Summary
Aloe vera is a species from Asphodelaceae family having characteristics like drought resistance and numerous medicinal properties. However, the genetic basis of these phenotypes is yet unknown primarily due to unavailability of its genome sequence. Thus, we report the first Aloe vera genome sequence comprising of 12.93 Gbp and harboring 86,177 protein-coding genes. It is the first genome from Asphodelaceae family and the largest angiosperm genome sequenced and assembled till date. We also report the first genome-wide phylogeny of monocots including Aloe vera to resolve its phylogenetic position. The comprehensive comparative analysis of Aloe vera with other available high-quality monocot genomes revealed adaptive evolution in several genes of drought stress response, CAM pathway, and circadian rhythm and positive selection in DNA damage response genes in Aloe vera. This study provides clues on the genetic basis of evolution of drought stress tolerance capabilities of Aloe vera.
Subject areas: Biological Sciences, Genetics, Phylogenetics, Evolutionary Biology
Graphical abstract

Highlights
-
•
First Aloe vera genome of 12.93 Gbp harboring 86,177 protein-coding genes
-
•
First genome from Asphodelaceae family and phylogeny of monocots with Aloe vera
-
•
Adaptive evolution in drought stress response, CAM pathway, circadian rhythm genes
-
•
Clues on the genetic basis of evolution of drought stress tolerance capabilities
Biological Sciences; Genetics; Phylogenetics; Evolutionary Biology
Introduction
Aloe vera is a succulent and drought-resistant plant belonging to the genus Aloe of family Asphodelaceae (Silva et al., 2010). More than 400 species are known in genus Aloe, of which four have medicinal properties, with Aloe vera being the most potent species (Grace et al., 2015). Aloe vera is a perennial tropical plant with succulent and elongated leaves having a transparent mucilaginous tissue consisting of parenchyma cells in the center referred to as Aloe vera gel (Reynolds and Dweck, 1999). The plant is extensively used as a herb in traditional practices in several countries and in cosmetics and skin care products due to its pharmacological properties including anti-inflammatory, anti-tumor, anti-viral, anti-ulcers, fungicidal, etc. (Gupta and Malhotra, 2012; Raksha et al., 2014). These medicinal properties emanate from the presence of numerous chemical constituents such as anthraquinones, vitamins, minerals, enzymes, sterols, amino acids, salicylic acids, and carbohydrates (Choudhri et al., 2018; Hamman, 2008; Joseph and Raj, 2010). These properties make it commercially important, with a global market worth 1.6 billion (Choudhri et al., 2018).
One of the key characteristics of this succulent plant is drought resistance that enables it to survive in adverse hot and dry climates (Silva et al., 2010). The plant has thick leaves arranged in an attractive rosette pattern to the stem. As an adaptation to the hot climate, the plant is able to perform a photosynthetic pathway known as crassulacean acid metabolism (CAM) that helps in limiting the water loss by transpiration (Nobel and Jordan, 1983). Moreover, the leaves have the capacity to store a large volume of water in their tissues (Jin et al., 2007). It is also known to synthesize more of soluble carbohydrates to make the osmotic adjustments under the limited water conditions, thus improving the water use efficiency (Delatorre-Herrera et al., 2010). The transcriptome and chloroplast genome of Aloe vera are available from previous studies, and a few studies have also highlighted the drought stress tolerance and potential benefits of Aloe vera (Choudhri et al., 2018; Ren et al., 2020). However, the unavailability of its reference genome sequence has been a deterrent in understanding the genetic basis and molecular mechanisms of the unique characteristics of this medicinal plant.
In addition to the functional analysis, the resolution of the phylogenetic position has the potential to reveal the evolutionary history and understand the correlations between phylogenetic diversity and important traits of interest. Multiple attempts have been made to resolve the phylogenetic position of Aloe genus and Aloe vera; however, these efforts only used a few conserved loci such as rbcL, psbA, matK, and ribosomal genes and were not performed at the genome-wide level due to the unavailability of the genomic sequence (Adams et al., 2000; Grace et al., 2015; Treutlein et al., 2003). The previous phylogenies have reported that Aloe vera shared the most common recent ancestor with the species of Poales and Zingiberales order, also within the Asparagales order; it was closest to the other succulent genera such as Haworthia, Gasteria, and Astroloba (Chase et al., 2016; Qian and Jin, 2016).
Aloe vera has an estimated genome size of 16.04 Gbp with a diploid ploidy level containing 14 (2n) chromosomes according to the Plant DNA c-value database (Zonneveld, 2002). The unavailability of the genome sequence of Aloe vera is noteworthy given the fact that the representative genomes of species from almost all the plant families including Brassicaceae, Cannabaceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Malvaceae, Rosaceae, Solanaceae, Poaceae, Orchidaceae, and Betulaceae have been sequenced and studied. However, no species from the Asphodelaceae plant family has been sequenced till date. Thus, the availability of Aloe vera genome sequence will help to reveal the genomic signatures of Asphodelaceae family and will also be useful in understanding the genetic basis of the important phenotypes such as medicinal properties and drought resistance in Aloe vera.
Therefore, in this study, we report the first draft genome sequence of Aloe vera using a hybrid sequencing and assembly approach by combining the Illumina short-read and Oxford Nanopore long-read sequences to construct the genome sequence. The transcriptome sequencing and analysis of two tissues, root and leaf, was carried out to gain deep insights into the gene expression and to precisely determine its gene set. The genome-wide phylogeny of Aloe vera with other available monocot genomes was also constructed to resolve its phylogenetic position. The comparative analysis of Aloe vera with other monocot genomes revealed adaptive evolution in its genes and provided insights on the stress tolerance capabilities of this species.
Results
Sequencing of Aloe vera genome and transcriptome
To comprehensively cover the large genome of Aloe vera species, a total of 506.4 Gbp (37.15X) of short-read and 146.8 Gbp (∼10.77X) of long-read data were generated using Illumina and nanopore platforms, respectively (Tables S1 and S2) (Dolezel et al., 2003; Zonneveld, 2002). For transcriptome, a total of 6.6 Gbp and 7.3 Gbp of RNA-seq data were generated from leaf and root, respectively. The transcriptome data from this study and the publicly available RNA-seq data from previous studies (Choudhri et al., 2018; Wickett et al., 2014) were combined together, resulting in a total of 37.1 Gbp of RNA-seq data for Aloe vera, which was used for the analysis (Table S3). All the short-read genomic and RNA-seq data were trimmed and filtered using Trimmomatic, and only the high-quality read data were used to construct the final genome and transcriptome assemblies. The complete workflow of the sequence analysis is shown in Figure S1.
Assembly of Aloe vera genome
The percent heterozygosity was estimated to be 11.3% for Aloe vera species. The final draft genome assembly of Aloe vera had the size of 12.93 Gbp and N50 of 14.6 kbp, of which 11.12 Gbp had length >500 bp and N50 of 20.4 kbp (Table S4). The genomic coverage and N50 attained in case of the first draft assembly of Aloe vera genome appears reasonable for such a challenging and a gigantic plant genome and is also comparable to the other large plant genomes assembled till date (Birol et al., 2013; Neale et al., 2014; Nystedt et al., 2013; Stevens et al., 2016). This was achieved by the hybrid assembly of short-read and long-read data, which was further polished by correction using SeqBug, RNA-seq-data-based scaffolding using Rascaf, and long-read-based gap-closing using LR-gap closer. The k-mer-count-distribution-based method using only the short Illumina reads estimated a genome size of 13.63 Gb, which was smaller than the c-value-based genome size estimation of 16.04 Gbp, conceivably due to the usage of only short-read data for the genome size estimation (Figure S2). The percent GC for the final assembly was 41.98%.
