaferin-A by HPLC were carried out as per the standard Pharmacopoeia methods and WHO guidelines for Ayurvedic formulations. Standardization promises constant composition of all herbals including analytical operations for identification of markers and assay of bioactive constituents. In the current study qualitative identification of Withaferin-A, Withanolide-A using HPTLC and quantitative estimation of specific biologically active Withaferin-A compound was conducted in the Ayurvedic Polyherbal formulation Ashwagandhadi lehyam using HPLC.
Evaluation of physical characters
The Organoleptic characters (colour, odour, taste and texture) of the Ashwagandhadi lehyam was evaluated and tabulated in Table 1.
Evaluation of physicochemical parameters
Table 1.
Organoleptic characteristics of Ashwagandhadi lehyam.
| Colour | Odour | Taste | Texture |
|---|---|---|---|
| Dark brown | Acceptable | Sweet | Semi solid/sticky |
Evaluation of physicochemical parameters such as pH, Total ash, Acid insoluble ash, Loss on drying at 105 °C, Alcohol soluble extractive value, Water soluble extractive value, Total sugar, Reducing sugar, Non reducing sugar and Fat content were carried out as per the Standard Pharmacopoeial methods and WHO guidelines for Ashwagandhadi lehyam results tabulated in Table 2.
HPTLC fingerprinting
Table 2.
Physico-chemical parameters of Ashwagandhadi lehyam formulation.
| S. No | Parameters | Results |
|---|---|---|
| 1. | pH (5% w/v aqueous solution) | 5.4 |
| 2. | Loss on drying at 105 °C (w/w) | 13.12 |
| 3. | Total ash % (w/w) | 1.82 |
| 4. | Acid insoluble ash % (w/w) | 0.08 |
| 5. | Water soluble extractive % (w/w) | 59.35 |
| 6. | Alcohol soluble extractive % (w/w) | 33.86 |
| 7. | Total sugar % | 44.91 |
| 8. | Reducing sugar % | 30.24 |
| 9. | Non reducing sugar % | 14.67 |
| 10. | Fat content% | 10.65 |
HPTLC method is an accurate, simple and specific method for quantitative estimation of bioactive markers. For optimization of different mobile phase compositions were employed to achieve good separation. The Ashwagandhadi lehyam formulation was analysed using HPTLC fingerprint profile to confirm the presence of bioactive constituents. Withania somnifera is a major ingredient of Ashwagandhadi lehyam formulation. Withanolides are the major constituents in the Withania somnifera plant compared with the rest of the other compounds. Withaferin-A and Withanolide-A bioactive marker compound were selected for Ashwagandhadi lehyam formulation and the formulation is evaluated for its presence.
