Abstract
Background
HIV, heavy drinking, and smoking are all pro-inflammatory and increase risk for coronary heart disease (CHD). Interventions that reduce alcohol use, smoking, or both in HIV-positive people could lower inflammation, CHD and death risk. Varenicline and cytisine are proven therapies for smoking cessation and may also reduce alcohol consumption. The comparative efficacy of varenicline and cytisine to reduce alcohol consumption has not been tested, nor has their comparative effectiveness been reported for smoking.
Objective
This paper describes the protocol of the Studying Partial agonists for Ethanol and Tobacco Elimination in Russians with HIV (St PETER HIV), a four-arm parallel-group randomized controlled trial comparing effects of varenicline, cytisine, and nicotine replacement therapy (NRT).
Methods
The study is recruiting four hundred HIV-positive heavy drinking smokers interested in cutting down on alcohol and/or tobacco in St. Petersburg, Russia. Participants are randomly assigned to receive either active varenicline + NRT placebo, varenicline placebo + active NRT, active cytisine + NRT placebo, cytisine placebo + active NRT. All participants receive evidence-based counseling for alcohol and tobacco use, one active medication, and one placebo. Outcomes are: 1) % heavy drinking days in the past month (primary study outcome at three months) and alcohol craving; 2) cigarettes per day (primary smoking outcome at 3 months) and 7-day point prevalence abstinence and; 3) inflammation, CHD risk, and mortality risk.
Conclusion
St PETER HIV addresses the paucity of randomized controlled trial data to guide treatment of alcohol consumption and smoking in HIV-positive heavy drinking smokers.
Keywords: Russia, HIV, Alcohol use, Smoking, Varenicline, Cytisine
1. Introduction
In the era of effective antiretroviral treatment (ART) for HIV infection, co-morbidities such as coronary heart disease (CHD) are increasingly contributing to mortality and morbidity in this population [1]. People living with HIV (PLWH) who drink heavily and smoke are at high risk for CHD and death [2]. The mechanisms driving increased CHD risk in PLWH are unclear, but are linked to inflammation [[3], [4], [5], [6]]. HIV, heavy drinking, and smoking are all pro-inflammatory [7,8]. HIV viral suppression with antiretroviral therapy does not eliminate the elevated CHD risk or the increased inflammation (i.e., pre-HIV infection levels are not restored) [1]. Interventions that reduce alcohol use, smoking, or both in PLWH could lower inflammation, CHD and death risk [9,10]. Varenicline and cytisine are proven therapies for smoking cessation [[11], [12], [13], [14], [15]]. When compared to placebo, varenicline has higher cessation rates than cytisine, suggesting that its efficacy may be superior to cytisine [12,16], which can only be confirmed by head-to-head comparisons that are currently underway in non-HIV populations [17,18].
Human trials have studied varenicline for alcohol craving and consumption [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]], and a recent meta-analysis concluded that varenicline is superior to placebo for decreasing alcohol consumption [32]. This study also concluded that varenicline may be useful to lower heavy drinking days in people with unhealthy levels of drinking, but called for additional studies to address this important question. In murine models, cytisine reduces alcohol consumption [[33], [34], [35]], but human data on cytisine and alcohol consumption are lacking [36]. The comparative efficacy of varenicline and cytisine to reduce alcohol consumption and by extension, inflammation, CHD, and mortality risk, in humans has not been tested. Neither drug has been tested for smoking cessation against nicotine replacement therapy (NRT) in PLWH who are heavy drinkers and smokers. This paper describes the protocol of the Studying Partial agonists for Ethanol and Tobacco Elimination in Russians with HIV (St PETER HIV) trial, a four-arm parallel-group randomized controlled trial among 400 PLWH reporting heavy drinking and smoking to compare the effects of varenicline, cytisine, and NRT for alcohol and smoking outcomes.