The analysis of repetitive sequences revealed 557,638,058 bp of tandem repeats corresponding to 3.41% of the complete genome. For interspersed repeat identification, a total of 1,820 repeat families identified using RepeatModeler were used as custom repeat library for repeat identification in Aloe vera genome. Out of the 1,820 repeat families, 1,550 families could not be annotated by RepeatModeler. Identification of repeats was carried out on short-read assembly using RepeatMasker. It revealed that 82.66% of Aloe vera genome is constituted by interspersed repeats, of which 55.57% was unclassified and 26.96% was identified as retroelements. Among the retroelements, 26.71% was LTR repeats (7.34% Ty1/Copia and 19.37% Gypsy/DIRS1 elements) and 0.13% was DNA transposons. Similarly, the identification of repeats in hybrid assembly revealed that interspersed repeats constitute 78.70% of Aloe vera genome, of which 51.26% was unclassified and 27.28% was identified as retroelements. Among the retroelements, 26.94% was LTR repeats (8.87% Ty1/Copia and 18.07% Gypsy/DIRS1 elements) and 0.16% was DNA transposons.
Transcriptome assembly
The Trinity assembly of transcriptomic reads resulted in a total size of 163,190,792 bp with an N50 value of 1,268 bp and an average contig length of 796 bp (Table S5). The mapping of filtered RNA-seq reads on the Trinity transcripts using hisat2 resulted in the overall percentage mapping of 92.49%. The complete BUSCO score (addition of single copy and duplicates) on the transcripts was 90.5%. A total of 205,029 transcripts were predicted, corresponding to 108,133 genes with the percent GC of 43.69. The clustering of gene sequences using CD-HIT-EST to remove the redundancy resulted in 107,672 unigenes. The coding genes (CDS) from the unigenes were predicted using TransDecoder resulting in 34,269 coding genes.
Genome annotation and gene set construction
A total of 1,978 standard amino-acid-specific tRNAs and 378 hairpin miRNAs were identified in the Aloe vera genome (Table S6). The MAKER-pipeline-based gene prediction resulted in a total of 114,971 coding transcripts, of which 63,408 transcripts (≥300 bp) were considered further for clustering at 95% identity resulting in 57,449 unique coding gene transcripts. Application of the same length-based selection criteria (≥300 bp) on trinity-identified 34,269 coding gene transcripts resulted in 33,998 coding gene transcripts. The merging of these two coding gene transcript sets resulted in the final gene set of 86,177 genes for Aloe vera, which had the complete BUSCO score of 74.6% and single-copy BUSCO score of 72.4%.
Identification of orthologs across selected plant species
A total of 104,543 orthogroups were identified using OrthoFinder across the selected 16 plant species. Of which, only a total of 5,472 orthogroups had sequences from all the 16 plant species and were used for the identification of orthologs. For these 5,472 orthogroups, in case of presence of more than one gene from a species in an orthogroup, the longest gene representative from that species was selected to construct the final orthologous gene set for any orthogroup. Thus, including one gene from each of the 16 species in an orthogroup, a total of 5,472 orthologs were identified. In addition, the fuzzy one-to-one orthologs finding approach applied using KinFin resulted in a total of 1,440 fuzzy one-to-one orthologs that were used for constructing the maximum likelihood species phylogenetic tree.
Resolving the phylogenetic position of Aloe vera
Each of the 1,440 fuzzy one-to-one orthologous gene set was aligned and concatenated, and the resultant concatenated alignment had a total of 1,453,617 alignment positions. The concatenated alignment was filtered for the undetermined values, which were treated as missing values, and a total of 1,157,550 alignment positions were retained. The complete alignment data and the filtered alignment data were both used to construct maximum likelihood species trees using RAxML with the bootstrap value of 100, and both the alignment data resulted in the same phylogeny. Thus, the phylogeny based on the filtered data was considered as the final genome-wide phylogeny of Aloe vera including all the representative monocot genomes available on Ensembl plants database with Arabidopsis thaliana as an outgroup (Figure 1). This phylogeny also corroborated with the earlier reported phylogenies by Silvera et al. (2014), Dunemann et al. (2014), and Wang and Deng, 2016, which were constructed using a limited number of genetic loci (Dunemann et al., 2014; Silvera et al., 2014; Wang and Deng, 2016). It is apparent from the phylogeny that Dioscorea rotundata and Musa acuminata are the most closely related to Aloe vera and share the same clade (Figure 1). All other selected monocots are distributed in separate clade, with Triticum aestivum and Aegilops tauschii being the most distantly related to Aloe vera.
Figure 1.
The phylogenetic tree of the selected 14 monocot species, Aloe vera, and Arabidopsis thaliana as an outgroup
The values mentioned at the nodes are the bootstrap values. The scale mentioned is the nucleotide substitutions per base.
See also Figure S3.
Recently an updated plant megaphylogeny has been reported for the vascular plants (Qian and Jin, 2016). The species of Poales order showed similar relative positions in our reported phylogeny and this megaphylogeny. In the megaphylogeny, Musa acuminata was reported to share the most common recent ancestor with the species of Poales order, but in our phylogeny we observed that Musa acuminata shared the most common recent ancestor with Dioscorea rotundata from Dioscoreales order (Figures 1 and S3). Also, among the selected monocots, the species of Dioscoreales order was reported to show the earliest divergence. However, in our genome-wide phylogeny, Aloe vera showed the earliest divergence.
With reference to the reported phylogeny of angiosperms, at the order level the Poales and Zingiberales formed a clade, and their ancestor shared the most recent common ancestor with Asparagales, then all three shared a recent ancestor with Dioscoreales (Chase et al., 2016). In our genome-wide phylogeny, Zingiberales and Dioscoreales shared the most recent common ancestor, and their ancestor shared the most recent common ancestor with Asparagales, and the three shared a recent ancestor with Poales.
The divergence time for Aloe vera and clade formed by Dioscorea rotundata and Musa acuminata was estimated to be 104.97 Mya with equal-tailed confidence interval of 101.32–112.22 Mya, which is in agreement with the previous studies (Bremer, 2000; Fiz-Palacios et al., 2011; Pouget et al., 2016). Further, the divergence time of the clade leading to Aloe vera, Dioscorea rotundata, and Musa acuminata and the clade formed by the other species of Poales order was estimated to be 107.66 Mya with equal-tailed confidence interval of 103.47–116.73 Mya.
Gene families with expansion and contraction in Aloe vera
For the identification of gene family expansion and contraction, a total of 52,357 families were obtained by clustering. Of these, 23,016 families having <100 gene copies for every species were used for further analysis. A total of 5,143 families were found to be expanded, and 2,977 families showed contraction in Aloe vera in comparison to the recent ancestor (Figure S4). Among the top 20 expanded families in Aloe vera, ABC transporter, RNA-mediated transposition, transcription initiation factor TFIIB, ATPases, ribonuclease H, and serine arginine-rich splicing factor were the families with known function. In contrast, the top 20 contracted families in Aloe vera included the functions related to MADS-box transcription factor, synthase, RNA polymerase II transcription regulator recruiting activity, peptidase S10, cysteine-rich receptor-like protein kinase, galactoside, WRKY transcription factor, AP2-like ethylene-responsive transcription factor, and reverse transcriptase.
