Abstract
Background
Microvascular decompression has been established as a primary treatment for hemifacial spasm. Intraoperative monitoring is used during the surgery to guide neurosurgeons to determine whether the decompression of facial nerve from the vessel is sufficient. We performed a systematic review to assess the role of lateral spread response (LSR) monitoring in predicting hemifacial spasm outcomes after microvascular decompression.
Method
A systematic search of PubMed, ScienceDirect, Cochrane, and Google Scholar was conducted. We included studies that performed microvascular decompression surgery with intraoperative monitoring analyzing the correlation between lateral spread response and spasm relief. A critical appraisal was conducted for selected studies.
Result
Twenty-two studies comprising 6404 cases of hemifacial spasm, which underwent microvascular decompression surgery with intraoperative monitoring, were included. Of 15 articles that assessed symptoms shortly after surgery, 12 studies showed a significant correlation between lateral spread response resolution and disappearance of spasm. Four of six studies that evaluated the outcome at 3-month follow-up showed significant relationship between LSR and outcome, so did five of six articles that assessed spasm relief at 6-month follow-up. As much as 62.5% of studies (10 of 16) showed the result at long-term follow-up (≥1-year) was not significant.
Conclusion
Intraoperative monitoring during microvascular decompression surgery can be a useful tool to predict hemifacial spasm resolution. Though long-term outcomes of patients with LSR relief and persistence are similar, resolution of symptoms shortly after surgery will provide comfort to patients thereby improving their quality of life.
Keywords: Hemifacial spasm, Microvascular decompression, Intraoperative monitoring, Lateral spread response, Abnormal muscle response
Hemifacial spasm, Microvascular decompression, Intraoperative monitoring, Lateral spread response, Abnormal muscle response.
1. Introduction
Hemifacial spasm (HFS) is not a life-threatening disease which may make patients suffer intensely and tend to withdraw socially. This disorder, characterized by involuntary contraction of the muscle innervated by facial nerve (cranial nerve [CN] VII), triggers involuntary short or longer contractions of the facial muscles [1]. The symptom usually begins in muscle of orbicularis oculi and then spread to other muscle which innervated by CN VII [2].
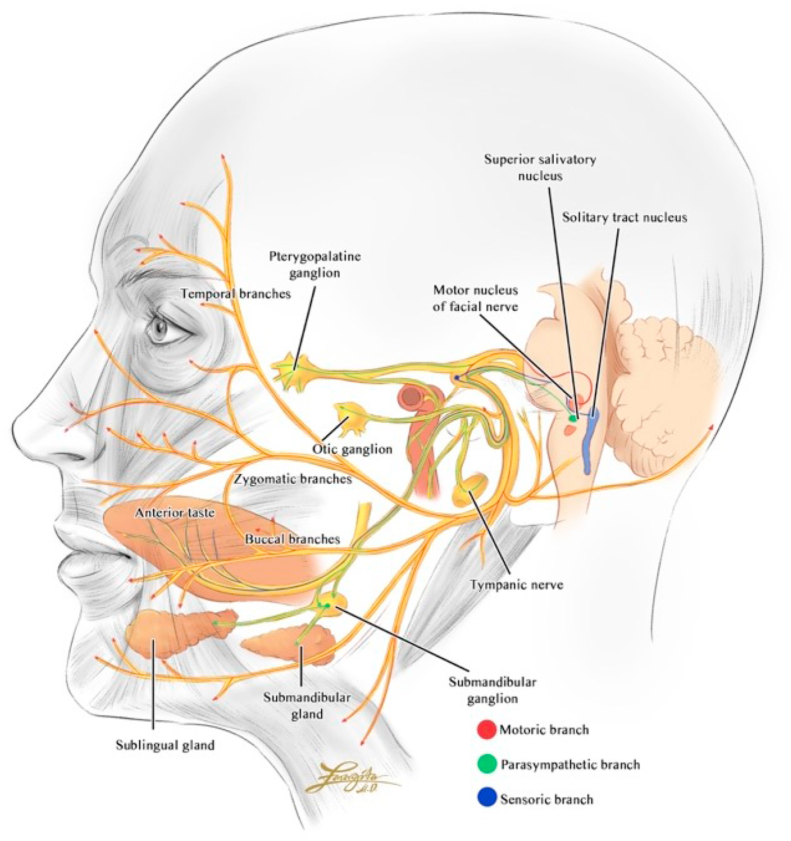
The facial nerve arises from pons, extends posteriorly and courses through facial canal in the temporal bone then exits through stylomastoid foramen. The nerve provides motor innervation of facial muscles, parasympathetic innervation of the glands of oral cavity and lacrimal glands, and sensory innervation of the anterior two-third of the tongue. The intracranial part of seventh nerve begins in facial motor area of precentral gyrus, which the axons travel ipsilaterally along the corticobulbar tract to lower pons. The facial nerve begins its extracranial part when it passes through internal acoustic meatus to enter the facial canal within the petrous part of temporal bone. After giving branch to greater petrosal nerve, nerve to stapedius muscle, and chorda tympani, it leaves the cranium via stylomastoid foramen and gives innervation to pinna of the ear, external auditory meatus, and parotid gland [3]. The upper motor neuron part received blood supply from middle cerebral artery, while facial nucleus located in the pons had supply from anterior inferior cerebellar artery. Within the facial canal, it received supply from middle meningeal artery and stylomastoid artery, and after exiting the stylomastoid foramen, it is supplied by branch of stylomastoid artery [4] (see Figure 1).
Figure 1.
Facial nerve course of motor, sensory, parasympathetic innervation.
Hemifacial spasm can be either primary or secondary. Anterior inferior cerebellar artery is the most common involved vessel in primary hemifacial spasm, in which it causes facial nerve compression [5]. The causes of secondary HFS includes cerebellopontine angle tumors, arteriovenous malformation, brainstem lesions such as stroke or demyelinating disorders, mastoid and ear infection, parotid tumors, and Bell's palsy [6].
