Summary
Granulosa cells (GCs) play a critical role in folliculogenesis. It remains unclear how GCs expand during follicle development and whether there is a subpopulation of cells that is responsible for GCs growth. Here, we observed that a small population of GCs expressed stem cell surface marker Procr (Protein C receptor). Procr GCs displayed higher proliferation ability and lower levels of hormone receptors compared with Procr- GCs. Knockdown of Procr inhibited proliferation. Lineage tracing experiments demonstrated that they contribute to increasing numbers of GCs during folliculogenesis. Targeted ablation of Procr+ cells disrupted ovarian follicle development, leading to phenotypes of polycystic ovary syndrome. Our findings suggest that Procr-expressing GCs are endowed with high proliferative capacity that is critical for follicle development.
Subject areas: Reproductive Medicine, Developmental Genetics, Molecular Biology, Female Reproductive Endocrinology
Graphical abstract

Highlights
-
•
Develop a new FACS isolation method for mouse GCs
-
•
Procr marks a subset of GCs that have higher proliferative capacity
-
•
Procr+ cells contribute to increasing numbers of GCs during follicle development
-
•
Generate a PCOS mouse model with targeted ablation of Procr+ cells
Reproductive Medicine; Developmental Genetics; Molecular Biology; Female Reproductive Endocrinology
Introduction
Follicles are the basic functional units of the ovary, composed of an immature oocyte, surrounded by granulosa cells (GCs) and theca cells. Reproduction requires controlled development of the oocyte and the somatic cells. Once a follicle is activated, the single layer of flattened GCs first proliferate and differentiate into cuboidal GCs, awakening the dormant oocyte (Zhang and Liu, 2015; Zhang et al., 2014). In the growing follicles, GCs divide for at least 10 times to support follicle growth and oocyte maturation, reaching a total amount of over 2,000 cells at the mature antral stage (Hirshfield, 1991). At the pre-ovulatory stage, the well-differentiated GCs also provide the hormones and other factors to ensure successful ovulation (Stocco et al., 2007). Despite the significant progress made in the regulation of folliculogenesis, it remains unclear how GCs expand during follicle development and whether there is a subpopulation of cells that is responsible for GCs growth.
GCs play a critical role in folliculogenesis through their direct communication with oocytes and theca cells as well as their ability to produce and respond to hormones. Deletion of Forkhead box L2 (Foxl2), a key transcription factor for the development of GCs, results in mammalian ovarian failure by pervasive blockage of follicle development (Pisarska et al., 2004; Uda et al., 2004). GC-specific knockout of Pten gene, the negative regulator of PI3K pathway, leads to enhanced proliferation of GCs, ovulation, and the formation of corpus luteum (Fan et al., 2008). Expansion of GCs is regulated by various ovarian factors via different molecular mechanisms (Lu, 2005). For example, GDF-9 and BMP-15 secreted by oocyte are essential for GCs' proliferation in early follicular development (Eppig, 2001; Su et al., 2004). Follicle-stimulating hormone (FSH) promotes GCs' proliferation by increasing proliferating cell nuclear antigen expression and activating ERK1/2 signal pathway (Yu et al., 2005). Nerve growth factor promotes GCs' expansion by inhibiting ESR2-mediated down-regulation of CDKN1A (Wang et al., 2015b). Wnt2 acts through beta-catenin to regulate mouse GCs' proliferation (Wang et al., 2010).
Dysregulation of GC is involved in a great number of ovarian pathologies. Polycystic ovary syndrome (PCOS), the most common reproductive disorder in women of reproductive age, affects about 5%–20% of the population (Azziz et al., 2016). It is characterized by hyperandrogenism, polycystic ovarian morphology, chronic anovulation, and metabolic disorders including insulin resistance and dyslipidemia (Azziz et al., 2016; Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004). Because of the complicated clinical manifestations and the insufficient research models of PCOS, its pathogenesis is largely unknown and remains to be elucidated.
Protein C receptor (Procr) marks adult stem/progenitor cells in various tissues, including the mammary gland (Wang et al., 2015a), vascular endothelial cells (Yu et al., 2016), pancreatic islet (Wang et al., 2020), ovarian surface epithelium (OSE) (Wang et al., 2019), and hematopoietic system (Balazs et al., 2006). Procr encodes a single-pass transmembrane protein (Fukudome and Esmon, 1994). Procr expression is positively regulated by Wnt signaling (Wang et al., 2015a). Upon binding to its ligand Protein C, Procr activates several intracellular signaling, resulting in increased proliferation and survival in various tissues (Cheng et al., 2003; Gramling et al., 2010; Wang et al., 2018). Indeed, Procr+ OSE cells proliferate faster than Procr- OSE cells (Wang et al., 2019) and Procr+ endothelial cells exhibited more robust proliferation than Procr- endothelial cells (Yu et al., 2016).