2. Methods/design
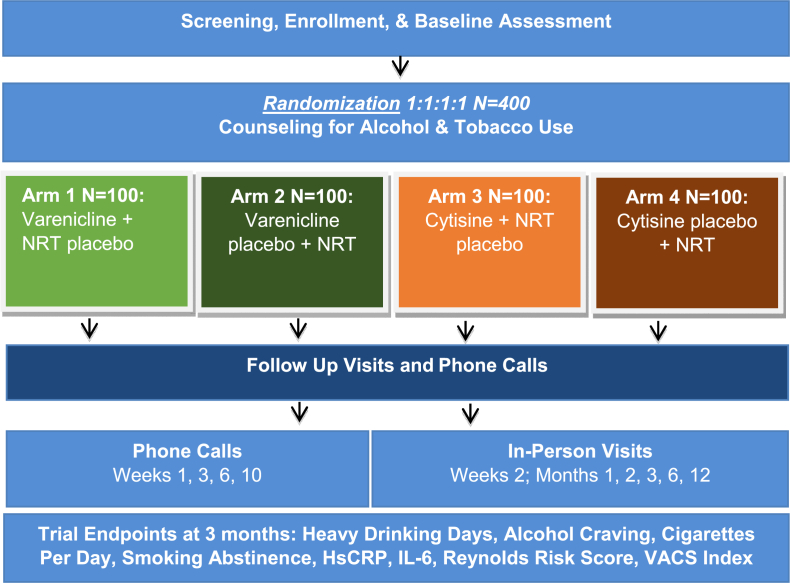
St PETER HIV will compare the effects of varenicline, cytisine, and NRT on: 1) alcohol use and craving, 2) smoking, and 3) inflammation and risk for CHD and mortality. Self-reported alcohol and smoking outcomes will be assessed at 1, 3 (primary), 6, and 12 months. Eligible participants are randomly assigned into one of four study arms: 1) active varenicline + NRT placebo; 2) varenicline placebo + active NRT; 3) active cytisine + NRT placebo; 4) cytisine placebo + active NRT. All participants receive evidence-based counseling for alcohol and tobacco use, 1 active medication, and 1 placebo (Fig. 1).
Fig. 1.
Study design of the St PETER HIV trial.
2.1. Study setting
Recruitment was initiated in July 2017. Participants are recruited from a recently completed cohort study, as well as from HIV clinical care sites and non-clinical sites, and via snowball recruitment in St. Petersburg, Russia. All study enrollment and assessment activities take place at the Laboratory of Clinical Pharmacology of Addictions at Pavlov University. All Research Assessors (RAs) are trained medical personnel (MD, MD/PhD) with prior experience conducting research studies and all study team members are trained in the Protection of Human Subjects and Good Clinical Practice (GCP).
2.2. Participants and recruitment
St PETER participants must meet the following inclusion criteria: 1) 18–70 years old; 2) HIV-positive; 3) ≥5 heavy drinking days (i.e., National Institute on Alcohol Abuse and Alcoholism [NIAAA] at-risk drinking levels) in the past 30 days; 4) smoking an average of at least 5 cigarettes per day; 5) provision of contact information for 2 contacts to assist with follow-up; 6) stable address within 100 km of St. Petersburg; 7) possession of a telephone; 8) interest in cutting down alcohol or tobacco; 9) able and willing to comply with study protocols and procedures. The following serve as exclusion criteria: 1) not fluent in Russian; 2) cognitive impairment resulting in inability to provide informed consent; 3) pregnancy, planning to become pregnant in next 3 months, or breastfeeding; 4) unstable psychiatric illness (i.e., answering yes to any of the following: past three month active hallucinations, mental health symptoms prompting a visit to the emergency room or hospital, mental health medication changes due to worsening symptoms, presence of suicidal ideations); 5) history of seizures; 6) acute coronary syndrome within 1 month of enrollment; 7) taking smoking cessation medications in the past 30 days; 8) history of pheochromocytoma; 9) history of Buerger's disease; 10) systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 105 mm Hg; 11) currently taking anti-tuberculosis medications; 12) currently taking galantamine or physostigmine; 13) breath alcohol content (BAC) level of 0.10% or higher; 14) known allergy to varenicline or cytisine.