The common cause of HFS is microvascular compression of CN VII by offending vessels at the root exit zone (REZ). Pressure and irritation of facial nerve cause local demyelination and shortening between nerve fibers, leading to ectopic bioelectric transmission [7]. Microvascular decompression (MVD) surgery was first proposed by Jannetta, suggesting that decompression of CN VII from offending vascular could solve the problem that was caused by neural circuitry shortening [8]. A report by Chung et al. of 1169 cases of MVD for HFS with a mean of follow-up of 28.3 months showed an overall effective rate of 95% [9]. Another review conducted by Miller et al. found complete resolution of symptoms as much as 91.1% with an average follow-up of 2.9 years [10]. However, several reports showed the recurrence of spasm due to inadequate nerve decompression or neglected offending vessels, as well as vascular change and slippage of Teflon [11, 12].
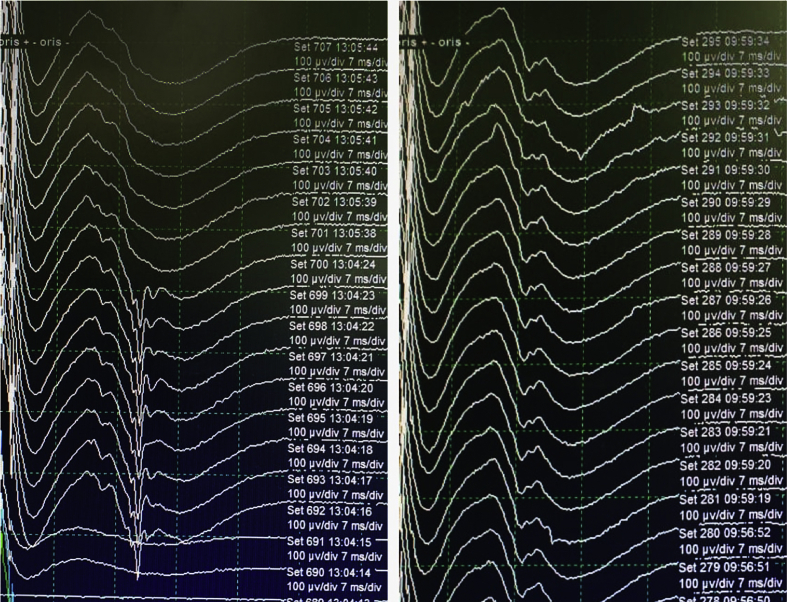
Intraoperative monitoring (IOM) has been widely used during MVD surgery to guide neurosurgeons to determine whether the decompression is sufficient, or the facial nerve has been released from all offending vessels. Classically, HFS in electromyography (EMG) is represented as lateral spread response (LSR), a paradoxical evoked response of facial muscle to the stimulation of CN VII [13] Figure 2. LSR, or abnormal muscle response (AMR), is believed to be responsible in HFS due to ephaptic transmission of neural impulses between different branches of the facial nerve [14]. Some reports showed a significant relationship of LSR resolution with relief of symptoms, while other reports failed to find the similar result [15, 16, 17, 18].
Figure 2.
Change of LSR during MVD. Intraoperative monitoring depicting partial resolution of LSR (left), gradually disappearing completely after vascular detachment and Teflon insertion (right).
This study aims to systematically review the role of IOM during MVD surgery in predicting the outcome of HFS, and to give insight especially for neurosurgeons to decide whether it is useful to have IOM while performing MVD surgery for HFS.
2. Method
2.1. Study search
We conduct a study according to Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (available from http://www.prisma-statement.org/). A thorough search was performed for available articles from PubMed, ScienceDirect, Cochrane Library, and Google Scholar from the date of inception until June 2, 2020. We used keywords “microvascular decompression” AND “hemifacial spasm” AND “intraoperative monitoring OR lateral spread response OR abnormal muscle response” AND “outcome” to find relevant articles.
2.2. Inclusion and exclusion criteria
Inclusion criteria were studies reporting MVD for HFS with monitoring of LSR/AMR intraoperatively, contained follow-up report on HFS status post-surgery, and performed analysis of relationship between LSR status post-surgery and outcome. Exclusion criteria were non-English literature, review study, case report study, and sample size <30 patients (see Tables 1 and 2).
Table 1.
Research question.
| Study Component | |
|---|---|
| Patient | Hemifacial spasm patients underwent microvascular decompression with IOM |
| Intervention/Exposure | Lateral Spread Response free after decompression |
| Comparison | Lateral Spread Response persistence after decompression |
| Outcome | Spasm resolution |
Table 2.
Inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
| Microvascular decompression surgery of HFS with intraoperative monitoring of LSR | Non-English article |
| Follow-up report of HFS status postoperatively | Review study |
| Analyzing relationship between LSR status post-surgery and outcome | Case report study |
| Sample size <30 patients |
2.3. Data extraction and quality assessment
Two reviewers (YNM and SAHP) independently screened titles and abstracts, and read the full-text to filter literatures that met our research question and inclusion criteria. If inconsistencies were encountered, the problems were resolved through discussion with the first author (SWN). Selected articles were appraised using critical appraisal tool for prognostic studies (available from https://cebm.net/wp-content/uploads/2018/11/Prognosis.pdf). Discussion between two reviewers or consultation with the first author was conducted if there was any disagreement during evaluation.