Genes with a higher rate of evolution
A total of 85 genes showed higher rates of evolution in Aloe vera in comparison to the other monocot species. These genes belonged to several eggNOG categories and KEGG pathways as mentioned in Tables S7 and S8, with a higher representation of ribosomal genes. The distribution of enriched (p value<0.05) biological process GO terms is mentioned in Table S9. Also, among these 85 genes three molecular function GO terms, rRNA binding, structural constituent of cytoskeleton, and structural constituent of ribosome showed an enrichment (p value<0.05) (Table S10). Five transcription factors—WRKY, MYB, bHLH, CPP, and LBD—showed higher rates of evolution in Aloe vera. Among these, WRKY, MYB, and bHLH are known to be involved in drought stress tolerance (Fei et al., 2019; Waseem and Li, 2019; Zhao et al., 2018b). There were six chloroplast-functioning-related genes, namely EMB3127, PnsB3, TL29, IRT3, PDV2, and SIRB, that showed a higher rate of evolution. Notably, the chloroplast-function-related genes have been implicated in different abiotic stress conditions in plants, including drought (Yoo et al., 2019; Zhao et al., 2018a).
Identification of positively selected genes
A total of 199 genes showed positive selection in Aloe vera with the FDR q-value threshold of 0.05. The distribution of these genes in eggNOG categories, KEGG pathways, and GO term categories are mentioned in Tables S11–S15. Among the genes with positive selection, several genes were involved in key functions with specific phenotypic consequences (Figure S5). These included flowering-related genes that are important for the reproductive success, calcium-ion-binding and transcription factors/sequence-specific DNA-binding genes involved in signal transduction for response to external stimulus, carbohydrate catabolism genes required for energy production, and genes involved in abiotic stress response (Agarwal and Jha, 2010; Takatsuji, 1998; Tuteja and Mahajan, 2007). Among the abiotic stress response genes, there were four categories of genes: water-related stress response genes, DNA damage response genes involved in reactive oxidative species (ROS) stress response, nuclear pore complex genes involved in plant stress response by regulating the nucleo-cytoplasmic trafficking, and secondary metabolites-biosynthesis-related genes that deal with different types of biotic and abiotic stresses (Naik and Al-Khayri, 2016; Roldán-Arjona and Ariza, 2009; Yang et al., 2017). The robust and efficient DNA damage response mechanism is essential for biotic and abiotic stress tolerance and for the genomic stability (Nisa et al., 2019). Thus, adaptive evolution in this pathway seemingly contributes toward the stress tolerance capabilities and genomic stability in Aloe vera.
Another gene G6PD5 that showed positive selection in Aloe vera protects plants against different types of stress such as salinity stress by producing nitric oxide (NO) molecule, which leads to the expression of defense response genes (Arasimowicz and Floryszak-Wieczorek, 2007; Liu et al., 2007). Regulation of osmotic potential under drought stress is acquired by different ion channels, transporters, and carrier proteins (Bray, 1993). In this study, K+ transporter 1(KT1), bidirectional amino acid transporter 1(BAT1), and “BASS6,” a sodium/metabolite co-transporter gene, were found to be positively selected in Aloe vera.
The abscisic acid (ABA) responsive element binding factor (ABF) gene was found to be positively selected. This gene is differentially expressed under drought and other abiotic stress and alters specific target gene expression by binding to ABRE (abscisic-acid-response element), the characteristic element of ABA-inducible genes (Feng et al., 2019). A previous study showed that mutations in this gene lead to increased sensitivity to drought (Yoshida et al., 2015). ABA also regulates stomatal closure and solute transport and thus have implications in drought tolerance (Yamaguchi-Shinozaki and Shinozaki, 2006). The trehalase 1 (TRE1) gene was also found to be positively selected, and the overexpression of this gene causes better drought tolerance through ABA-guided stomatal closure (Van Houtte et al., 2013).
Genes with site-specific signs of evolution
Two types of site-specific signatures of adaptive evolution, i.e., positively selected codon sites and unique amino acid substitutions with significant functional impact, were identified in Aloe vera. The unique substitutions analysis has the potential to reveal the amino acid substitutions, which are specific to the species of interest and can significantly affect the protein function. However, the number of genes with unique substitutions may increase with the increase in genetic distance, and thus the usage of closely related species is desired to make the analysis more reliable. Therefore, for this analysis we have only used the monocot genomes that were available on the Ensembl plant genome database to make the analysis more robust and reliable. The positively selected codon sites analysis identifies the codon sites that are under positive selection in our species of interest; the setup of using monocot genomes along with Arabidopsis thaliana as outgroup also helped in effectively identifying the positively selected codon sites with higher accuracy.
A total of 1,848 genes had positively selected codon sites, and a total of 2,669 genes had unique amino acid substitutions with functional impact. The distribution of genes with positively selected codon sites and unique amino acid substitutions with functional impact in eggNOG categories, KEGG pathways, and GO term categories are mentioned in Supplemental Tables S16–S25.
One of the characteristics of succulent plants such as Aloe vera is the ability to efficiently assimilate the atmospheric CO2 and reduce water loss by transpiration through the CAM pathway, a specific mode of photosynthesis. The evolution of CAM is an adaptation to the limited CO2 and limited water condition, and a significant correlation between higher succulence and increased magnitude of CAM metabolism has been observed (Teeri et al., 1981). In this study, several crucial genes of CAM metabolism showed site-specific signatures of adaptive evolution in Aloe vera (Figure 2A). The potassium channel involved in stomatal opening/closure (KAT2), malic enzyme (ME) that converts malic acid to pyruvate, and phosphoenolpyruvate carboxylase (PEPC) that converts phosphoenolpyruvate to oxaloacetate and assimilates the environmental CO2 showed both the signs of site-specific adaptive evolution. In addition, the other CAM genes including potassium transport 2/3 (KT2/3), pyruvate orthophosphate dikinase (PPDK), phosphoenolpyruvate carboxylase kinase 1 (PPCK1), carbonic anhydrase 1 (CA1), peroxisomal NAD-malate dehydrogenase 2 (PMDH2), tonoplast dicarboxylate transporter (TDT), and aluminum-activated malate transporter family protein (ALMT9) showed unique substitutions with functional impact on Aloe vera.
Figure 2.
The adaptive evolution of CAM pathway, plant circadian rhythm, and plant hormone signaling in Aloe vera
The important genes of the CAM pathway, plant circadian rhythm, and plant hormone signaling are shown with their function: (A) for CAM pathway, (B) for plant circadian rhythm, and (C) for plant hormone signaling. The genes in Lavender had positively selected codon sites, the genes in Green had unique substitutions with function impact, and the genes in Red showed both the signs of site-specific adaptive evolution in Aloe vera. There were no CAM pathway genes that had only positively selected codon sites.
See also Tables S16–S25.