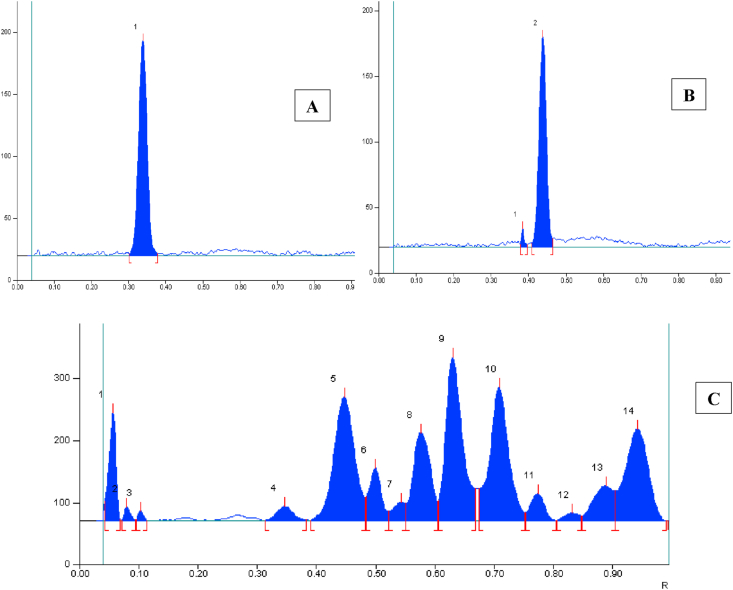
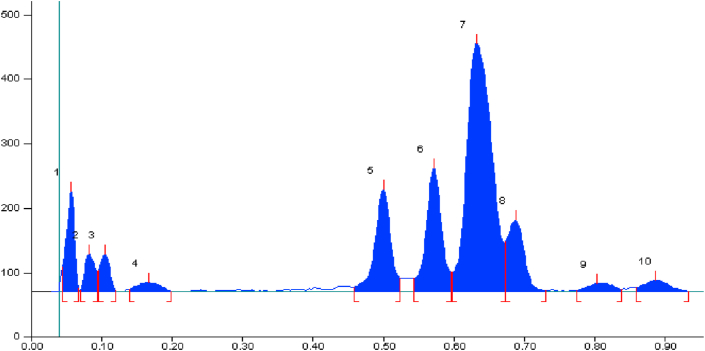
The solvent system containing Toluene: Ethyl acetate: Formic acid in the volume ratio of (6:4:1) (v/v/v) resulted in good separation of the Markers and formulation (Figure 1). TLC plate was observed under UV light and white light for the presence of Withaferin-A and Withanolide-A, which were detected by prominent Green spot at UV 254 nm, dark grey spots appeared After Derivatization with Vanillin sulphuric acid reagent at 540 nm. The Rf value of Withaferin-A is 0.35 and Withanolide-A is 0.45 in formulation and reference standards were found comparable under UV light at 254 nm and 540 nm. The chromatograms of HPTLC finger print profiling of Withaferin-A, Withanolide-A and Ashwagandhadi lehyam formulation are shown in Figures 2, 3, and 4 and Rf value and peak area of the standards and formulations are given in Tables 3, 4, and 5.
Figure 1.
HPTLC profile of Methanol extract of Ashwagandhadi lehyam and Withaferin-A and Withanolide-A as a biomarker compounds. Track 1- Withaferin A; Track 2&4- Ashwagandhadi lehyam; Track 3 –Withanolide A;(10 μl). Solvent system: Toluene: Ethylacetate: Formic acid (6:4:1 v/v).
Figure 2.
Chromatogram of HPTLC fingerprint profile of Withaferin-A (A),Withanolide-A (B) and Ashwagandhadi lehyam (C) at UV 254nm.
Figure 3.
HPTLC fingerprint profile of Ashwagandhadi lehyam at UV 366nm.
Figure 4.
HPTLC finger print profile of Withaferin-A (A),Withanolide-A (B) and Ashwagandhadi lehyam (C) at 540nm.
Table 3.
HPTLC fingerprint profile of Withaferin A, Withanolide A and Ashwagandhadi lehyam at UV 254 nm.
| Track | Peak No. |
Rf | Height | % | Area | Area % |
|---|---|---|---|---|---|---|
| Track-1 Withaferin A |
1 | 0.35 | 173.6 | 100.0 | 3744.8 | 100.0 |
| Track-3 Withanolide A |
1 | 0.45 | 159.9 | 91.78 | 2987.1 | 96.87 |
| Track 2&4 Ashwagandhadi lehyam | 1 | 0.06 | 187.9 | 14.21 | 2352.7 | 6.82 |
| 2 | 0.09 | 18.9 | 1.43 | 189.5 | 0.55 | |
| 3 | 0.11 | 20.1 | 1.52 | 197.5 | 0.57 | |
| 4 | 0.35 | 23.8 | 1.80 | 568.0 | 1.65 | |
| 5 | 0.45 | 192.5 | 14.56 | 6179.6 | 17.91 | |
| 6 | 0.51 | 78.9 | 5.97 | 1534.7 | 4.45 | |
| 7 | 0.56 | 25.2 | 1.91 | 502.4 | 1.46 | |
| 8 | 0.59 | 126.8 | 9.59 | 3391.0 | 9.83 | |
| 9 | 0.65 | 233.8 | 17.68 | 6475.9 | 18.77 | |
| 10 | 0.73 | 193.1 | 14.60 | 5697.6 | 16.51 | |
| 11 | 0.80 | 36.0 | 2.72 | 898.1 | 2.60 | |
| 12 | 0.89 | 53.7 | 4.06 | 1701.0 | 4.93 | |
| 13 | 0.94 | 131.5 | 9.95 | 4812.6 | 13.95 |
Table 4.