3. Result
3.1. Search result
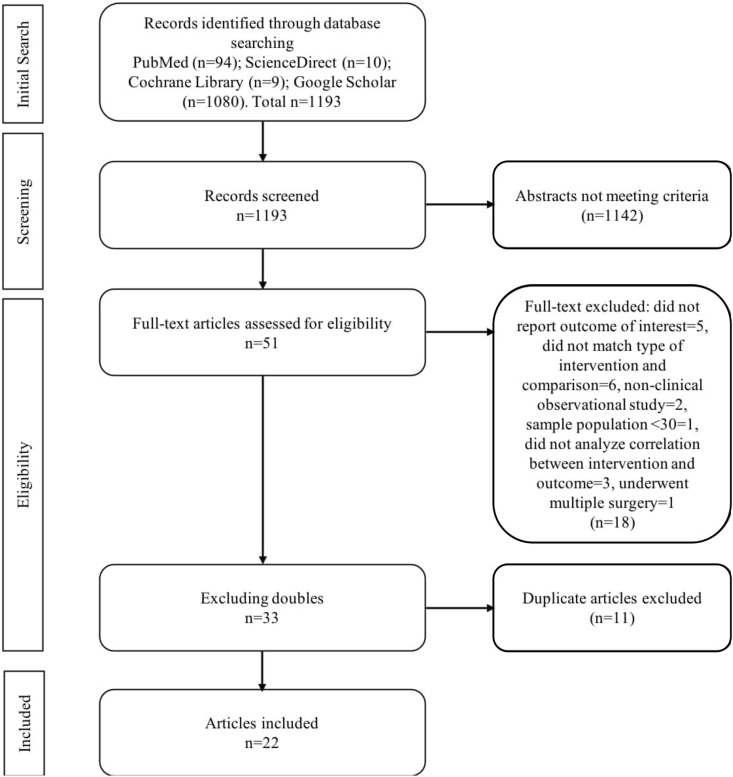
Figure 3 presented a detailed process of literature selection. At initial search of combined keywords, we found 94 articles form PubMed, 10 articles from ScienceDirect, 9 articles from Cochrane Library, and 1080 articles from Google Scholar, total 1193. After screening titles and abstracts, we selected 52 papers, and after reading full-text and excluding duplicates, we included 22 studies.
Figure 3.
Flowchart of screening strategy for included studies.
3.2. Studies characteristic
Twenty-two studies comprising 6404 patients with HFS, who underwent MVD with monitoring of LSR intraoperatively, were selected. Eight articles were prospective studies, and the rest were retrospective, published between 1996-2019. Of 22 articles, eight studies were conducted in China, five in South Korea, four in Japan, two in Germany, one in France, and two in USA. The sample sized ranged from 32-1172, all received MVD surgery with IOM from 1985-2017. The range of follow-up of outcomes was one day – 82.3 months. The definition of LSR resolution and HFS relief were varied; some studies defined them as a decrease of amplitude of LSR and partial elimination of symptoms, others defined them as complete LSR disappearance and resolution of spasm. The detailed characteristics of studies was presented in Table 3.
Table 3.
Characteristic of eligible studies.
| Author | Year | Duration of Study | Country | Type of Study | Population | Time of Follow Up | Definition of LSR Resolution | Definition of HFS Relief |
|---|---|---|---|---|---|---|---|---|
| Eckardstein [17] | 2013 | 2000–2010 | Germany | Retrospective Study | 38 | 1-year | Complete disappearance | Complete elimination of symptoms |
| El Damaty [18] | 2016 | 2013–2015 | Germany | Prospective Study | 100 | 1-year | Total or partial relief | ≥90% improvement |
| Hatem [19] | 2001 | N/A | France | Prospective Study | 33 | 3-month, >1 year | Complete disappearance | Complete elimination of symptoms |
| Huang [16] | 2017 | 2013–2015 | China | Retrospective Study | 1138 | 1-day, 3-month, 1-year | Complete disappearance | Symptoms improved >75% |
| Isu [20] | 1996 | Undefined | Japan | Prospective Study | 40 | 1-day, >1 year | Complete disappearance | Complete elimination of symptoms |
| Jo [21] | 2012 | 1997–2007 | South Korea | Retrospective Study | 801 | >1 year | Complete disappearance | Less than complete elimination of symptoms |
| Joo [22] | 2007 | 2001–2005 | South Korea | Retrospective Study | 72 | 1-week, 6-month | Complete disappearance | Complete elimination of symptoms |
| Kiya [23] | 2001 | 1998–1999 | Japan | Prospective Study | 38 | 1-day, 3-month | Complete disappearance | Complete elimination of symptoms |
| Kong [24] | 2007 | 2000–2005 | South Korea | Retrospective Study | 263 | 1-week, 3-month, 1-year | Complete disappearance | Complete elimination of symptoms |
| Lee [25] | 2016 | 2009–2013 | South Korea | Retrospective Study | 885 | 1-day, 6-month, 1-year | Complete disappearance | Complete elimination of symptoms |
| Li [26] | 2012 | 2006–2007 | China | Prospective Study | 104 | >1 year | Complete disappearance | Undefined |
| Liu [27] | 2017 | 2010–2014 | China | Retrospective Study | 332 | 1-day, >1 year | Amplitude decreased >50% of baseline | Symptoms improved >75% |
| Shin [28] | 1997 | 1985–1994 | South Korea | Prospective Study | 132 | 6-month | Undefined | Complete elimination of symptoms |
| Song [15] | 2019 | 2013–2016 | China | Retrospective Study | 73 | 1-week, 1-year | Amplitude decreased >50% of baseline | Complete elimination of symptoms |
| Thirumala [29] | 2015 | 2000–2007 | USA | Retrospective Study | 235 | Immediate post-surgery, discharge, >1 year | Complete disappearance | Less than complete elimination of symptoms |
| Thirumala [30] | 2011 | 2000–2007 | USA | Retrospective Study | 259 | Immediate post-surgery, discharge, >1 year | Complete disappearance | Less than complete elimination of symptoms |
| Tobishima [31] | 2013 | 2005–2009 | Japan | Retrospective Study | 131 | 1-week, 1-year | Complete disappearance | Complete disappearance or occasional slight spasm |
| Wei [32] | 2018 | 2013–2014 | China | Prospective Study | 145 | 1-week, 1-year | Complete disappearance | Symptoms improved ≥90% |
| Yamashita [33] | 2005 | 1994–2001 | Japan | Retrospective Study | 60 | 1-day, >1 year | Complete disappearance | Complete elimination of symptoms |
| Ying [34] | 2011 | 2010 | China | Retrospective Study | 241 | 1-day, 3-month, 6-month | Complete disappearance | Symptoms improved >75% |
| Zhang [35] | 2017 | 2014–2016 | China | Retrospective Study | 1172 | 1-day, 3-month | Amplitude decreased >50% of baseline | Complete disappearance or occasional slight spasm |
| Zhu [36] | 2019 | 2015–2017 | China | Prospective Study | 112 | 6-month | Complete disappearance | Undefined |
3.3. Association between LSR and outcome
3.3.1. One-day to one-week follow-up
Fifteen papers assessed patients’ outcome shortly after surgery. Studies that conducted evaluation immediately post-surgery were included in 1-day to 1-week window period in our systematic review. Two studies that assessed outcome at discharge (mean: 3.91 ± 1.98 days) were also considered to be within this follow-up period.