Interested participants are screened via phone or in-person at Pavlov University following the administration and receipt of verbal consent for the screening process. For participants screened by phone and found eligible, a visit to the study site is scheduled, where all screening questions are repeated, followed by additional screening procedures (i.e., rapid HIV test, urine pregnancy test, measurement of BAC, blood pressure). Prior to performing the additional screening procedures, RA administers and documents informed consent and enrolls participants into the study. If participants do not meet eligibility criteria based on laboratory testing, they are disenrolled at this stage. Once eligibility is confirmed, the RA collects contact information for the participant and at least two alternative contacts and verifies phone numbers are working and valid.
Participants then meet with the study nurse for phlebotomy. Inability to obtain blood after repeated attempts precludes randomization given the importance of samples in the determination of study outcomes. Upon completion of a successful blood draw, the RA administers the baseline assessment and randomizes participants to a study group.
2.3. Randomization and blinding
Participants are randomized to study groups in a 1:1:1:1 ratio using blocked randomization stratified by three factors: alcohol consumption (≥3 vs. <3 heavy drinking days in the past week according to NIAAA risky drinking criteria), average daily cigarettes (≤1 vs. >1 pack per day), and current ART use to ensure balance across arms. Randomization is conducted and monitored by the URBAN ARCH Biostatistics and Data Management (BDM) core. The software package SAS was used to generate randomization lists to assign participants as they are enrolled into the study. Study participants, investigators, staff, and physicians administering the medications are unaware to which of the 4 arms a participant is assigned. Although theoretically possible to double blind this study, it would not have been practical or feasible because the regimens for varenicline and cytisine are very different and would therefore require complex regimens to incorporate the necessary number of placebos. Participants assigned to either nicotinic acetylcholine receptor (nAChR) partial agonist arm (varenicline or cytisine) are blinded to whether the study medication is active or placebo.
2.4. Intervention
Participants are assigned to one of 4 study groups: 1) active varenicline + NRT placebo; 2) varenicline placebo + active NRT; 3) active cytisine + NRT placebo; 4) cytisine placebo + active NRT (Table 1).
Table 1.
St PETER HIV intervention arms.
| Study Arm | Intervention |
|---|---|
| Active Varenicline + NRT Placebo | 12-week regimen of active varenicline and eight weeks of NRT placebo mouth spray |
| Varenicline Placebo + Active NRT | 12-weeks regimen of placebo varenicline and eight weeks of active NRT mouth spray |
| Active Cytisine + NRT Placebo | 25-day regimen of active cytisine and eight weeks of NRT placebo mouth spray |
| Cytisine Placebo + Active NRT | 25-day regimen of placebo cytisine and eight weeks of active NRT mouth spray |
All participants receive study pills (active or placebo) and a study mouth spray (active NRT or placebo). Active varenicline and cytisine study pills are purchased from a distributor in St. Petersburg and delivered to a compounding pharmacy to be encapsulated with 50 mg of riboflavin, a biologic adherence measure. The procedure is the same for placebo pills, which are composed of lactose and contain riboflavin. The placebo and active study medications are indistinguishable by appearance and taste. This study administers NRT in the form of a mouth spray. NRT mouth spray is purchased locally in St. Petersburg and delivered to Vertex Pharmaceuticals for repackaging, where an identical placebo is manufactured. Placebo solution contains propylene glycol, anhydrous ethanol, trometamol, glycerol, sodium hydrogen carbonate, levomenthol, mint flavor, cooling flavor, sucralose, acesulfame potassium, hydrochloric acid, capsaicin and purified water. The taste and smell of placebo is identical to active NRT mouth spray. Study medication are provided by trained physicians, who instruct participants in proper medication administration and adherence. In accordance with FDA guidelines, the study did not require an Investigational New Drug (IND) application, as it was conducted outside of the United States. Participants are asked to set a date to stop smoking one week from the baseline visit and all participants receive brief (5-min) evidence-based counseling for alcohol and tobacco use at baseline [37,38]. Alcohol counseling follows guidelines from the NIAAA. For tobacco, counseling follows the Agency for Healthcare Research and Quality's Five Major Steps to intervention (”5 As”). Medication dosing starts on the day of the baseline visit.