CAM metabolism evolution is known to be a result of modified circadian regulation at the transcription and posttranscriptional levels (Mallona et al., 2011). CAM evolution is a well-characterized physiological rhythm in plants, and it is also a specific example of circadian clock-based specialization (Mallona et al., 2011; Silvera et al., 2010). Several plant circadian rhythm genes showed site-specific signs of adaptive evolution in Aloe vera (Figure 2B). Three essential genes of red light response—PHYB, ELF3, and LHY showed both the signs of site-specific adaptive evolution. Also, the FT gene important for flowering and under the control of circadian rhythm showed both the signs of site-specific adaptive evolution. The PHYA gene, which is also a part of the red light response, had unique substitutions with functional impact. Among the blue light response genes, three genes—GI, FKF1, and SPA2 had unique substitutions with functional impact, and two genes—HY5 and CHS had positively selected codon sites. The blue light response regulates the UV protection and photomorphogenesis.
Plant hormone signaling regulates plant growth, development, and response to different types of biotic and abiotic stress (Santner and Estelle, 2009). Multiple genes of auxin, cytokinin, and brassinosteroid hormone signaling involved in cellular growth and elongation having implications in cellular and tissue succulence showed site-specific signatures of adaptive evolution (Figure 2C). The genes of the abscisic acid hormone signaling involved in stomatal opening/closure required for CAM metabolism and different biotic and abiotic stress response (Feng et al., 2019) had positively selected codon sites and unique substitution sites with functional impact (Figure 2C). Also, the genes involved in salicylic acid signaling important for providing disease resistance and help in biotic stress response showed site-specific signatures of adaptive evolution (Figure 2C).
Genes with multiple signs of adaptive evolution
Among the three signatures of adaptive evolution i.e., positive selection, a higher rate of evolution, and unique amino acid substitutions with functional impact, a total of 148 genes showed two or more signs of adaptive evolution and were identified as the genes with multiple signs of adaptive evolution (MSA) (Chakraborty et al., 2020; Jaiswal et al., 2018; Mittal et al., 2019). The distribution of these genes in eggNOG categories, KEGG pathways, and GO categories are mentioned in Tables S26–S29. Another study that performed the proteomic analysis of drought stress response in wild peach also found similar categories to be enriched in the proteins that were differentially expressed under drought conditions (Cao et al., 2017). A total of 90 genes out of the 148 MSA genes in Aloe vera were from the specific categories that are associated with drought stress tolerance (Supplementary Data Sheet 1.). The literature references for MSA genes that were considered to be associated with drought stress tolerance mechanisms are mentioned in Supplementary Data Sheet 1. The specific groups of proteins and their relation with the drought stress tolerance are mentioned in Figure 3.
Figure 3.
The MSA genes in Aloe vera that are involved in drought stress response
The relation of specific categories of genes with drought stress response was determined from the literature. The standard Arabidopsis thaliana gene IDs were used in case of genes that did not have a standard gene symbol.
See also Tables S26–S30.
Several ribosomal genes and transcription factors genes were found to be MSA genes in this study, and these were also found to be overexpressed under drought conditions in different proteomic and transcriptomic studies and aid in better drought stress survival (Cao et al., 2017; Janiak et al., 2018; Moin et al., 2016). Mutational studies in these genes have also shown their role in drought resistance (Moin et al., 2016). Many nuclear genes are involved in the functioning of symbiotic organelles chloroplast and mitochondria. Some of these genes are also involved in the organellar gene expression (OGEs) regulation, and their mutants are known to show altered response to different abiotic stress, including high salinity stress (Leister et al., 2017; Robles and Quesada, 2019). Several of these genes belonging to two categories, RNA helicases and PPR domain proteins, were found to be MSA genes. Thus, in the Aloe vera species, these genes have been adaptively evolved to provide this species with better salt tolerance.
Two osmotic biosensor genes—“CPA” and “AT2G42100” were found to be among the MSA genes in Aloe vera. Different membrane transporters that can transport signaling molecules, osmolytes, and metals were also among the MSA genes (Figure 3). These included two peroxisomal transporters—“PNC1” a nucleotide carrier protein and “PEX14” a transporter for PTS1 and PTS2 domain containing signaling proteins and different heavy metal transporters such as “IRT3,” an iron transporter, “AT4G17650,” a lipid transporter, “AT2G40420,” an amino acid transporter, “ALA1,” a phospholipid transporter, “NAT8,” a nucleobase-ascorbate transporter, “NRT2.6,” a high-affinity nitrate transporter, and “BASS6,” a sodium/metabolite co-transporter. These osmotic sensors and transporters provide significant enhancement in function in drought stress condition and help in adjusting to the water scarcity (Iqbal, 2018; Jarzyniak and Jasiński, 2014).
The genes for several kinases and WD-40 repeat proteins were also found to be among the MSA genes in Aloe vera. These proteins are involved in signaling and transcription regulation required for the drought stress tolerance (Feyissa et al., 2019; Janiak et al., 2018; Liu et al., 2017; Ranjan and Sawant, 2015). Also, the genes involved in energy generation and are part of the thylakoid membrane showed MSA. The stability of thylakoid membrane proteins has been associated with drought resistance, and these energy-production-related genes are crucial in survival during the drought stress (Janiak et al., 2018; Tian et al., 2013). Two genes that assist in protein folding were found to show MSA (Figure 3), and these proteins are very important in protecting the macromolecules of the cells under drought stress conditions (Shinozaki and Yamaguchi-Shinozaki, 2007). Four genes involved in plant hormone signaling were also among the MSA genes. The plant hormone signaling is central to the signaling pathways required for drought stress tolerance (Tiwari et al., 2017). Four genes involved in flowering and reproduction regulation were also found to be among the MSA genes in Aloe vera. The flowering and reproduction-related genes are known to be regulated for better reproductive success under drought stress conditions as part of the drought tolerance strategy used by many plants (Monroe et al., 2018; Song et al., 2017).
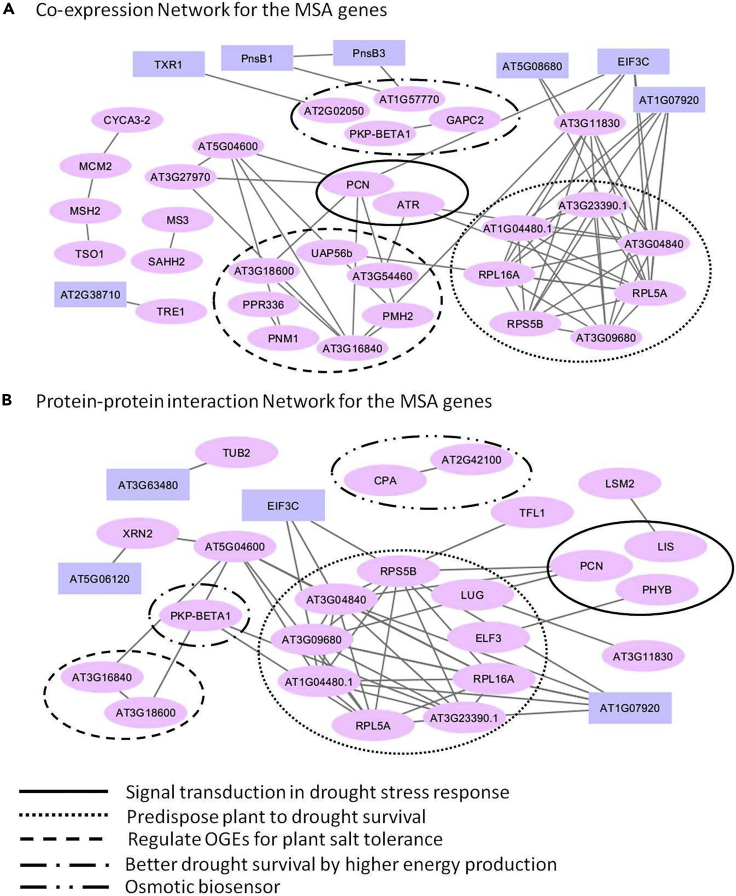
The co-expression of MSA genes was examined using the co-expression data from the STRING database (Szklarczyk et al., 2017), and the MSA genes that co-express with at least one other MSA gene are displayed as a network diagram (Figure 4A). From the network, it is evident that majority of co-expressing MSA genes are associated with drought stress tolerance mechanisms, and the genes forming the dense network are also related to drought stress tolerance. Predominantly, three categories of drought-stress-tolerance-related MSA genes have shown co-expression: genes involved in energy production, genes involved in OGEs regulation, and genes that predispose plants to drought stress tolerance.