In this study, we investigate the role of Procr+ GCs in folliculogenesis. We utilized Procr reporter mice and RNA in situ hybridization to analyze Procr expression during follicle development. We developed new protocol to isolate primary GCs and compare the proliferative abilities between Procr+ and Procr- GCs. Furthermore, through in vivo genetic lineage tracing and targeted ablation, we examine the contribution and significance of Procr+ GCs during follicle development.
Results
Procr is expressed in a subpopulation of granulosa cells
To analyze the expression of Procr in the ovarian follicle, we utilized Procr-rtTA;TetO-H2B-GFP mice (Figure S1A) (Wang et al., 2019). Mice at postnatal day 10 were fed with doxycycline (Dox), and the ovaries were harvested 2 days later (Figure 1A). Shown by immunostaining of ovarian sections, Procr-expressing (GFP+) cells were detected in a small population of GCs in the follicles of different stages (Figure 1B). RNA in situ hybridization further validated the expression of Procr in developing ovarian follicles. Procr was detected in a small number of GCs in all follicular development stages, including primordial, primary, secondary, antral, and pre-ovulatory follicles (Figures 1C–1G). In primordial follicle, where GCs are flattened (Da Silva-Buttkus et al., 2008), Procr was readily detected in one of the GCs in the cross section of primordial follicle (Figure 1C). Quantification indicated that the percentage of Procr+ GC gradually declined to 12.42% ± 2.63% in primary follicle, 4.14% ± 0.31% in secondary follicle, 1.63% ± 0.08% in antral follicle, and 1.13% ± 0.04% in pre-ovulatory follicle (Figure 1H). This may imply that Procr+ cells exert their function in earlier stage of follicle development.
Figure 1.
Procr is expressed in a subpopulation of granulosa cells
(A and B) Procr-rtTA;TetO-H2B-GFP mice at postnatal day 10 were fed with doxycycline for 2 days to induce the expression of H2B-GFP protein in Procr+ cells (A). Confocal images of an ovary section exhibited H2B-GFP expression in the ovarian follicles (B). Scale bar, 100 μm. n = 3 mice and representative image was shown.
(C–H) In situ hybridization showing Procr mRNA expression in the GCs of primordial (C), primary (D), secondary (E), antral (F), and pre-ovulatory (G) follicles. Scale bars, 20 μm (C–E) and 50 μm (F and G). n = 3 mice and representative image was shown. Quantification showing the percentage of Procr+ GCs in different follicle stages (H); at least 10 follicles in different stages were counted, and data are presented as mean ± SEM.
Procr+ granulosa cells have higher proliferation ability and lower differentiation level than Procr- granulosa cells
To examine the behavior of Procr+ GCs, we seek to isolate live Procr+ GCs taking advantage that Procr is a surface protein. However, the purification of GCs from ovarian cells by fluorescence-activated cell sorting (FACS) has not been successfully attempted. In previous methods, GCs were enriched by either puncture of large follicles (ignore the smaller follicles) (Kipp et al., 2007) or simply a digestion of the whole ovary of 10-day old mice (Parvari et al., 2016). Neither ways harvest complete or pure GC cells. Interestingly, we observed that in R26-mTmG mice, the mTomato (mTom) fluorescence is dimmer in follicles compared with the rest of the ovarian section (Figure 2A). It is unclear why an R26-driven exogenous gene would have lower expression in follicles, i.e., GCs. Nevertheless, we exploited this feature to separate GCs from the rest of ovarian cells. Dissociated ovarian cells were separated based on mTom-low and mTom-high (Figure 2B). qPCR analysis indicated that mTom-low ovarian cells, compared with the mTom-high cells, have higher expression of GC markers, such as Cyp19a1, Foxl2, Esr2, Fshr, and lower expression of theca cells marker Esr1 (Figure 2C). These results suggest that using a R26-driven fluorescent reporter, GCs can be FACS-isolated.
Figure 2.