Of 15 studies, 12 articles (75%) found that LSR resolution was significantly correlated with spasm improvement in short-term follow-up. Twelve out of 15 defined LSR resolution as complete disappearance of abnormal muscle response wave, while the rest defined it as a decrease of amplitude >50% of baseline. Seven studies used complete elimination of spasm as definition HFS relief, improvement of spasm ≥75% in three studies, improvement of spasm ≥90% in one study, and less than complete elimination of symptom in four studies.
3.3.2. Three-month follow-up
Six articles evaluated patients’ outcome at 3-month follow-up. The result showed 67% studies had significant correlation between LSR disappearance and symptoms relief. One article could not be analyzed because all patients, whether had LSR resolution or persistence after surgery, did not complaint any spasm due to improvement over time. All but one studies defined LSR resolution as complete disappearance, and four articles described spasm relief as complete elimination of symptom.
3.3.3. Six-month follow-up
Only one out of six papers that found insignificant relationship between LSR status after surgery and spasm relief in 6-month follow-up. All but one study described LSR resolution as complete disappearance of the wave, whereas the other one did not mention the definition of LSR resolution. Complete elimination of spasm was described as HFS relief in four studies, symptoms improved >75% in one study, and undefined in other one.
3.3.4. Long-term follow-up
Of 22 studies, 16 articles re-assessed of outcomes in ≥1-year follow-up. Ten of 16 articles (62.5%) failed to show LSR resolution as a significant predictor of spasm relief. Amplitude decrease >50% of baseline was defined as LSR resolution in two studies, total or partial disappearance in one study, and the rest defined it as complete wave disappearance. Eight studies used complete elimination of symptom as definition of HFS relief, one study did not mention any definition, and the others described it as less than complete elimination.
4. Discussion
Hemifacial spasm is described as a disorder with paroxysmal, involuntary one side twitching of facial muscles innervated by ipsilateral CN VII. Some articles [37, 38] reported rare cases of severe hemifacial spasm (<5%) with bilateral involvement of facial muscles, but commonly it occurs as unilateral disorder. As the symptoms suggest, HFS is considered as a subtype of peripheral (neuromuscular) movement disorder [5].
Worldwide, it is estimated prevalence of HFS is 14.5 per 100,000 women and 7.4 per 100,000 men, showing that spasm more commonly occurs in females than males [39]. Some studies reported, for unknown reasons, Asians have a slightly higher prevalence as compared to Caucasians [40, 41]. The highest prevalence of HFS was between 40 and 59 years [39], while another report from Norway showed continual increase in the prevalence to a maximum of 39.7 per 100 000 in those older than 70 [42].
Previous reports suggested hyperexcitability of the facial nucleus as a substantial contributing factor in the pathogenesis of HFS [43]. Another article [7] postulated the common cause of HFS is microvascular compression of CN VII by offending vessels at REZ. Pressure and irritation of facial nerve causes local demyelination and shortening between nerve fibers, leading to ectopic bioelectric transmission.
Finding of LSR is indicated by impulses lateral spreading from one fiber to other facial nerve fibers, or ephaptic transmission, and fibers auto-excitation. In patients with blink reflex of HFS, there was synkinetic response in the mental muscle, and the response after-activity and late-activity was observed as LSR [13]. This monitoring has been used to assess if facial nerve decompression is sufficient during MVD surgery [25].
Botulinum toxin remains a popular treatment of choice for HFS. Derived from Clostridium botulinum, it is a potent biological toxin that acts on the presynaptic region of the neuromuscular junction and prevents the calcium-mediated release of acetylcholine at the nerve terminal preventing impulse generation [6]. A prospective blinded study including 9 patients with HFS by Yoshimura et al showed efficacy of botulinum toxin compared to placebo [44]. Both primary and secondary HFS responded well to botulinum toxin, according to an article by Pandey et al. [45] Although the toxin needs to be injected repeatedly because its effect is transient [6], a multicenter study by Defazio et al found that HFS relief remained unchanged in the first and tenth year of treatment [46]. Botulinum toxin injection is also treatment of choice for patients who cannot tolerate surgery [1].
Surgical treatment is a preferred option for compressive lesions because it directly addressed the cause, and for cases which are refractory to botulinum toxin [5]. Microvascular decompression aims to remove the vascular compression in the REZ of the nerve from the brainstem. It was first suggested by Jannetta that releasing CN VII from offending vascular could solve the HFS problem [8]. The patient is put under general anesthesia in lateral decubitus position, and the procedure is performed via a keyhole lateral retrosigmoid approach. After the cerebellopontine angle is fenestrated and the arachnoid membrane is dissected, the offending vessels are isolated away from REZ. A Teflon sponge is then placed into interspace between the facial nerve and offending vessels [47]. Despite some studies had reported effectiveness MVD in relieving spasm [9, 10], some reports showed recurrent of spasm due to inadequate nerve decompression or neglected offending vessels [11, 12].
Monitoring through EMG in MVD is performed from the time of administration of general anesthesia to dural closure. Prior to anesthesia induction, stimulating needle electrode is inserted intradermally to zygomatic branch of CN VII, and 0.1–0.2 ms pulse wave with intensity 5–25 ms is used. LSR recording is conducted at mentalis muscle during stimulation of zygomatic branch of facial nerve. During surgery, LSR is recorded before opening the dura, during discharge of cerebrospinal fluid, dissection of arachnoid membrane and blood vessels, Teflon insertion, and after suturing the dura mater [16, 18, 20, 22, 23, 24].
For the facial nerve to be decompressed adequately, the first area to be checked is REZ of the CN VII. LSR disappearance indicates sufficient decompression of facial nerve. If the LSR persists or reappears, central myeline of facial nerve or pontobulbar junction area is checked to find any additional offending vessels. Distal periphery of facial nerve then re-explored to ensure that CN VII is released from any compressing structure. The procedure is considered complete if no offending vessels remained despite the persistence of LSR [25, 26, 27, 29, 30, 31].