Figure 4.
Evaluating the co-expression and physical interaction of MSA genes in Aloe vera
(A) The co-expression network of the MSA genes is shown. Only the MSA genes that showed at least one co-expression connection are shown. The nodes represent the genes, and the edges represent the co-expression of the connected nodes.
(B) The protein-protein interaction network of the MSA genes is shown. Only the MSA genes that showed at least one protein-protein interaction are shown. The nodes represent the genes, and the edges represent the protein-protein interaction between the connected nodes.
The genes shown in Pink are the genes involved in drought stress tolerance mechanisms, and genes shown in Lavender had other functions. To check the literature reference for the genes associated with the drought stress tolerance mechanisms, please refer to Table S30. The standard Arabidopsis thaliana gene IDs were used in case of genes that did not have a standard gene symbol.
Similarly, a network diagram was constructed using the protein-protein interaction data of MSA genes from the STRING database (Szklarczyk et al., 2017). The genes with physical interaction known from the experimental studies are shown as a network diagram in Figure 4B. From the network, it is apparent that among the interacting MSA genes, all of them except four are associated with drought stress tolerance mechanisms. Further, among the MSA genes, primarily the genes that predispose plants to drought stress tolerance showed the physical interaction. In addition, three genes involved in signal transduction in drought stress response, two genes that function as osmotic biosensors, and two OGEs regulation genes also displayed physical interaction.
Discussion
In this work, we have reported the complete draft genome sequence of Aloe vera, which is an evolutionarily important, ornamental, and widely used plant species due to its medicinal properties, pharmacological applications, traditional usage, and commercial value. The availability of Aloe vera genome sequence is also important because it is the first genome sequenced from the Asphodelaceae plant family and is the largest angiosperm and the fifth largest genome sequenced so far. It is also the largest genome sequenced using the Oxford Nanopore technology till date.
The high level of heterozygosity is one of the key challenges in genome assembly because the de novo assembler tries to generate a single haploid output from the allelic differences, which is difficult to achieve with high levels of heterozygosity (Asalone et al., 2020). The estimated heterozygosity for the Aloe vera genome was 11.3%, which is even higher than the heterozygosity of wheat genome (10.1%) that required the combined effort of several research institutions across the world and more than a decade for its completion. Thus, the hybrid approach of using short-read (Illumina) and long-read (Nanopore) sequence data emerged as a successful assembly strategy to overcome the challenge of constructing one of the largest plant genomes. The study also reported the gene set of Aloe vera constructed using the combination of de novo and homology-based gene predictions, and also using the data from the genomic assembly and the transcriptomic assembly from multiple tissues, thus indicating the comprehensiveness of the approach.
This study reported the first genome-wide phylogeny of Aloe vera with all other monocot species available on the Ensembl plant database and with Arabidopsis thaliana as an outgroup. A few previous studies have also examined the phylogenetic position of Aloe vera with respect to other monocots but used a few genomic loci. Thus, this is the first genome-wide phylogeny of monocots that resolves the phylogenetic position of Aloe vera with respect to the other monocots by using 1,440 different loci distributed throughout their genomes. The very high bootstrap values for the internal nodes and existence of no polytomy in the phylogeny further attest to the correctness of the phylogeny. This phylogeny is mostly in agreement with the previously known phylogenies and also provided new insights (Dunemann et al., 2014; Grace et al., 2015; Qian and Jin, 2016; Silvera et al., 2014; Wang and Deng, 2016).
An earlier phylogeny constructed using “ppc-aL1a” gene showed that Sorghum bicolor, Zea mays, Setaria italica, Brachypodium distachyon, Hordeum vulgare, and Oryza sativa form a monophyletic group, which was also observed in our phylogeny (Silvera et al., 2014). Similarly, the relative positions of Hordeum vulgare, Saccharum officinarum, Zea mays, and Oryza sativa in another phylogeny based on “CENH3” gene were in agreement with our phylogeny (Dunemann et al., 2014). Using the “NORK” gene, another recent study reported the relative phylogenetic position of four monocot species: Oryza sativa, Zea mays, Sorghum bicolor, and Setaria italica (Wang and Deng, 2016). Zea mays, Sorghum bicolor, and Setaria italica were found to share a recent last common ancestor, and Oryza sativa had diverged earlier from their common ancestor, which is also supported by the genome-wide phylogeny reported in this study.
Although the genome-wide phylogeny showed the species of Poales order with similar topology as reported in earlier studies, a different topology was observed for the relative position of Musa acuminata, Dioscorea rotundata, and Aloe vera from the orders Zingiberales, Dioscoreales, and Asparagales, respectively (Chase et al., 2016; Qian and Jin, 2016). The observed differences could be due to the usage of a few genomic loci in the previous phylogenies, whereas the phylogeny reported in this study is a genome-wide phylogeny constructed using 1,440 one-to-one orthologs distributed across the genome. The availability of more complete genomes from monocots and the inclusion of more genomic loci in the phylogenetic analysis will help in explaining the observed differences and confirm the relative positions of these species.
One of the key highlights of the study was the revelation of adaptive evolution of genes involved in drought stress response, which provides a genetic explanation for the drought stress tolerance properties of Aloe vera. This plant is known to display a number of phenotypes such as perennial succulent leaves and CAM mechanism for carbon fixation that provide it with better drought stress survival (Jin et al., 2007). Several experimental studies have also reported that it can make adjustments such as increased production of sugars and increased expression of heat-shock and ubiquitin proteins for efficient water utilization and osmotic maintenance that eventually provide better drought survival (Delatorre-Herrera et al., 2010; Hazrati et al., 2017; Huerta et al., 2013). In this study, the majority (60.81%) of genes that showed multiple signs of evolution (MSA) were involved in drought-stress-tolerance-related functions. These genes were also found to be co-expressing and physically interacting with each other, which further point toward the adaptive evolution of the drought stress tolerance mechanisms in this species. The adaptive evolution of genes involved in drought stress tolerance provides insights into the genetic basis of drought resistance property of Aloe vera.