Procr+ granulosa cells display higher proliferation ability
(A) Section imaging of R26-mTmG mice ovary showing lower level of mTomato expression in GCs compared with non-GCs. Scale bar, 20 μm. F, follicle; CL, corpus luteum. n = 3 mice, and representative image was shown.
(B) FACS isolation of R26-mTmG ovarian cells with mTomato and Procr. The percentages of total granulosa cells (mTom-low) and Procr+ granulosa cells (mTom-low, Procr+) are as indicated. Data are pooled from three independent experiments and presented as mean ± SEM.
(C) qPCR analysis of Cyp19a1, Foxl2, Esr1, Esr2, and Fshr in isolated mTom-low and mTom-high ovarian cells verifying the granulosa cells' property of mTom-low. n = 3, and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗∗p < 0.001, ∗∗p < 0.01.
(D and E) Confocal imaging (D) and quantification (E) of EdU staining showing higher proliferation ability in Procr+ GCs compared with Procr- GCs. Scale bars, 100 μm. Total 317 Procr+ cells and 294 Procr- cells from three mice were counted, and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗∗p < 0.001.
(F and G) Isolated primary Procr+ GCs and Procr- GCs were cultured, and cell numbers were counted in each passage (F). Representative images were shown (G). The growth curve showing that Procr+ GCs are more proliferative compared with Procr- GCs. n = 3 times, and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗∗p < 0.001. Scale bars, 100 μm.
(H) qPCR analysis of Foxl2, Cyp19a1, Fshr, and Lhcgr using freshly isolated Procr+ and Procr- GCs. n = 3, and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗p < 0.01, ∗p < 0.05.
Next, we further separated GCs by surface Procr expression. FACS analysis indicated that Procr+ cells comprised 1.03% ± 0.21% of total GCs (Figure 2B), in line with the observations with GFP reporter mice and RNA in situ results. To investigate the proliferation ability, Procr+ and Procr- GCs were placed in culture and treated with EdU for 2 hours followed by detection of fluorescence. EdU staining indicated that Procr+ GCs have significantly higher proliferative ability than Procr- GCs (Figures 2D and 2E). This was further examined in isolated primary cell culture. Same amount of Procr+ and Procr- GCs were plated in culture, and cell numbers were counted during serial passages. Consistent with the in vivo results, Procr+ GCs in culture also proliferate faster than Procr- GCs (Figures 2E–2G).
Mature GCs have the capacity of steroidogenesis. Previous studies have found that genes encoding steroidogenic enzymes (such as Cyp19a1) and genes encoding hormone receptors (such as Esr, Fshr, and Lhcgr) were upregulated during folliculogenesis (Richards and Pangas, 2010; Zhang et al., 2018), whereas an important transcription factor Foxl2 was decreased in GCs along follicles development (Liu et al., 2015). Next we asked whether Prcor+ and Procr- GCs are different in these aspects. qPCR analysis revealed that Procr+ GCs expressed higher level of Foxl2 and lower levels of Cyp19a1, Esr2, Fshr, and Lhcgr than Procr- GCs (Figure 2H). This may reflect an immature state of Procr+ GCs. Taken together, these results suggest that Procr marks a subpopulation of GCs that have higher proliferative ability and may be in an immature/undifferentiated state.
Knockdown of PROCR inhibits AKT and ERK signaling pathway in granulosa cells
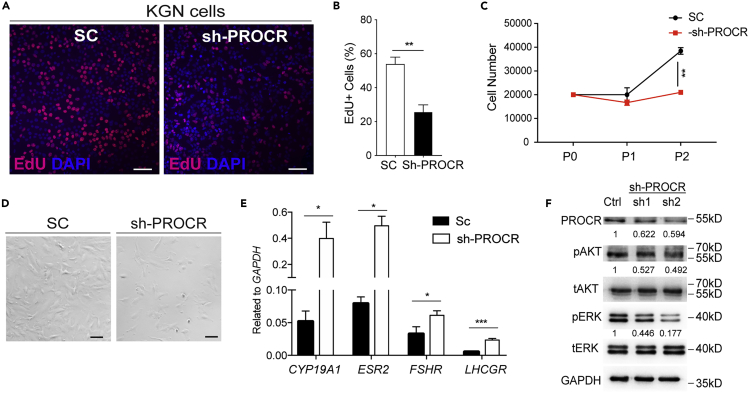
To gain molecular insights of the proliferative ability of Procr+ GCs, we inhibited PROCR expression by short haprpin RNAs (shRNAs) in KGN cells (a human ovarian GC-like tumor cell line). Knockdown of PROCR inhibited the proliferation as shown by decreased EdU staining (Figures 3A and 3B), as well as reduction in cell numbers (Figures 3C and 3D). Furthermore, knockdown of PROCR up-regulated the expression of Cyp19a1, Esr2, Fshr, and Lhcgr (Figure 3E), suggesting that the differentiation is skewed. Western analysis indicated the decreased phosphorylation of AKT and ERK upon PROCR knockdown (Figures 3F and S2). This is consistent with the known role of PI3K/Akt and MAPK signaling pathways in GC proliferation (Liu et al., 2020; Wu et al., 2019). Together, PROCR controlled PI3K/Akt and MAPK/Erk signaling pathways to regulate GCs and follicle development.