2.5. Varenicline
Participants are provided with a 12-week regimen of varenicline (active and placebo). Active varenicline follows recommended dosing, beginning at 0.5 mg for men and women (one pill on days 1–3 and two pills per day [morning and evening] on days 4–7) with a target dose of 1 mg twice daily during weeks 2–12. Participants are asked to take the study medication with a full glass of water and to take the medication at the same time each day.
2.6. Cytisine
Participants receive cytisine (active and placebo) for 25 days. Medication dosing follows recommended standards and is administered perorally according to the following schedule:
First three days: one tablet (1.5 mg) six times daily (every 2 h, up to six tablets per day).
4th to 12th day: one tablet every two and a half hours (five tablets daily).
13th to 16th day: one tablet every 3 h (four tablets daily).
17th to 20th day: one tablet every 5 h (three tablets daily).
21st to 25th day: one to two tablets daily.
2.7. Nicotine replacement therapy
NRT and placebo mouth spray consist of a small bottle (30 ml) of solution held in a dispenser with a mechanical spray pump. Each dispenser contains approximately 300 sprays. Participants are instructed to use enough spray to control cravings. They are asked to use one spray first when they would normally smoke a cigarette or have cravings to smoke. If cravings do not disappear within a few minutes, they are asked to use a second spray. Participants are instructed not to use more than two sprays per dose or four sprays every hour and are asked not to use more than 64 sprays per day. For the first four weeks participants are asked to use at least eight sprays per day (every 2 h while awake) as a minimum. After four weeks, participants are instructed to use the spray as much as they need within the recommended dosing for a total of eight weeks.
2.8. Adherence
Medication adherence is assessed at each study visit using objective (Riboflavin, pill count) and subjective (self-report) measures.
2.9. Direct adherence measure
Riboflavin (50 mg), a vitamin yielding a change in urine color, is added to study capsules. Participants are informed that the color change is harmless. At this dose, Riboflavin is expected to remain in the system at detectable levels for up to 24 h. At each study visit post-baseline, participants are asked to provide a urine sample, which is visually inspected for the presence of Riboflavin in a room with low ambient light, using ultraviolet (UV) light at the long wave setting (33 mm) [39].
2.10. Indirect adherence measures
Pill counts and self-report are also used as adherence measures. Participants are instructed to return any unused medication at each study visit post-baseline. The assessor counts and records the number of remaining pills. At each post-baseline study visit, participants are asked to draw a line on a paper ruler numbered from 0 to 100, to indicate the number that best reflects how much of the study medication they have taken in the past week. Participants are also asked if they stopped taking the medication for any duration in the past week. For the purposes of study analyses, participants will be considered adherent to study medication if they self-report taking at least 80% of their assigned study medication in the past week [40].
2.11. Adherence aids
During each study visit at which medication is provided, strategies for adherence are discussed with the participant. To further increase medication adherence, an automated text message is sent daily (on weekdays) reminding participants to take their study medication. Participants may reduce the frequency or opt out of text message reminders entirely at any time throughout the study. Participants are also encouraged to set a reminder in their phone to take the study medication each day. A paper calendar is provided to participants to assist with tracking medications taken.
2.12. Assessments
Questionnaires.
Questionnaires are administered at baseline, 1-, 3-, 6-, and 12-month study visits with shorter assessments involving medication adherence check-ins at 1-week, 2-weeks, 3-weeks, 6-weeks, 2-months, and 10-weeks (See Table 2).
Table 2.
St PETER HIV data and sample collection.