Several crucial genes of CAM pathway and circadian rhythm have also shown site-specific signs of adaptive evolution in Aloe vera in comparison to the other monocot species. The CAM pathway has very high water use efficiency and is known to have evolved convergently in many arid regions for better drought survival (Ming et al., 2015). Also, the CAM pathway is a physiological rhythm with temporal separation of atmospheric CO2 assimilation and Calvin-Benson cycle and is under the control of plant circadian rhythm (Mallona et al., 2011; Yin et al., 2018). This CAM pathway evolution is known to be a specific type of circadian rhythm specialization (Hartwell, 2018; Silvera et al., 2010). Thus, the observed adaptive evolution of CAM pathway and its controller circadian rhythm in this study point toward its role in providing this species an evolutionary advantage for efficient drought stress survival. Furthermore, it should be noted that among the genes, which showed adaptive evolution in Aloe vera such as the ones involved in drought stress response, CAM pathway, and circadian rhythm, only the genes with previously known functional role from experimental studies were used for the interpretation. Therefore, it is likely that the genes with adaptive evolution in Aloe vera may have phenotypic consequences.
The evolutionary success of the Aloe genus is also known to be due to the succulent leaf Mesophyll tissue (Grace et al., 2015). Particularly, the medicinal use of Aloe vera is much associated with the succulent leaf mesophyll tissue, and a loss of this tissue leads to the loss of medicinal properties (Reynolds and Dweck, 1999). The plant species with CAM pathway have large vacuoles in comparison to the non-CAM plants, and therefore, the leaf succulence is also higher in CAM plants. Thus, it is tempting to speculate that the observed evolution of CAM pathway in Aloe vera may also be crucial for the higher leaf mesophyll succulence contributing to its medicinal properties. Also previously, it has been proposed that the specific properties of Aloe vera such as the high leaf succulence, medicinal properties, and drought resistance are the consequences of evolutionary processes such as selection and speciation rather than due to phylogenetic diversity or isolation (Grace et al., 2015). The signatures of adaptive evolution in drought tolerance and CAM pathway genes in Aloe vera further substantiate this notion.
Limitations of the study
Because of the unavailability of any other genome from the Aloe genus or from the Asphodelaceae family, the phylogenetic and adaptive evolution analysis was performed on the available species that were distantly related to Aloe vera. Thus, the availability of more genomes from closely related plant species will provide more insights into the unique properties of this species and its family. Further, the large genome size and high heterozygosity in plant genomes such as Aloe vera are among the key challenges for their complete assembly, and the availability of more data, improved algorithms, and tools are likely to help in generating a more comprehensive and contiguous assembly of this species.
Resource availability
Lead contact
Further information, requests, and inquiries should be directed to the Lead Contact, Vineet K. Sharma (vineetks@iiserb.ac.in).
Materials availability
All the materials and methods used for the generation of data and analysis are mentioned in the manuscript text. The generated data were deposited to the public repositories, and accession numbers are mentioned in “Data and code availability” section.
Data and code availability
The accession number for the Aloe vera sequence data (DNA and RNA) reported in this paper are NCBI BioProject accession number: PRJNA634897, NCBI Biosample accession number: SAMN15010737, and NCBI accession codes: SRR11842980, SRR11842979, SRR11842978, and SRR11842977.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
The author SKJ thanks Department of Science and Technology, India for the DST-INSPIRE fellowship. AC and SM thank Council for Scientific and Industrial Research (CSIR), India for fellowship. SK thanks University Grants Commission (UGC), India for the fellowship. The authors thank the intramural research funds provided by IISER Bhopal, India. We also acknowledge the help of Mr. Vishnu Prasoodanan PK in preparing Figures 1 and 3.
Author contributions
VKS conceived and coordinated the project. SM prepared the DNA and RNA samples, performed sequencing, and the species identification assay. SKJ and VKS designed the computational framework of the study. SKJ and AC performed the genome assembly, transcriptome assembly, genome annotation, gene set construction, orthology analysis, and species phylogenetic tree construction. SKJ performed the root-to-tip branch length, positive selection, unique substitution with functional impact, network, and statistical analysis. SKJ, AC, SK, and SM performed the functional annotation of gene sets. SKJ, AC, and VKS analyzed the data. SKJ, AC, and VKS interpreted the results. SKJ constructed the figures. SKJ, AC, SM, SK, and VKS wrote and revised the manuscript. All the authors have read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102079.
Supplemental information
The gene IDs, description, different signs of adaptive evolution, and the involvement of these genes in the drought stress response are mentioned in separate columns. (If a gene is considered to be involved in drought stress tolerance/response, the corresponding reference is also mentioned in the last column).
References
- Adams S.P., Leitch I.J., Bennett M.D., Chase M.W., Leitch A.R. Ribosomal DNA evolution and phylogeny in Aloe (Asphodelaceae) Am. J. Bot. 2000;87:1578–1583. [PubMed] [Google Scholar]
- Agarwal P., Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010;54:201–212. [Google Scholar]
- Arasimowicz M., Floryszak-Wieczorek J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007;172:876–887. [Google Scholar]
- Asalone K.C., Ryan K.M., Yamadi M., Cohen A.L., Farmer W.G., George D.J., Joppert C., Kim K., Mughal M.F., Said R. Regional sequence expansion or collapse in heterozygous genome assemblies. PLoS Comput. Biol. 2020;16:e1008104. doi: 10.1371/journal.pcbi.1008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birol I., Raymond A., Jackman S.D., Pleasance S., Coope R., Taylor G.A., Yuen M.M.S., Keeling C.I., Brand D., Vandervalk B.P. Assembling the 20 Gb white spruce (Picea glauca) genome from whole-genome shotgun sequencing data. Bioinformatics. 2013;29:1492–1497. doi: 10.1093/bioinformatics/btt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E.A. Molecular responses to water deficit. Plant Physiol. 1993;103:1035. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer K. Early Cretaceous lineages of monocot flowering plants. Proc. Natl. Acad. Sci. U S A. 2000;97:4707–4711. doi: 10.1073/pnas.080421597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Luo Q., Tian Y., Meng F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017;17:53. doi: 10.1186/s12870-017-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A., Mahajan S., Jaiswal S.K., Sharma V.K. Genome sequencing of turmeric provides evolutionary insights into its medicinal properties. bioRxiv. 2020 doi: 10.1101/2020.09.07.286245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M.W., Christenhusz M., Fay M., Byng J., Judd W.S., Soltis D., Mabberley D., Sennikov A., Soltis P.S., Stevens P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016;181:1–20. [Google Scholar]