Figure 3.
Knockdown of PROCR inhibits AKT and ERK signaling activities in granulosa cells
(A and B) Confocal imaging in KGN cells (A) and quantification (B) showing fewer EdU+ cells in sh-PROCR group compared with the scramble (SC). Scale bars, 100 μm. Representative image was shown. Total 1,087 KGN SC cells and 976 sh-PROCR cells from three different experiments were counted. Data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗p < 0.01.
(C and D) Cell numbers were counted in first three passages (C), and representative images (D) of KGN cells infected by scramble and PROCR shRNA. n = 3 times and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗p < 0.01. Scale bars, 50 μm.
(E) qPCR analysis showing increased CYP19A1, ESR2, FSHR, and LHCGR with PROCR knockdown. n = 3 times, and data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗∗p < 0.001, ∗p < 0.05.
(F) Western blots showing the decreased p-AKT and p-ERK levels in KGN cells with PROCR knockdown. One of three experiments was shown.
Procr+ cells contribute to granulosa cell expansion during follicle development
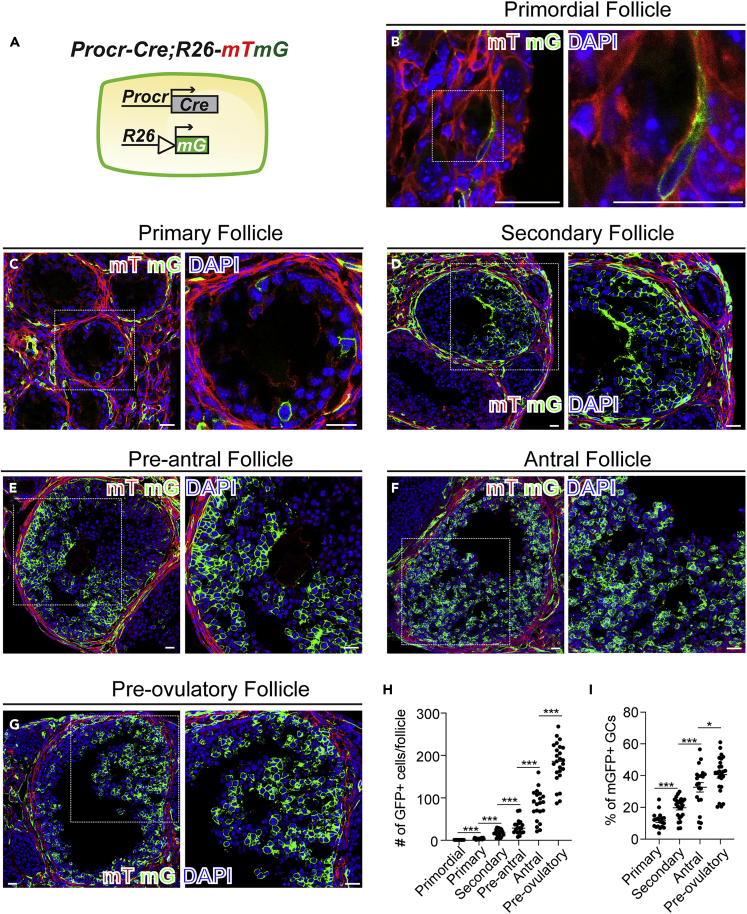
To investigate the contribution of Procr+ GCs during follicle development in vivo, we utilized a Procr-Cre knock-in line, with a Cre inserted after the fourth (last) exon of Procr gene (Q.C. Yu and Y.A. Zeng, unpublished data). Lineage tracing was performed using Procr-Cre;R26-mTmG mice (Figure S1B). Procr+ GCs and their progeny were labeled with mGFP expression, reflecting the contribution of Procr+ GCs in folliculogenesis (Figure 4A). Ovaries were harvested and the mGFP+ cells were analyzed by section imaging. In contrast to the decreased proportion of Procr+ cells along the follicle development (shown in Figure 1), the progeny of Procr+ cells increased while the follicle advances. We observed single mGFP+ cell in primordial follicles (Figure 4B), and 3∼4 mGFP+ cells in primary follicles (Figure 4C). In large follicles, including second, pre-antral, antral, and pre-ovulatory follicle, the number of mGFP+ GCs was dramatically increased (Figures 4D–4H). The proportion of mGFP+ GCs was also increased along follicle development (Figure 4I). The lineage tracing results validated the in vivo proliferative and differentiation ability of Procr+ GCs and visualized their contribution to GC expansion during follicle development.