HPTLC fingerprint profile of Withaferin A, Withanolide A and Ashwagandhadi lehyam at UV 366nm.
| Track No. |
Peak No. |
Rf | Height | % | Area | Area % |
|---|---|---|---|---|---|---|
| Track 2&4 Ashwagandhadi lehyam | 1 | 0.06 | 155.6 | 13.39 | 1666.0 | 6.25 |
| 2 | 0.08 | 58.3 | 5.02 | 779.1 | 2.92 | |
| 3 | 0.11 | 57.8 | 4.97 | 731.6 | 2.74 | |
| 4 | 0.17 | 14.3 | 1.23 | 425.7 | 1.60 | |
| 5 | 0.50 | 158.8 | 13.66 | 3248.7 | 12.18 | |
| 6 | 0.57 | 191.0 | 16.43 | 3877.3 | 14.54 | |
| 7 | 0.63 | 384.7 | 33.11 | 12556.8 | 47.09 | |
| 8 | 0.69 | 110.7 | 9.52 | 2366.5 | 8.87 | |
| 9 | 0.81 | 13.2 | 1.13 | 437.1 | 1.64 | |
| 10 | 0.89 | 17.9 | 1.54 | 577.6 | 2.17 |
Table 5.
HPTLC fingerprint profile of Withaferin-A, Withanolide-A and Ashwagandhadi lehyam at 540nm.
| Track No. |
Peak No. |
Rf | Height | % | Area | Area % |
|---|---|---|---|---|---|---|
|
Track-1 Withaferin A |
1 | 0.35 | 353.7 | 76.12 | 9243.9 | 74.46 |
|
Track-3 Withanolide A |
1 | 0.45 | 273.9 | 84.10 | 5627.4 | 73.81 |
| Track-2&4 Ashwagandhadi lehyam | 1 | 0.07 | 259.0 | 24.41 | 9563.7 | 24.47 |
| 2 | 0.25 | 35.9 | 3.39 | 615.5 | 1.57 | |
| 3 | 0.35 | 50.2 | 4.73 | 1656.0 | 4.24 | |
| 4 | 0.45 | 83.6 | 7.87 | 3169.6 | 8.11 | |
| 5 | 0.52 | 62.3 | 5.87 | 1399.0 | 3.58 | |
| 6 | 0.57 | 48.4 | 4.56 | 2176.3 | 5.57 | |
| 7 | 0.65 | 72.4 | 6.82 | 3420.4 | 8.75 | |
| 8 | 0.75 | 112.1 | 10.57 | 3718.0 | 9.51 | |
| 9 | 0.84 | 252.0 | 23.75 | 10937.6 | 27.98 | |
| 10 | 0.91 | 72.8 | 6.86 | 2292.7 | 5.87 | |
| 11 | 0.95 | 12.4 | 1.17 | 135.7 | 0.35 |
3.1. Quantitative estimation of Withaferin-A in Ashwagandhadi lehyam formulation by HPLC
The standard and sample solutions were run in different mobile phase systems. Various proportions of mobile phase were tried using Water, Acetonitrile, Methanol and Phosphate Buffer. It was found that the combination of Acetonitrile and Buffer in proportion of 35:65 v/v has given a good resolution, sharp and symmetric peak at retention time 5.050 min for standard and 5.015 for formulation.
Calibration curve
From 0.17 mg/ml of standard stock solution, aliquots (1, 0.5, 0.25, 0.12 ml) were pipette out and diluted up to 2ml to make 0.085 mg/ml, 0.0425 mg/ml, 0.02125 mg/ml, 0.010625 mg/ml concentration of Withaferin-A respectively. Five different concentrations of standard (0.010625, 0.02125, 0.0425, 0.085 and 0.17 mg/ml) were used for preparing five points calibration curve. Each of the standard solution was run through the HPLC and recorded the respective peak areas. Calibration curve was established for peak area vs concentration of Withaferin-A applied. Calibration peak summary was tabulated in Table 6 and the Calibration curve was depicted in Figure 5.
Table 6.
Five point calibration table.
| Level | Amount (mg/ml) | Area | Amount/Area |
|---|---|---|---|
| 1 | 1.70000e-1 | 148.56769 | 7.15162e-5 |
| 2 | 8.50000e-2 | 294.93130 | 7.20507e-5 |
| 3 | 4.25000e-2 | 577.98193 | 7.35317e-5 |
| 4 | 2.12500e-2 | 294.93130 | 7.20507e-5 |
| 5 | 1.06250e-2 | 148.56769 | 7.15162e-5 |
Figure 5.