- Choudhri P., Rani M., Sangwan R.S., Kumar R., Kumar A., Chhokar V. De novo sequencing, assembly and characterisation of Aloe vera transcriptome and analysis of expression profiles of genes related to saponin and anthraquinone metabolism. BMC Genomics. 2018;19:427. doi: 10.1186/s12864-018-4819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatorre-Herrera J., Delfino I., Salinas C., Silva H., Cardemil L. Irrigation restriction effects on water use efficiency and osmotic adjustment in Aloe vera plants (Aloe barbadensis Miller) Agric. Water Manage. 2010;97:1564–1570. [Google Scholar]
- Dolezel J., Bartos J., Voglmayr H., Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytometry A J. Int. Soc. Anal. Cytol. 2003;51:127–128. doi: 10.1002/cyto.a.10013. author reply 129. [DOI] [PubMed] [Google Scholar]
- Dunemann F., Schrader O., Budahn H., Houben A. Characterization of centromeric histone H3 (CENH3) variants in cultivated and wild carrots (Daucus sp.) PLoS One. 2014;9:e98504. doi: 10.1371/journal.pone.0098504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X., Hou L., Shi J., Yang T., Liu Y., Wei A. Patterns of drought response of 38 WRKY transcription factors of Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci. 2019;20:68. doi: 10.3390/ijms20010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R.-J., Ren M.-Y., Lu L.-F., Peng M., Guan X., Zhou D.-B., Zhang M.-Y., Qi D.-F., Li K., Tang W. Involvement of abscisic acid-responsive element-binding factors in cassava (Manihot esculenta) dehydration stress response. Scientific Rep. 2019;9:1–12. doi: 10.1038/s41598-019-49083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa B.A., Arshad M., Gruber M.Y., Kohalmi S.E., Hannoufa A. The interplay between miR156/SPL13 and DFR/WD40–1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019;19:1–19. doi: 10.1186/s12870-019-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiz-Palacios O., Schneider H., Heinrichs J., Savolainen V. Diversification of land plants: insights from a family-level phylogenetic analysis. BMC Evol. Biol. 2011;11:341. doi: 10.1186/1471-2148-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace O.M., Buerki S., Symonds M.R., Forest F., van Wyk A.E., Smith G.F., Klopper R.R., Bjorå C.S., Neale S., Demissew S. Evolutionary history and leaf succulence as explanations for medicinal use in aloes and the global popularity of Aloe vera. BMC Evol. Biol. 2015;15:29. doi: 10.1186/s12862-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V.K., Malhotra S. Pharmacological attribute of Aloe vera: Revalidation through experimental and clinical studies. Ayu. 2012;33:193. doi: 10.4103/0974-8520.105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J. The circadian clock in CAM plants. Annu. Plant Rev. Online. 2018;21:211–236. [Google Scholar]
- Hazrati S., Tahmasebi-Sarvestani Z., Mokhtassi-Bidgoli A., Modarres-Sanavy S.A.M., Mohammadi H., Nicola S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Management. 2017;181:66–72. [Google Scholar]
- Huerta C., Freire M., Cardemil L. Expression of hsp70, hsp100 and ubiquitin in Aloe barbadensis Miller under direct heat stress and under temperature acclimation conditions. Plant Cell Rep. 2013;32:293–307. doi: 10.1007/s00299-012-1363-4. [DOI] [PubMed] [Google Scholar]
- Iqbal M.J. Role of osmolytes and antioxidant enzymes for drought tolerance in wheat. Glob. Wheat Prod. 2018;51 doi: 10.5772/intechopen.75926. [DOI] [Google Scholar]
- Jaiswal S.K., Gupta A., Saxena R., Prasoodanan V.P.K., Sharma A.K., Mittal P., Roy A., Shafer A.B.A., Vijay N., Sharma V.K. Genome sequence of peacock reveals the peculiar case of a glittering bird. Front. Genet. 2018 doi: 10.3389/fgene.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak A., Kwasniewski M., Sowa M., Gajek K., Żmuda K., Kościelniak J., Szarejko I. No time to waste: transcriptome study reveals that drought tolerance in barley may be attributed to stressed-like expression patterns that exist before the occurrence of stress. Front. Plant Sci. 2018;8:2212. doi: 10.3389/fpls.2017.02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzyniak K.M., Jasiński M. Membrane transporters and drought resistance–a complex issue. Front. Plant Sci. 2014;5:687. doi: 10.3389/fpls.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.M., Wang C.H., Liu Z.P., Gong W.J. Physiological and ecological characters studies on Aloe vera under soil salinity and seawater irrigation. Process Biochem. 2007;42:710–714. [Google Scholar]
- Joseph B., Raj S.J. Pharmacognostic and phytochemical properties of Aloe vera linn an overview. Int. J. Pharm. Sci. Rev. Res. 2010;4:106–110. [Google Scholar]
- Leister D., Wang L., Kleine T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017;8:387. doi: 10.3389/fpls.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Li Y.H., Yuan H.M., Zhang B.L., Zhai S., Lu Y.T. WD40-REPEAT 5a functions in drought stress tolerance by regulating nitric oxide accumulation in Arabidopsis. Plant Cell Environ. 2017;40:543–552. doi: 10.1111/pce.12723. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wu R., Wan Q., Xie G., Bi Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007;48:511–522. doi: 10.1093/pcp/pcm020. [DOI] [PubMed] [Google Scholar]
- Mallona I., Egea-Cortines M., Weiss J. Conserved and divergent rhythms of crassulacean acid metabolism-related and core clock gene expression in the cactus Opuntia ficus-indica. Plant Physiol. 2011;156:1978–1989. doi: 10.1104/pp.111.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming R., VanBuren R., Wai C.M., Tang H., Schatz M.C., Bowers J.E., Lyons E., Wang M.-L., Chen J., Biggers E. The pineapple genome and the evolution of CAM photosynthesis. Nat. Genet. 2015;47:1435–1442. doi: 10.1038/ng.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal P., Jaiswal S.K., Vijay N., Saxena R., Sharma V.K. Comparative analysis of corrected tiger genome provides clues to its neuronal evolution. Sci. Rep. 2019 doi: 10.1038/s41598-019-54838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin M., Bakshi A., Saha A., Udaya Kumar M., Reddy A.R., Rao K., Siddiq E., Kirti P. Activation tagging in indica rice identifies ribosomal proteins as potential targets for manipulation of water-use efficiency and abiotic stress tolerance in plants. Plant Cell Environ. 2016;39:2440–2459. doi: 10.1111/pce.12796. [DOI] [PubMed] [Google Scholar]
- Monroe J.G., Powell T., Price N., Mullen J.L., Howard A., Evans K., Lovell J.T., McKay J.K. Drought adaptation in Arabidopsis thaliana by extensive genetic loss-of-function. Elife. 2018;7:e41038. doi: 10.7554/eLife.41038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P.M., Al-Khayri J.M. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. In: Shanker A., Shanker C., editors. Abiotic and Biotic Stress in Plants: Recent Advances and Future Perspectives. 2016. pp. 247–277. [Google Scholar]
- Neale D.B., Wegrzyn J.L., Stevens K.A., Zimin A.V., Puiu D., Crepeau M.W., Cardeno C., Koriabine M., Holtz-Morris A.E., Liechty J.D. Decoding the massive genome of loblolly pine using haploid DNA and novel assembly strategies. Genome Biol. 2014;15:R59. doi: 10.1186/gb-2014-15-3-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisa M.-U., Huang Y., Benhamed M., Raynaud C. The plant DNA damage response: signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P.S., Jordan P.W. Transpiration stream of desert species: resistances and capacitances for a C3, a C4, and a CAM plant. J. Exp. Bot. 1983;34:1379–1391. [Google Scholar]
- Nystedt B., Street N.R., Wetterbom A., Zuccolo A., Lin Y.-C., Scofield D.G., Vezzi F., Delhomme N., Giacomello S., Alexeyenko A. The Norway spruce genome sequence and conifer genome evolution. nature. 2013;497:579–584. doi: 10.1038/nature12211. [DOI] [PubMed] [Google Scholar]