Figure 4.
Procr+ cells contribute to granulosa cells' expansion
(A) Illustration of lineage tracing using Procr-Cre;R26-mTmG mice. Procr+ cells and their progeny were labeled by mGFP expression.
(B and C) Section imaging showing single mGFP+ GC in primordial follicles (B) and 3–4 mGFP+ GCs in primary follicle (C). Scale bars, 20 μm. n = 3 mice and representative image was shown.
(D–G) Section imaging showing the number of mGFP+ GCs expanded along follicular development, i.e., secondary (D), pre-antral (E), antral (F), and pre-ovulatory (G) follicles. Scale bar, 20 μm. n = 3 mice and representative image was shown.
(H and I) Quantification of mGFP+ GC numbers (H) and percentage of mGFP+ GCs (mGFP + GCs/all GCs) (I) of different stages per follicle exhibited the continuous expansion of mGFP+ GCs during tracing. At least 20 follicles in different stages of follicles from three mice were counted as the number of GFP+ cells per follicle. Unpaired two-tailed Student's t test is used for comparison. Data are presented as mean ± SEM. ∗∗∗p < 0.001, ∗p < 0.05.
Ablation of Procr+ cells disrupts ovarian follicle development and leads to polycystic ovary syndrome phenotype
Next we investigated the significance of Procr+ GCs in folliculogenesis in vivo. We performed targeted ablation of Procr+ cells using Procr-CreER;R26-DTA mice (Figure S1C). Diphtheria toxin (DTA) was expressed to ablate Procr+ cells, and we examined its impact in follicle development. Two-week old mice were administered Tamoxifen (TAM) every other day for a total of three times, and the ovaries were examined after 1 month (Figure 5A). By whole-mount imaging, we observed fewer corpus luteum in the ablation group compared with the control (Figure 5B). By section imaging, the ablation group exhibited accumulation of small follicles and lack of corpus luteum (Figures 5C and 5D). Quantification revealed the increased percentage of immature follicles (secondary follicle, pre-antral follicle) and decreased proportion of mature follicles (antral follicle, pre-ovulatory follicle) in the ablation group compared with the control (Figure 5E). The lack of corpus luteum in the ablation group was a sign of anovulation (Figures 5C–5E). In addition, plasma testosterone, anti-Müllerian hormone (AMH) level, and luteinizing hormone (LH)/FSH ratio was significantly increased after ablation of Procr+ cells (Figures 5F–5H). qPCR analysis indicated the downregulation of Cyp19a1 (Figure 5I). Upon PMSG/HCG-stimulated superovulation, the ablated group yielded more oocytes in numbers compared with the control (Figure 5J). The polar bodies in the ablation group also appeared abnormal (Figure 5K). Interestingly, in many cases, it appeared that GCs adhered to the surface of the oocytes (Figure 5K). These observations are reminiscent of the clinical features of PCOS. Together, targeted ablation of Procr+ cells suppressed follicle development and induced phenotypes of PCOS.
Figure 5.
Ablation of Procr+ cells disrupts ovarian follicle development and leads to polycystic ovary syndrome phenotype
(A) Illustration of Procr+ cells ablation using Procr-CreER;R26-DTA mice. TAM was administered to 2-week-old mice, and the ovaries were analyzed at 1 month post injection.
(B–D) Whole-mount images (B), H&E section staining (C), and confocal section imaging (D) showing Procr+ cells ablation resulted in more immature follicles and less corpus luteum compared with control (Ctrl). Scale bars, 200 μm in (B) and 100 μm in (C and D). n = 3 mice in each experimental group, and representative image was shown.