Calibration curve and regression coefficient of Withaferin-A.
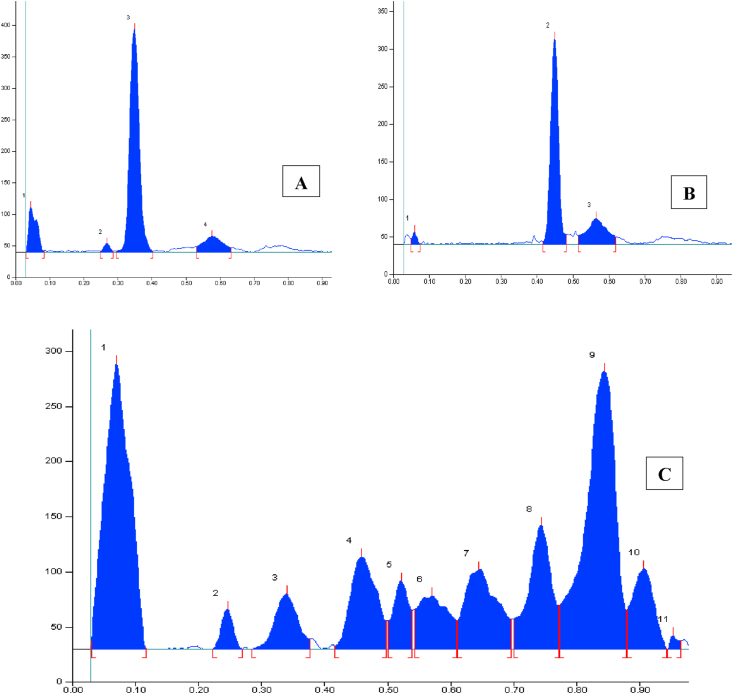
A characteristic HPLC chromatogram was obtained using isocratic elution of the formulation extract, which exhibited a clean and smooth baseline with excellent resolution where marker peak identified clearly. The marker compounds Withaferin-A is exhibited at retention time 5.050 for standard and 5.015 for formulation in chromatogram. By measuring the peak area, calibration curve was constructed, which were linear (r2 > 0.9999) in the concentration range of 0.17 mg/ml to 0.010625 mg/ml. The HPLC chromatogram of Ashwagandhadi lehyam corresponding to standard Withaferin-A was showed at a retention time of 5.015 min, at 227 nm wavelength shown in Figure 6. The quantitative evaluation of Withaferin-A in Ashwagandhadi lehyam formulation was found to be 0.092% using the formula,
Figure 6.
HPLC Chromatograms of Withaferin-A standard (A) and Ashwagandhadi lehyam formulation (B).
4. Conclusion
Standardization and development of reliable quality protocols for Ayurvedic polyherbal formulations using modern techniques of analysis is extremely important. The generated physical characteristics, physiochemical parameters, HPTLC fingerprint profile with bioactive marker compounds will be used as a consistent analytical tool in the routine quality control or standardization of Ayurvedic formulations. Estimation of Withaferin-A can be used by pharmaceutical industry as an appropriate bioactive marker compound for standardization and quality control of Ashwagandhadi lehyam. Standardization protocol of herbal formulation is essential in order to assess the quality, purity, efficacy and safety of the Ayurvedic formulations.
The Rf value of Withaferin-A is 0.35 and Withanolide-A is 0.45 in formulation and reference standards were found comparable under UV light at 254 nm and 540 nm. The HPLC chromatogram of Ashwagandhadi lehyam corresponding to standard Withaferin-A was showed at a retention time of 5.015 min, at 227 nm wavelength. The quantitative evaluation of Withaferin-A present in Ashwagandhadi lehyam formulation was 0.092%.
Declarations
Author contribution statement
Ajay Kumar Meena: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
R. Ilavarasan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
P. Rekha, Ayyam Perumal: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Gokul, K. N. Swathi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are very grateful to Director General and Deputy Director General, Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, New Delhi for their encouragement and providing facilities to conduct this study.