Available studies in our systematic review showed different results of LSR monitoring usefulness in MVD for HFS. Most of the papers found a significant IOM role for predicting outcome in the short-term period [15, 16, 20, 22, 25, 27, 29, 30, 31, 32, 34, 35]. However, some patients who had LSR and symptom persistence at initial had spasm improvement spontaneously overtime. This condition made LSR resolution role insignificant for a long-term follow-up in some studies [25, 29, 30, 31, 32] (see Table 4).
Table 4.
Summary table of relationship between LSR resolution after decompression of facial nerve and HFS relief.
| Author, Year | Effect Size in Defined Time of Follow-Up |
|||
|---|---|---|---|---|
| 1-Day to 1-Week | 3-Month | 6-Month | ≥1-Year | |
| Eckardstein, 2013 | _ | _ | _ | p = 0.7225 |
| El Damaty, 2016 | _ | _ | _ | p = 0.9 |
| Hatem, 2001 | _ | p = 0.245 | _ | p = 0.697 |
| Huang, 2017 | p = 0.0000∗ | p = 0.0000∗ | _ | p = 0.0000∗ |
| Isu, 1996 | p = 0.001∗ | _ | _ | p = 0.008∗ |
| Jo, 2012 | _ | _ | _ | p = 0.048∗ |
| Joo, 2007 | p = 0.044∗ | _ | p = 0.323 | _ |
| Kiya, 2001 | p = 0.062 | N/A∗∗ | _ | _ |
| Kong, 2007 | Not significant | p = 0.04∗ | _ | p = 0.01∗ |
| Lee, 2016 | p = 0.048∗ | _ | p = 0.02∗ | p = 0.608 |
| Li, 2012 | _ | _ | _ | p = 0.791 |
| Liu, 2017 | p < 0.01∗ | _ | _ | p < 0.01∗ |
| Shin, 1997 | _ | _ | p < 0.05∗ | _ |
| Song, 2019 | p < 0.05∗ | _ | _ | p < 0.05∗ |
| Thirumala, 2015 | p < 0.05∗ | _ | _ | p = 0.69 |
| Thirumala, 2011 | p < 0.05∗ | _ | _ | p = 1.00 |
| Tobishima, 2013 | p < 0.05∗ | _ | _ | p = 0.56 |
| Wei, 2018 | p = 0.017∗ | _ | _ | p = 0.249 |
| Yamashita, 2005 | p = 0.281 | _ | _ | p = 0.399 |
| Ying, 2011 | p < 0.01∗ | p < 0.01∗ | p < 0.01∗ | _ |
| Zhang, 2017 | p < 0.05∗ | p < 0.05∗ | _ | _ |
| Zhu, 2019 | _ | _ | p < 0.05∗ | _ |
∗p < 0.05.
∗∗Unable to be analyzed due to absence of patient with spasm in LSR resolution and LSR persistence group.
Fifteen studies in our review analyzed the correlation between LSR change and HFS status in short-term follow-up. Three of them showed no significant relationship in 1-day to 1-week evaluation. This may be due to intraoperative finding of multiple vessels compression, in addition to not easily seen vessel behind the facial nerve or vessels coursing around the root exit zone without compressing the nerve, which cause of residual spasm after MVD, as hypothesized by Kong et al. [24] High proportion of patients with LSR persistence but HFS free was found in paper by Kiya et al [23] and Yamashita et al [33] which cause insignificant correlation between LSR resolution and HFS relief. This may occur due to small sample size included in the studies. Kiya et al finding in 3-month follow-up even could not be analyzed because no spasm remained in both LSR disappeared and persistence groups, and Yamashita et al result in 1-year evaluation was also not significant.
There were five articles [25, 29, 30, 31, 32] that found significant relationship in 1-day to 1-week follow-up but insignificant in ≥1-year. All studies showed compelling conversion of patients with LSR residue from persistence spasm to HFS-free in long-term follow-up. This change of outcome made the correlation not significant in one year or more evaluation.
One study showed insignificant result at 1-week follow-up but significant at 1-year follow-up. An article by Kong et al [24] reported some patients who still had residual spasm despite LSR disappearance. Various and complicated findings of the offending vessels, as stated in the report, may be the cause of spasm persistence. However, in 1-year follow-up, number of patients that included in HFS-free status increased remarkably so that the correlation between LSR changed and outcome became significant.
We found four papers that resulted in significant correlation at short-term and long-term follow-up. A study by Huang et al showed significant relationship between LSR resolution and symptom relief in short-term (1-day post-surgery, p = 0.000) and long-term (1-year follow-up, p = 0.000). The authors re-examined of LSR at the time of follow-up, showing that 53% of patients with initial persistent LSR intraoperatively evolved into LSR free. At 1-day assessment, 97% patients with LSR free had negative HFS, whereas 35% patient with LSR persistent had positive HFS. Number of patients with LSR free and negative HFS increased into 99% at 1-year follow-up, while subjects with LSR persistent and HFS positive decreased into 12% [16]. Another study authored by Liu et al showed in 1-day evaluation, 98% patients with LSR resolution intraoperatively had symptom relief, whereas 72% patients with non-resolved intraoperative LSR had symptom persistence. In 1-year follow-up, only one patient who was in non-resolved LSR and spasm persistence group had improvement of symptom, hence the correlation between LSR change and HFS status still significant [27]. Song et al found significant relationship between LSR resolution and complete elimination of spasm in short-term and long-term. Patients with disappearance of LSR, defined as reduce of amplitude >50% from baseline, had spasm free as much as 83% in 1-week evaluation. After one year, only eight patients was added into group of patients with resolved LSR and HFS free [15]. A report by Isu et al found similar result in 1-day and >1-year follow-up. The study contained 40 patients which only showed little different result between short-term and long-term evaluation, therefore the statistical outcome remained significant [20].
Overall, LSR was significantly correlated with spasm relief in 1-day to 1-week, 3-month, and 6-month follow-up. However, due to spontaneous improvement overtime in patients who initially had HFS persistence post-MVD, most studies found the correlation had been turned into not significant in 1-year or more follow-up.