(E) Quantification of the percentage of follicle in different stages. Data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. n = 3 mice in each experimental group. n.s, not significant. ∗∗p < 0.01. ∗∗∗p < 0.001.
(F–H) Plasma testosterone (F), AMH (G) concentration, and LH/FSH ratio (H) in the serum of control and ablation mice. Data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. n = 3 mice in each experimental group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(I) qPCR analysis of Cyp19a1 expression in the ovary of control and ablation mice. Data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗∗p < 0.01.
(J) Illustration of superovulation strategy, and quantification of the number of ovulated oocytes from control and ablation mice. n = 3 mice in each experimental group. Data are presented as mean ± SEM. Unpaired two-tailed Student's t test is used for comparison. ∗p < 0.05.
(K) Representative images showed sticky granulosa cells to the oocytes and abnormal polar bodies in ablation mice after superovulation. Scale bars, 100 μm.
Discussion
In this study, we identified a subpopulation of GCs that expressed Procr, exhibited higher proliferation compared with the rest GCs, and were important for follicle development. Knockdown of PROCR suppressed AKT and ERK signaling activities, rendering reduced proliferation and increased expression of differentiated genes in GCs. In vivo, Procr+ GCs contributed to increasing numbers of GCs shown by lineage tracing. Finally, targeted ablation of Procr+ cells disrupted ovarian follicle development, leading to PCOS phenotype.
The rapid proliferation of the GCs in the growing follicles as well as their exertion of various specialized functions have brought about the notion that the population of GCs in a single healthy follicle is heterogeneous, consisting of subpopulations of differentiated and less differentiated cells (Kossowska-Tomaszczuk et al., 2009). Several studies have reported that a subpopulation of GCs have proliferative capacity and can form colonies in culture (Lavranos TC et al., 1994; Rodgers HF et al., 1995); they can divide for at least 10 times to support follicle growth and oocyte maturation in the growing follicles (Hirshfield, 1991). Yet, little is known about such a subpopulation. In this study, our findings suggest that Procr+ GCs to some extend exhibit progenitor-like characteristics. They are highly proliferative and have lower expression of differentiation gene, e.g., Cyp19a1, Fshr, and Lhcgr.
The in vivo contribution and significance of Procr+ GCs during folliculogenesis was demonstrated by lineage tracing and targeted ablation experiments. Using Procr-Cre;R26-mTmG mouse model, we observed that the majority of GCs were derived from Procr+ cells during folliculogenesis. Upon targeted ablation of Procr+ cells using Procr-CreER;R26-DTA mouse model, the ovarian follicle development was disrupted, manifesting the PCOS phenotype. Ablation of Procr+ GCs led to accumulation of secondary and pre-antral follicles, and appearance of anovulation. It is worth noting that Cyp19a1 was shown to be downregulated after Procr+ cells ablation, resulting in increase in plasma testosterone level. This may be because GCs in early follicles do not express Cyp19a1 (Stocco, 2008). These findings reveal an indispensable role of Procr+ GCs during follicle development.
Owing to the heterogeneity of PCOS, it has been challenging to establish a genetic animal model that displays the main characteristics of PCOS. Common PCOS models are induced models with chronic exposure to testosterone or the aromatase inhibitor letrozole (Kauffman et al., 2015; van Houten et al., 2012). In this study, we established a new PCOS mouse model, Procr-CreER;R26-DTA, by targeted ablation of Procr+ GCs. Our model recapitulates the clinical features of PCOS, such as hyperandrogenism, polycystic ovarian morphology, and chronic anovulation. The advantages brought by the new mouse model will be of broad value in studies of PCOS. Of note, the role of Procr goes beyond a surface marker. It plays functional roles in GCs proliferation. Knockdown of PROCR suppressed AKT and ERK signaling activities, rendering reduced proliferation and increased expression of differentiated genes in GCs. Future study may investigate the association of PROCR signaling pathway gene mutations with PCOS in human patients.
In conclusion, we reveal for the first time that Procr+ GCs plays a key role in ovarian follicle development by promoting proliferation and suppressing differentiation of GCs. Ablation of Procr+ cells disrupts ovarian follicle development and leads to a PCOS phenotype. Our findings not only shed new light on the role of Procr+ GCs in folliculogenesis but also extend knowledge of potential genes associated with PCOS in human patients.
Limitations of the study
The results of this study were obtained from mouse models. Further studies on human ovarian tissues would advance our understanding on the role of PROCR in follicle development and PCOS.