| Study Time Point (in months) |
|||||||
|---|---|---|---|---|---|---|---|
| Administered Assessment | Description | Baseline | Short Check Ins | 1 Mo. | 3 Mo. |
6 Mo. |
12 Mo. |
| Demographics [41,42] | Participant demographics and socio-economic status | X | X | ||||
| 30-day Alcohol Timeline Followback (TLFB) [43] | Estimates of participant's daily drinking over the past 30 days | X | X | X | X | X | |
| Alcohol Use Disorders Identification Test (AUDIT) [44] | Identifies persons with hazardous alcohol use | X | X | X | |||
| DSM-5 Alcohol Use Disorder [45] | Diagnosis of alcohol use disorders, with classifications of mild, moderate, and severe | X | X | ||||
| Short Inventory of Problems (SIP) for Alcohol Use [46,47] | Assessment of adverse consequences of alcohol use | X | X | X | X | X | |
| Penn Alcohol Craving Scale [48] | Instrument for assessing alcohol craving. Measures frequency, intensity, and duration of thoughts about drinking, as well as ability to resist drinking | X | X | X | X | X | |
| Readiness to Quit Alcohol [49] | Assesses readiness to quit drinking via a readiness to change ruler | X | X | X | X | X | |
| 30-day Cigarette Timeline Followback (TLFB) [50,51] | Estimates of participant's daily cigarette smoking over the past 30 days | X | X | X | X | X | |
| Tobacco Use (Fagerström) [52,53] | A measure of nicotine dependence | X | X | X | X | X | |
| Other Tobacco Use [54] | Assesses tobacco use other than cigarette use | X | X | X | X | X | |
| Tobacco Craving (QSU) [[55], [56], [57]] | Instrument for assessing craving to smoke | X | X | X | X | X | |
| Readiness to Quit Tobacco [58] | Assesses readiness to quit smoking via a readiness to change ruler | X | X | X | X | X | |
| Smoking Quit Attempts | Assesses whether participant attempted to quit smoking in past 6 months | X | X | X | |||
| HIV Risk Categories [59] | Asks about participant's potential exposure to HIV | X | |||||
| Depressive Symptoms (CES-D) [60,61] | A measure of depressive symptomology | X | X | X | X | X | |
| Anxiety (GAD-7)62 | A measure of severity of anxiety | X | X | X | X | X | |
| Trauma | Questions about traumatic experiences | X | X | ||||
| HIV Testing and HCV Diagnosis | Dates and locations of HIV/HCV testing and HIV/HCV treatment | X | X | ||||
| ART Use and Adherence [63] | Questions on ART and 30-day adherence | X | X | X | |||
| Opportunistic Infections [64] | Assesses any history of candida or yeast infection of the esophagus, TB, pneumonia, or toxoplasmosis | X | X | ||||
| Co-Morbidities [65] | Participant and family medical history | X | X | ||||
| Brief Chronic Pain Questionnaire [66,67] | Two questions about severity of body pain in the past week and duration of bodily pain | X | X | X | |||
| HIV Symptom Index [68] | A 20-item HIV symptom index includes patient-reported symptoms | X | X | ||||
| Smoking-related symptoms [[69], [70], [71]] | Assesses smoking-related respiratory symptom severity | X | X | X | X | X | |
| Reproductive Health | Questions regarding reproductive health | X | X | ||||
| Falls [72] | Questions about falls in the past 6 months | X | X | ||||
| TB testing and treatment | Questions about TB testing, diagnosis, and treatment | X | X | ||||
| Healthcare Utilization [73] | How often participants see a physician and if they receive treatment for HIV and addiction | X | X | X | |||
| Medications | Prescription and non-prescription medication taken in the past 7 days | X | X | X | X | X | |
| Brief Pain Inventory- SF [74] | Participant pain severity in the past 7 days | X | X | X | |||
| Drug Use (modified RBS) & Overdose [[75], [76], [77]] | Participant's self-reported drug use, modified to adapt to drug practices in Russia, and questions regarding history of overdose | X | X | X | X | X | |
| 24 Hour Activities | Participant past 24-h activities that may impact inflammatory biomarkers measured | X | X | X | X | X | |
| Social Support Scale [78,79] | Measures access to companionship, assistance, or other types of support | X | X | ||||
| Veterans Rand 12-Item Health Survey plus 4 cognitive questions from MOS-HIV [80,81] | Assessment of overall health and cognitive function | X | X | ||||
| Smartphones | Asks whether participant owns a smartphone and whether they would be willing to use a free app to help quit drinking or smoking | X | |||||
| Medication Adherence [63] | Adherence to study medication in the past week, as indicated by measurement on a ruler ranging from 0 to 100 and additional questions on adherence patterns | X | X | X | |||