In 2020, there are two articles that present systematic review and meta-analysis of similar topic with this study. The first study was conducted by Thirumala et al [48] which contained 26 reports Our review contained two studies [15, 36], published in 2019, that was not included in Thirumala analysis. The study found that LSR provided high specificity (89%–90%) and low sensitivity (40%–41%) for predicting HFS relief at discharge, 3-month follow-up, and 1-year follow-up. Specificity defined as proportion of patients who will be free of HFS postoperatively whose LSR was eliminated intraoperatively; sensitivity referred to proportion of patients who will continue to have HFS postoperatively whose LSR could not be eliminated intraoperatively. Our study had slightly different result that showed LSR might not be a reliable predictor of HFS-free status at 1-year follow-up.
The second meta-analysis was conducted by Zhang et al [49] contained 14 studies, which had eight similar articles [15, 16, 17, 25, 26, 29, 31, 34] with ours. Short-term was referred to no more than one week, whereas long-term was described as no less than half a year. Overall effect showed that disappearance of AMR during MVD was associated with a favorable short-term outcome (pooled RR, 1.42; 95% CI, 1.24–1.62). Regarding long-term outcome, the result was almost similar with ours, as the resolution of LSR was almost ineffective in predicting long-term outcome (pooled RR, 10.9; 95% CI, 1.02–1.17). This finding showed delayed relief of HFS after surgery was common and improvement of spasm occurred over time.
One of the purposes of our review was to give insight to neurosurgeons regarding the usefulness of IOM. This review showed the role of IOM in guiding surgeons to explore the surgical area further to ensure adequate facial nerve decompression. However, the no difference result of outcome between LSR disappearance and LSR persistence in long-term follow-up might raise question to neurosurgeons whether to or not to use IOM in surgery. Wei et al had compared the outcome of HFS patients who received MVD with or without IOM, and the result showed no difference between groups in 1-week and 1-year follow-up [32]. A study by Shin et al also found similar results between monitored and non-monitored groups in 6-month follow-up [50]. This findings may provide input to neurosurgeons who work in facilities lacking resources for IOM. Nevertheless, relief of spasm since completion of the surgery will make patients feel comfortable, and this might be something surgeons like to consider if IOM is available.
There are several limitations in our systematic review. First, only English articles were included. Second, there was variability in defining “LSR resolution” and “HFS relief”; some papers mentioned complete LSR and spasm disappearance, while others did not use those definitions strictly. Different result could be found if criteria in one study applied in other studies. Third, some articles [17, 19, 20, 23] contained small samples. Fourth, most of included articles were in retrospective fashion [15, 16, 17, 21, 22, 24, 25, 27, 29, 30, 31, 33, 34, 35], which risked unavoidable bias. Our result should be viewed with caution owing to some limitations.
5. Conclusion
Intraoperative monitoring is a useful tool to predict spasm improvement after MVD. Available literature showed that LSR disappearance was an independent predictor of outcome in short-term follow-up. However, as symptoms can improve spontaneously over time, LSR disappearance or persistence after decompression have similar prognosis in 1-year or more follow-up. Even though long-term outcomes of patients with LSR relief and persistence are similar, resolution of symptoms shortly after surgery will provide comfort to patients thereby improving their quality of life.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Nadya Zaragita for providing us an original illustration depicting facial nerve course in Figure 1.
Contributor Information
Sayyid Abdil Hakam Perkasa, Email: sayyidperkasa@gmail.com.
Kevin Gunawan, Email: dr.med.kgunawan@gmail.com.
References
- 1.Rosenstengel C., Matthes M., Baldauf J., Fleck S., Schroeder H. Hemifacial spasm. Dtsch Aerzteblatt. 2012 Oct 12 doi: 10.3238/arztebl.2012.0667. https://www.aerzteblatt.de/10.3238/arztebl.2012.0667 Online [Internet]; [cited 2020 Aug 12]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaltho T.C., Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms: differential Diagnosis of Hemifacial Spasm. Mov. Disord. 2011 Aug 1;26(9):1582–1592. doi: 10.1002/mds.23692. [DOI] [PubMed] [Google Scholar]
- 3.Dulak D., Naqvi I.A. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2020. Neuroanatomy, cranial nerve 7 (facial)http://www.ncbi.nlm.nih.gov/books/NBK526119/ [cited 2020 Oct 12]. Available from: [PubMed] [Google Scholar]
- 4.Seneviratne S.O., Patel B.C. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2020. Facial nerve anatomy and clinical applications.http://www.ncbi.nlm.nih.gov/books/NBK554569/ [cited 2020 Oct 12]. Available from: [PubMed] [Google Scholar]
- 5.Chopade T.R., Bollu P.C. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2020. Hemifacial spasm.http://www.ncbi.nlm.nih.gov/books/NBK526108/ [cited 2020 Aug 13]. Available from: [Google Scholar]
- 6.Chaudhry N., Srivastava A., Joshi L. Hemifacial spasm: the past, present and future. J. Neurol. Sci. 2015 Sep;356(1–2):27–31. doi: 10.1016/j.jns.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J., Li S.-T., Zhong J., Guan H.-X., Ying T.-T., Yang M. Role of arterioles in management of microvascular decompression in patients with hemifacial spasm. J. Clin. Neurosci. 2012 Mar;19(3):375–379. doi: 10.1016/j.jocn.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Jannetta P.J., Abbasy M., Maroon J.C., Ramos F.M., Albin M.S. Etiology and definitive microsurgical treatment of hemifacial spasm: operative techniques and results in 47 patients. J. Neurosurg. 1977 Sep;47(3):321–328. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- 9.Chung S.S., Chang J.H., Choi J.Y., Chang J.W., Park Y.G. Microvascular decompression for hemifacial spasm: a long-term follow-up of 1,169 consecutive cases. Stereotact. Funct. Neurosurg. 2001;77(1–4):190–193. doi: 10.1159/000064620. [DOI] [PubMed] [Google Scholar]