- Pouget M., Youssef S., Dumas P.-J., Baumberger T., San Roman A., Torre F., Affre L., Médail F., Baumel A. Spatial mismatches between plant biodiversity facets and evolutionary legacy in the vicinity of a major Mediterranean city. Ecol. Indicators. 2016;60:736–745. [Google Scholar]
- Qian H., Jin Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 2016;9:233–239. [Google Scholar]
- Raksha B., Pooja S., Babu S. Bioactive compounds and medicinal properties of Aloe vera L.: an update. J. Plant Sci. 2014;2:102–107. [Google Scholar]
- Ranjan A., Sawant S. Genome-wide transcriptomic comparison of cotton (Gossypium herbaceum) leaf and root under drought stress. 3 Biotech. 2015;5:585–596. doi: 10.1007/s13205-014-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.-J., Wang J., Lee K.-K., Deng H., Xue H., Zhang N., Zhao J.-C., Cao T., Cui C.-L., Zhang X.-H. The complete chloroplast genome of Aloe vera from China as a Chinese herb. Mitochondrial DNA B. 2020;5:1092–1093. doi: 10.1080/23802359.2020.1726229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T., Dweck A. Aloe vera leaf gel: a review update. J. Ethnopharmacology. 1999;68:3–37. doi: 10.1016/s0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Robles P., Quesada V. Transcriptional and post-transcriptional regulation of organellar gene expression (OGE) and its roles in plant salt tolerance. Int. J. Mol. Sci. 2019;20:1056. doi: 10.3390/ijms20051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán-Arjona T., Ariza R.R. Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. Rev. Mutat. Res. 2009;681:169–179. doi: 10.1016/j.mrrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Santner A., Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–1078. doi: 10.1038/nature08122. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Silva H., Sagardia S., Seguel O., Torres C., Tapia C., Franck N., Cardemil L. Effect of water availability on growth and water use efficiency for biomass and gel production in Aloe vera (Aloe barbadensis M.) Ind. Crops Prod. 2010;31:20–27. [Google Scholar]
- Silvera K., Neubig K.M., Whitten W.M., Williams N.H., Winter K., Cushman J.C. Evolution along the crassulacean acid metabolism continuum. Funct. Plant Biol. 2010;37:995–1010. [Google Scholar]
- Silvera K., Winter K., Rodriguez B.L., Albion R.L., Cushman J.C. Multiple isoforms of phospho enol pyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): implications for the evolution of crassulacean acid metabolism. J. Exp. Bot. 2014;65:3623–3636. doi: 10.1093/jxb/eru234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Kim H.C., Shin S., Kim K.-H., Moon J.-C., Kim J.Y., Lee B.-M. Transcriptome analysis of flowering time genes under drought stress in maize leaves. Front. Plant Sci. 2017;8:267. doi: 10.3389/fpls.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K.A., Wegrzyn J.L., Zimin A., Puiu D., Crepeau M., Cardeno C., Paul R., Gonzalez-Ibeas D., Koriabine M., Holtz-Morris A.E. Sequence of the sugar pine megagenome. Genetics. 2016;204:1613–1626. doi: 10.1534/genetics.116.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic. Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuji H. Zinc-finger transcription factors in plants. Cell Mol. Life Sci. CMLS. 1998;54:582–596. doi: 10.1007/s000180050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeri J., Tonsor S., Turner M. Leaf thickness and carbon isotope composition in the Crassulaceae. Oecologia. 1981;50:367–369. doi: 10.1007/BF00344977. [DOI] [PubMed] [Google Scholar]
- Tian F., Gong J., Zhang J., Zhang M., Wang G., Li A., Wang W. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J. Exp. Bot. 2013;64:1509–1520. doi: 10.1093/jxb/ert004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Lata C., Singh Chauhan P., Prasad V., Prasad M. A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr. Genomics. 2017;18:469–482. doi: 10.2174/1389202918666170605083319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J., Smith G.F., Van Wyk B.-E., Wink M. Phylogenetic relationships in Asphodelaceae (subfamily Alooideae) inferred from chloroplast DNA sequences (rbcL, matK) and from genomic fingerprinting (ISSR) Taxon. 2003;52:193–207. [Google Scholar]
- Tuteja N., Mahajan S. Calcium signaling network in plants: an overview. Plant Signal. Behav. 2007;2:79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtte H., Vandesteene L., López-Galvis L., Lemmens L., Kissel E., Carpentier S., Feil R., Avonce N., Beeckman T., Lunn J.E. Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol. 2013;161:1158–1171. doi: 10.1104/pp.112.211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Deng L. GmACP expression is decreased in GmNORK knockdown transgenic soybean roots. Crop J. 2016;4:509–516. [Google Scholar]
- Waseem M., Li Z. Dissecting the role of a basic helix-loop-helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front. Plant Sci. 2019;10:734. doi: 10.3389/fpls.2019.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett N.J., Mirarab S., Nguyen N., Warnow T., Carpenter E., Matasci N., Ayyampalayam S., Barker M.S., Burleigh J.G., Gitzendanner M.A. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. U S A. 2014;111:E4859–E4868. doi: 10.1073/pnas.1323926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang W., Chu Z., Zhu J.-K., Zhang H. Roles of nuclear pores and nucleo-cytoplasmic trafficking in plant stress responses. Front. Plant Sci. 2017;8:574. doi: 10.3389/fpls.2017.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Guo H.-B., Weston D.J., Borland A.M., Ranjan P., Abraham P.E., Jawdy S.S., Wachira J., Tuskan G.A., Tschaplinski T.J. Diel rewiring and positive selection of ancient plant proteins enabled evolution of CAM photosynthesis in Agave. BMC Genomics. 2018;19:588. doi: 10.1186/s12864-018-4964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Y.-H., Hong W.-J., Jung K.-H. A Systematic view exploring the role of chloroplasts in plant abiotic stress responses. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/6534745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Fujita Y., Maruyama K., Mogami J., Todaka D., Shinozaki K., Yamaguchi-Shinozaki K. Four A rabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015;38:35–49. doi: 10.1111/pce.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Haigh A.M., Holford P., Chen Z.-H. Roles of chloroplast retrograde signals and ion transport in plant drought tolerance. Int. J. Mol. Sci. 2018;19:963. doi: 10.3390/ijms19040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Cheng X., Liu X., Wu H., Bi H., Xu H. The wheat MYB transcription factor TaMYB31 is involved in drought stress responses in Arabidopsis. Front. Plant Sci. 2018;9:1426. doi: 10.3389/fpls.2018.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B.J. Genome size analysis of selected species of Aloe (Aloaceae) reveals the most primitive species and results in some new combinations. Bradleya. 2002;2002:5–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The gene IDs, description, different signs of adaptive evolution, and the involvement of these genes in the drought stress response are mentioned in separate columns. (If a gene is considered to be involved in drought stress tolerance/response, the corresponding reference is also mentioned in the last column).
Data Availability Statement
The accession number for the Aloe vera sequence data (DNA and RNA) reported in this paper are NCBI BioProject accession number: PRJNA634897, NCBI Biosample accession number: SAMN15010737, and NCBI accession codes: SRR11842980, SRR11842979, SRR11842978, and SRR11842977.