Resource availability
Lead contact
Professor Wen Li (liwen@smmu.edu.cn).
Materials availability
Requests for further information or materials should be directed to the lead contact.
Data and code availability
Requests for biological datasets should be directed to the lead contact.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This research was supported by grants from National Natural Science Foundation of China (81873821 and 82071605 to W.L.; 31625020, 31530045, 31830056, and 31861163006 to Y.A.Z.; 81570759 and 81270938 to J.F.), National Key Research and Development Program of China (2020YFA0509002, 2019YFA0802002 to Y.A.Z.; 2016YFC1305301 to J.F.), the Chinese Academy of Sciences (XDB19020200 and XDA16020200 to Y.A.Z.), Zhejiang Provincial Key Disciplines of Medicine (Innovation Discipline, 11-CX24 to J.F.), Fundamental Research Funds for the Central Universities (2020XZZX002-22 to J.F.), China Postdoctoral Science Foundation (2020TQ0260 to J.W.), and Zhejiang Provincial Preferential Postdoctoral Foundation (ZJ2020150 to J.W.).
Author contributions
W.L. and Y.A.Z. designed the project. J.W. performed lineage tracing, target ablation, primary cell culture, staining, and qPCR. K.C. performed RNA in situ, western blotting, KGN cell line culture, and staining. Y.W. performed superovulation and oocytes collection. J.F. and J.L. helped project design. J.W., K.C., W.L., and Y.A.Z. analyzed the data and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102065.
Contributor Information
Junfen Fu, Email: fjf68@zju.edu.cn.
Yi Arial Zeng, Email: yzeng@sibcb.ac.cn.
Wen Li, Email: liwen@smmu.edu.cn.
Supplemental information
References
- Azziz R., Carmina E., Chen Z., Dunaif A., Laven J.S., Legro R.S., Lizneva D., Natterson-Horowtiz B., Teede H.J., Yildiz B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- Balazs A.B., Fabian A.J., Esmon C.T., Mulligan R.C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Liu D., Griffin J.H., Fernandez J.A., Castellino F., Rosen E.D., Fukudome K., Zlokovic B.V. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- Eppig J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Fukudome K., Esmon C.T. Identification, cloning, and regulationof a novel endothelial cell protein C/activated ProteinC receptor. J. Biol. Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- Fan H.Y., Liu Z., Cahill N., Richards J.S. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol. Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramling M.W., Beaulieu L.M., Church F.C. Activated protein C enhances cell motility of endothelial cells and MDA-MB-231 breast cancer cells by intracellular signal transduction. Exp. Cell Res. 2010;316:314–328. doi: 10.1016/j.yexcr.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- van Houten E.L., Kramer P., McLuskey A., Karels B., Themmen A.P., Visser J.A. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153:2861–2869. doi: 10.1210/en.2011-1754. [DOI] [PubMed] [Google Scholar]
- Kauffman A.S., Thackray V.G., Ryan G.E., Tolson K.P., Glidewell-Kenney C.A., Semaan S.J., Poling M.C., Iwata N., Breen K.M., Duleba A.J. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol. Reprod. 2015;93:69. doi: 10.1095/biolreprod.115.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp J.L., Kilen S.M., Woodruff T.K., Mayo K.E. Activin regulates estrogen receptor gene expression in the mouse ovary. J. Biol. Chem. 2007;282:36755–36765. doi: 10.1074/jbc.M705143200. [DOI] [PubMed] [Google Scholar]
- Kossowska-Tomaszczuk K., De Geyter C., De Geyter M., Martin I., Holzgreve W., Scherberich A., Zhang H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009;27:210–219. doi: 10.1634/stemcells.2008-0233. [DOI] [PubMed] [Google Scholar]
- Lavranos TC R.H., Bertoncello I., Rodgers R.J. Anchorage-independent culture of bovine granulosa cells: the effects of basic fibroblast growth factor and dibutyryl cAMP on cell division and differentiation. Exp. Cell Res. 1994;211:245–251. doi: 10.1006/excr.1994.1084. [DOI] [PubMed] [Google Scholar]
- Liu C., Peng J., Matzuk M.M., Yao H.H. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015;6:6934. doi: 10.1038/ncomms7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Qiu Y., Xue Z., Wu R., Li J., Niu X., Yuan J., Wang Y., Wu Q. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2020;11:3. doi: 10.1186/s13287-019-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. Granulosa cell proliferation differentiation and its role in follicular development. Chin. Sci. Bull. 2005;50:2665. [Google Scholar]