- 10.Miller L.E., Miller V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: a systematic review. Br. J. Neurosurg. 2012 Aug;26(4):438–444. doi: 10.3109/02688697.2011.641613. [DOI] [PubMed] [Google Scholar]
- 11.Lee S., Park S.-K., Lee J.-A., Joo B.-E., Park K. Missed culprits in failed microvascular decompression surgery for hemifacial spasm and clinical outcomes of redo surgery. World Neurosurg. 2019 Sep;129:e627–e633. doi: 10.1016/j.wneu.2019.05.231. [DOI] [PubMed] [Google Scholar]
- 12.Shu W., Zhu H., Li Y., Liu R. Clinical analysis of repeat microvascular decompression for recurrent hemifacial spasm. Acta Neurol. Belg. 2019 Sep;119(3):453–459. doi: 10.1007/s13760-019-01103-9. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen V.K. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology. 1984 Apr 1;34(4):427. doi: 10.1212/wnl.34.4.427. [DOI] [PubMed] [Google Scholar]
- 14.Kameyama S., Masuda H., Shirozu H., Ito Y., Sonoda M., Kimura J. Ephaptic transmission is the origin of the abnormal muscle response seen in hemifacial spasm. Clin. Neurophysiol. 2016 May;127(5):2240–2245. doi: 10.1016/j.clinph.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Song H., Xu S., Fan X., Yu M., Feng J., Sun L. Prognostic value of lateral spread response during microvascular decompression for hemifacial spasm. J. Int. Med. Res. 2019 Dec;47(12):6120–6128. doi: 10.1177/0300060519839526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Miao S., Chu H., Dai C., Wu J., Wang J. An optimized abnormal muscle response recording method for intraoperative monitoring of hemifacial spasm and its long-term prognostic value. Int. J. Surg. 2017 Feb;38:67–73. doi: 10.1016/j.ijsu.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Eckardstein K., Harper C., Castner M., Link M. The significance of intraoperative electromyographic “lateral spread” in predicting outcome of microvascular decompression for hemifacial spasm. J. Neurol. Surg. Part B Skull Base. 2014 Mar 12;75(3):198–203. doi: 10.1055/s-0034-1368145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Damaty A., Rosenstengel C., Matthes M., Baldauf J., Schroeder H.W.S. The value of lateral spread response monitoring in predicting the clinical outcome after microvascular decompression in hemifacial spasm: a prospective study on 100 patients. Neurosurg. Rev. 2016 Jul;39(3):455–466. doi: 10.1007/s10143-016-0708-9. [DOI] [PubMed] [Google Scholar]
- 19.Hatem J., Sindou M., Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br. J. Neurosurg. 2001 Jan;15(6):496–499. doi: 10.1080/02688690120105101. [DOI] [PubMed] [Google Scholar]
- 20.Isu T., Kamada K., Mabuchi S., Kitaoka A., Ito T., Koiwa M. Intra-operative monitoring by facial electromyographic responses during microvascular decompressive surgery for hemifacial spasm. Acta Neurochir (Wien) 1996 Jan;138(1):19–23. doi: 10.1007/BF01411718. [DOI] [PubMed] [Google Scholar]
- 21.Jo K.W., Kong D.-S., Park K. Microvascular decompression for hemifacial spasm: long-term outcome and prognostic factors, with emphasis on delayed cure. Neurosurg. Rev. 2013 Apr;36(2):297–302. doi: 10.1007/s10143-012-0420-3. [DOI] [PubMed] [Google Scholar]
- 22.Joo W.-I., Lee K.-J., Park H.-K., Chough C.-K., Rha H.-K. Prognostic value of intra-operative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J. Clin. Neurosci. 2008 Dec;15(12):1335–1339. doi: 10.1016/j.jocn.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Kiya N., Bannur U., Yamauchi A., Yoshida K., Kato Y., Kanno T. Monitoring of facial evoked EMG for hemifacial spasm: a critical analysis of its prognostic value. Acta Neurochir (Wien) 2001 May 16;143(4):365–368. doi: 10.1007/s007010170091. [DOI] [PubMed] [Google Scholar]
- 24.Kong D.-S., Park K., Shin B., Lee J.A., Eum D.-O. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J. Neurosurg. 2007 Mar;106(3):384–387. doi: 10.3171/jns.2007.106.3.384. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.H., Park B.J., Shin H.S., Park C.K., Rhee B.A., Lim Y.J. Prognostic ability of intraoperative electromyographic monitoring during microvascular decompression for hemifacial spasm to predict lateral spread response outcome. J. Neurosurg. 2017 Feb;126(2):391–396. doi: 10.3171/2016.1.JNS151782. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Zhang Y., Zhu H., Li Y. Prognostic value of intra-operative abnormal muscle response monitoring during microvascular decompression for long-term outcome of hemifacial spasm. J. Clin. Neurosci. 2012 Jan;19(1):44–48. doi: 10.1016/j.jocn.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Liu M.X., Zhong J., Xia L., Dou N.-N., Sun H., Li B. The significance of abnormal muscle response monitoring during microvascular decompression for hemifacial spasm. In: Visocchi M., Mehdorn H.M., Katayama Y., von Wild K.R.H., editors. Springer International Publishing; Cham: 2017. pp. 297–301.http://link.springer.com/10.1007/978-3-319-39546-3_43 (Trends in Reconstructive Neurosurgery [Internet]). [cited 2020 Aug 12]; (Acta Neurochirurgica Supplement; vol. 124). Available from: [Google Scholar]
- 28.Shin J.C., Chung U.H., Kim Y.C., Park C.I. Prospective study of microvascular decompression in hemifacial spasm. Neurosurgery. 1997 Apr 1;40(4):730–735. doi: 10.1097/00006123-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Thirumala P.D., Wang X., Shah A., Habeych M., Crammond D., Balzer J.R. Clinical impact of residual lateral spread response after adequate microvascular decompression for hemifacial spasm: a retrospective analysis. Br. J. Neurosurg. 2015 Nov 2;29(6):818–822. doi: 10.3109/02688697.2015.1054351. [DOI] [PubMed] [Google Scholar]