References
- 1.The Ayurvedic Formulary of India . second ed. Government of India, Ministry of Health and Family Welfare; New Delhi: 2003. Part I. [Google Scholar]
- 2.The Ayurvedic Pharmacopoeia of India . first ed. III. Government of India, Ministry of health and family welfare, Department of AYUSH; New Delhi: 2010. Part II (Formulations) pp. 27–28. [Google Scholar]
- 3.Al-Hindawi M.K., Al-Khafaji S., Abdul-Nabi M. Anti-granuloma activity of Iraqi Withania somnifera. J. Ethnopharmacol. 1992;37:113–116. doi: 10.1016/0378-8741(92)90069-4. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni S.K., George B., Mathur R. Protective effect of Withania somnifera root extract on electrographic activity in a Lithium pilocarpine model of status epilepticus. Phytother Res. 1998;12:451. [Google Scholar]
- 5.Devi P.U., Sharada A.C., Soloman F.E., Kamath M.S. In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor sarcoma-180. Indian J. Exp. Biol. 1992;30:169–172. [PubMed] [Google Scholar]
- 6.Bhattacharya S.K., Satyan K.S., Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J. Exp. Biol. 1997;35:236. [PubMed] [Google Scholar]
- 7.Vibhavari Sail, Hadden M., Kyle . 2012. Annual Reports in Medicinal Chemistry. [Google Scholar]
- 8.Rasheed Arun, Satyanarayana K.V., Gulabi, Swetha P., Rao M., Sindhura Chemical and pharmacological standardization of Ashwagandhadi lehyam: an ayurvedic formulation. J. Compl. Integr. Med. 2013;10(1):1–9. doi: 10.1515/jcim-2012-0026. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, Mansi, Bisht Deepa, Pandey Madan M, Ojha SK, Khatoon Sayyada, Rastogi Subha and Rawat AKS. Standardization of Ashwagandhadi lehya”An important Ayurvedic formulation of Withania somnifera, Ind. J. Trad. Knowledge 10(4):594-598.
- 10.Meena A.K., Simha G.V., Mangal A.K., Sannd R., Panda P., Rao M.M., Padhi M.M. Evaluation of quality control parameters for srngyadi Churna “ A potential ayurvedic formulation. Res. J. Pharmacogn. Phytochem. 2013;5(1):42–46. [Google Scholar]
- 11.Quality Control Methods for Medicinal Plant Materials. 16thedition. World Health Organization; Geneva: 1998. pp. 25–28. 21. AOAC official methods of analysis of AOAC International. [Google Scholar]
- 12.AOAC International . Wilson Doulevard; Arlington, Virginia, USA: 1995. Suite 400, 2200. [Google Scholar]
- 13.Indian Pharmacopoeia. Ministry of Health and Family Welfare. Government of India; New Delhi, India: 2018. [Google Scholar]
- 14.Sethi P.D. first ed. CBS Publishers and Distributors; New Delhi: 1996. High Performance Thin Layer Chromatography. X. [Google Scholar]
- 15.Stahl I. Student Edition. Springer-Verlag; Berlin: 1969. Thin Layer Chromatography. A Laboratory Hand Book. [Google Scholar]
- 16.Wagner H., Baldt S., Zgainski E.M. Berlin Springer; 1996. Plant Drug Analysis. [Google Scholar]
- 17.Meena A.K., Narasimhaji Ch V., Velvizhi D., Singh Arjun, Rekha P., Kumar Vijay, Ilavarasan R., Narayanam Srikanth, Dhiman K.S. Determination of gallic acid in ayurvedic polyherbal formulation triphalachurna and its ingredients by HPLC and HPTLC. Res. J. Pharm. Technol. 2018;11(8):3243–3249. [Google Scholar]
- 18.Kumar Meena Ajay, Rekha P., Poorna V., Jayarega V., Swathi K.N., Ilavarasan R. Standardisation and HPTLC finger print profile of poly herbal churna, an Ayurvedic Formulation. Res. J. Pharm. Technol. 2020;13(3):1361–1367. [Google Scholar]
- 19.Kumar Meena Ajay, Narasimhaji Ch.V., Rekha P., Velvizhi D., Ilavarasan R. Comparative preliminary phytochemical and HPTLC fingerprint profile studies of two cinnamon species commonly used in ASU formulations. Asian J. Res. Chem. 2018;11(2):344–350. [Google Scholar]
- 20.Gurav N., Solanki B., Gadhvi I., Patel P., Sen D. RP-HPLC method development and validation for estimation of withaferin-A in ranger capsule. IJPSR. 2015;6(12):5141–5146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.