- Parvari S., Yazdekhasti H., Rajabi Z., Gerayeli Malek V., Rastegar T., Abbasi M. Differentiation of mouse ovarian stem cells toward oocyte-like structure by coculture with granulosa cells. Cell. Reprogram. 2016;18:419–428. doi: 10.1089/cell.2016.0013. [DOI] [PubMed] [Google Scholar]
- Pisarska M.D., Bae J., Klein C., Hsueh A.J. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology. 2004;145:3424–3433. doi: 10.1210/en.2003-1141. [DOI] [PubMed] [Google Scholar]
- Richards J.S., Pangas S.A. The ovary: basic biology and clinical implications. J. Clin. Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers HF L.T., Vella C.A., Rodgers R.J. Basal lamina and other extracellular matrix produced by bovine granulosa cells in anchorage-independent culture. Cell Tissue Res. 1995;282:463–471. doi: 10.1007/BF00318878. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum. Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Da Silva-Buttkus P., Jayasooriya G.S., Mora J.M., Mobberley M., Ryder T.A., Baithun M., Stark J., Franks S., Hardy K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J. Cell Sci. 2008;121:3890–3900. doi: 10.1242/jcs.036400. [DOI] [PubMed] [Google Scholar]
- Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73:473–487. doi: 10.1016/j.steroids.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C., Telleria C., Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- Su Y.Q., Wu X., O'Brien M.J., Pendola F.L., Denegre J.N., Matzuk M.M., Eppig J.J. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev. Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Uda M., Ottolenghi C., Crisponi L., Garcia J.E., Deiana M., Kimber W., Forabosco A., Cao A., Schlessinger D., Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Li T.Y., Kidder G.M. WNT2 regulates DNA synthesis in mouse granulosa cells through beta-catenin. Biol. Reprod. 2010;82:865–875. doi: 10.1095/biolreprod.109.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Cai C., Dong X., Yu Q.C., Zhang X.O., Yang L., Zeng Y.A. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015;517:81–84. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu W., Du J., Yu Y., Liang N., Liang M., Yao G., Cui S., Huang H., Sun F. NGF promotes mouse granulosa cell proliferation by inhibiting ESR2 mediated down-regulation of CDKN1A. Mol. Cell. Endocrinol. 2015;406:68–77. doi: 10.1016/j.mce.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Wang D., Liu C., Wang J., Jia Y., Hu X., Jiang H., Shao Z.M., Zeng Y.A. Protein C receptor stimulates multiple signaling pathways in breast cancer cells. J. Biol. Chem. 2018;293:1413–1424. doi: 10.1074/jbc.M117.814046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang D., Chu K., Li W., Zeng Y.A. Procr-expressing progenitor cells are responsible for murine ovulatory rupture repair of ovarian surface epithelium. Nat. Commun. 2019;10:4966. doi: 10.1038/s41467-019-12935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang J., Bai L., Pan H., Feng H., Clevers H., Zeng Y.A. Long-Term expansion of pancreatic islet organoids from resident Procr(+) progenitors. Cell. 2020;180:1198–1211.e19. doi: 10.1016/j.cell.2020.02.048. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xiao H., Pi J., Zhang H., Pan A., Pu Y., Liang Z., Shen J., Du J. EGFR promotes the proliferation of quail follicular granulosa cells through the MAPK/extracellular signal-regulated kinase (ERK) signaling pathway. Cell Cycle. 2019;18:2742–2756. doi: 10.1080/15384101.2019.1656952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.Q., Han C.-S., Yang W., Jin X., Hu Z.Y., Liu Y.X. Role of erk1/2 in fsh-induced pcna expression and steroidogenesis in granulosa cells. Front. Biosci. 2005;10:896–904. doi: 10.2741/1584. [DOI] [PubMed] [Google Scholar]
- Yu Q.C., Song W., Wang D., Zeng Y.A. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26:1079–1098. doi: 10.1038/cr.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update. 2015;21:779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- Zhang H., Risal S., Gorre N., Busayavalasa K., Li X., Shen Y., Bosbach B., Brännström M., Liu K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 2014;24:2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan Z., Qin Q., Nisenblat V., Chang H.M., Yu Y., Wang T., Lu C., Yang M., Yang S. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol. Cell. 2018;72:1021–1034.e4. doi: 10.1016/j.molcel.2018.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for biological datasets should be directed to the lead contact.