- 30.Thirumala P.D., Shah A.C., Nikonow T.N., Habeych M.E., Balzer J.R., Crammond D.J. Microvascular decompression for hemifacial spasm: evaluating outcome prognosticators including the value of intraoperative lateral spread response monitoring and clinical characteristics in 293 patients. J. Clin. Neurophysiol. 2011 Feb;28(1):56–66. doi: 10.1097/WNP.0b013e3182051300. [DOI] [PubMed] [Google Scholar]
- 31.Tobishima H., Hatayama T., Ohkuma H. Relation between the persistence of an abnormal muscle response and the long-term clinical course after microvascular decompression for hemifacial spasm. Neurol. Med.-Chir. 2014;54(6):474–482. doi: 10.2176/nmc.oa2012-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y., Yang W., Zhao W., Pu C., Li N., Cai Y. Microvascular decompression for hemifacial spasm: can intraoperative lateral spread response monitoring improve surgical efficacy? J. Neurosurg. 2018 Mar;128(3):885–890. doi: 10.3171/2016.11.JNS162148. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita S., Kawaguchi T., Fukuda M., Watanabe M., Tanaka R., Kameyama S. Abnormal muscle response monitoring during microvascular decompression for hemifacial spasm. Acta Neurochir (Wien) 2005 Sep;147(9):933–938. doi: 10.1007/s00701-005-0571-x. [DOI] [PubMed] [Google Scholar]
- 34.Ying T.-T., Li S.-T., Zhong J., Li X.-Y., Wang X.-H., Zhu J. The value of abnormal muscle response monitoring during microvascular decompression surgery for hemifacial spasm. Int. J. Surg. 2011;9(4):347–351. doi: 10.1016/j.ijsu.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Zhao H., Ying T.-T., Tang Y.-D., Zhu J., Li S.-T. The effects of dual abnormal muscle response monitoring on microvascular decompression in patients with hemifacial spasm. World Neurosurg. 2017 May;101:93–98. doi: 10.1016/j.wneu.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W., Sun C., Zhang Y., Xu J., Wu S. AMR monitoring in microvascular decompression for hemifacial spasm: 115 cases report. J. Clin. Neurosci. 2020 Mar;73:187–194. doi: 10.1016/j.jocn.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Tan E.K., Jankovic J. Bilateral hemifacial spasm: a report of five cases and a literature review. Mov. Disord. 1999 Mar;14(2):345–349. doi: 10.1002/1531-8257(199903)14:2<345::aid-mds1023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Felício A.C., Godeiro-Junior C. de O., Borges V., Silva SM. de A., Ferraz H.B. Bilateral hemifacial spasm: a series of 10 patients with literature review. Park. Relat. Disord. 2008 Mar;14(2):154–156. doi: 10.1016/j.parkreldis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Auger R.G., Whisnant J.P. Hemifacial spasm in rochester and olmsted county, Minnesota, 1960 to 1984. Arch. Neurol. 1990 Nov 1;47(11):1233–1234. doi: 10.1001/archneur.1990.00530110095023. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y., Davidson A.L., Pan T., Jankovic J. Asian over-representation among patients with hemifacial spasm compared to patients with cranial–cervical dystonia. J. Neurol. Sci. 2010 Nov;298(1–2):61–63. doi: 10.1016/j.jns.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Poungvarin N., Devahastin V., Viriyavejakul A. Treatment of various movement disorders with botulinum A toxin injection: an experience of 900 patients. J Med Assoc Thail Chotmaihet Thangphaet. 1995 Jun;78(6):281–288. [PubMed] [Google Scholar]
- 42.Nilsen B., Le K.-D., Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology. 2004 Oct 26;63(8):1532–1533. doi: 10.1212/01.wnl.0000142080.85228.e8. [DOI] [PubMed] [Google Scholar]
- 43.Møller A.R., Jannetta P.J. Microvascular decompression in hemifacial spasm: intraoperative electrophysiological observations. Neurosurgery. 1985 May 1;16(5):612–618. doi: 10.1227/00006123-198505000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura D.M., Aminoff M.J., Tami T.A., Scott A.B. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve. 1992 Sep;15(9):1045–1049. doi: 10.1002/mus.880150909. [DOI] [PubMed] [Google Scholar]
- 45.Pandey S., Jain S. Clinical features and response to botulinum toxin in primary and secondary hemifacial spasm. Neurol. India. 2018;66(4):1036. doi: 10.4103/0028-3886.236959. [DOI] [PubMed] [Google Scholar]
- 46.Defazio G., Abbruzzese G., Girlanda P., Vacca L., Currà A., De Salvia R. Botulinum toxin A treatment for primary hemifacial spasm: a 10-year multicenter study. Arch. Neurol. 2002 Mar 1;59(3):418. doi: 10.1001/archneur.59.3.418. [DOI] [PubMed] [Google Scholar]
- 47.Nan-xiang X., Lv-an C., Zhi-jun C., Hong-yang Z. Placement of Teflon sponges in microvascular decompression procedure for treatment of hemifacial spasm. J Neurol Surg Part Cent Eur Neurosurg. 2015 Mar 23;77(4):321–325. doi: 10.1055/s-0034-1543957. [DOI] [PubMed] [Google Scholar]
- 48.Thirumala P.D., Altibi A.M., Chang R., Saca E.E., Iyengar P., Reddy R. The utility of intraoperative lateral spread recording in microvascular decompression for hemifacial spasm: a systematic review and meta-analysis. Neurosurgery [Internet] 2020 Apr 16 doi: 10.1093/neuros/nyaa069. https://academic.oup.com/neurosurgery/advance-article/doi/10.1093/neuros/nyaa069/5820806 [cited 2020 Aug 28]; Available from: [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Li Z.-H., Wang J.-F., Chen Y.-H., Wang N., Wang Y. Prognostic value of abnormal muscle response during microvascular decompression for hemifacial spasm: a meta-analysis. World Neurosurg. 2020 May;137:8–17. doi: 10.1016/j.wneu.2020.01.166. [DOI] [PubMed] [Google Scholar]
- 50.Shin J.C., Kim Y.C., Park C.I., Chung U.H. Intraoperative monitoring of microvascular decompression in hemifacial spasm. Yonsei Med. J. 1996;37(3):209. doi: 10.3349/ymj.1996.37.3.209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.