| Medication Satisfaction [82] | Participant satisfaction with study medication | X | X | ||||
| Blinding Questions | Questions on which study medication the participant believes to be taking | X | X | ||||
| Clinical and Laboratory Values/Testing | Baseline | Short Check Ins | 1 Mo. |
3 Mo. |
6 Mo. |
12 Mo. |
|
| Height (m/cm) | X | X | X | X | X | ||
| Weight (kg) | X | X | X | X | X | ||
| Breath Alcohol Content (%) | X | X | X | X | X | ||
| Exhaled Carbon Monoxide (abstinence threshold < 10 ppm)_ | X | X | X | X | X | ||
| Urine Pregnancy Testing | X | X | X | ||||
| Blood Pressure (mmHG) | X | X | X | X | X | ||
| Urine Nicotine Metabolite Testing | X | X | |||||
| Reynolds Risk Score (Cholesterol [total and HD], hsCRP) | X | X | |||||
| VACS Index (HIV Viral Load, CD4 count, AST/ALT, Creatinine, Hemoglobin & Platelets, HCV Aba) | X | X | |||||
| Nicotine Metabolite Ratio (NMR) (Whole blood) | X | ||||||
| Available Repository Samples | Baseline | Short Check Ins | 1 Mo. |
3 Mo. |
6 Mo. |
12 Mo. |
|
| DBS Cards (PEth) | X | X | X | ||||
| Serum and Plasma | X | X | X | X | X | ||
HCV Ab will be measured at baseline, and if negative at baseline, again at 3 months.
Full assessments capture information about participant demographics, general and mental health and health-related behaviors such as substance use. Most sections of the study questionnaire are interviewer-administered, with potentially sensitive sections on HIV risk behaviors, depressive symptoms, anxiety, and trauma, self-administered by the participant. RAs measure and recorde participants’ height, weight, BAC, exhaled carbon monoxide, and blood pressure. Participants receive the equivalent of US $33 in goods or cash for their participation at baseline 1-, 3-, and 6-month study visits and US $40 for the final 12-month study visit. Participants also receive in total the equivalent of US $14 for short medication check phone calls (4 phone calls, $3.50/call) and the equivalent of US $15 for short in-person medication visits at 2 weeks and 2-months post-baseline.
2.13. Laboratory procedures and testing
This study requires the collection of blood from each participant at baseline, 1, 3, 6, and 12 months for storage and testing. At baseline and 3 months, fresh blood is tested for hemoglobin, platelets, CD4, HCV Ab, HIV RNA, HS CRP, cholesterol (total and HDL), eGFR (creatinine), and AST/ALT. Stored samples will be used for IL-6 testing (baseline and 3 months); nicotine metabolites to calculate the nicotine metabolite ratio, which will be used to help interpret study results (baseline), and phosphatidylethanol (PEth) testing (Table 1).
Urine is collected at study visits up to and including the 3 month visit. It is used to administer a pregnancy test to all women. Participants found to be pregnant will discontinue their study medication but will still be followed-up for the duration of the study. Urine is also used to conduct nicotine metabolite testing on all participants at baseline and 3-month study visits to validate participant smoking self-report. The urine nicotine metabolite testing panel includes nicotine, cotinine, 3-hydroxy cotinine, nornicotine, and anabasine.
2.14. Adverse events
Participant symptoms are assessed weekly for the first month and biweekly thereafter while the participants are administered study medication. For rare instances in which a participant experiences severe symptoms, staff call more frequently to ensure safety. A standardized symptom checklist is used for symptom monitoring and is administered by trained clinical staff. Any chronic conditions or symptoms that existed prior to introduction of study medication are documented. Any event that meets the criteria for an adverse event (AE), serious adverse event (SAE), or unanticipated problem is recorded. All events are presented to and reviewed by an external Data and Safety Monitoring Board (DSMB).
2.15. Data Management
The URBAN ARCH Biostatistics and Data Management (BDM) Core provided active statistical collaboration in the design and analysis of the St PETER trial. Electronic questionnaires include programmed skip patterns and range checks, minimizing errors at data capture, and allowing for timely electronic data transfer to the Boston team at regular intervals. Participant screening, tracking and randomization utilize web-based systems, in which data elements necessary to determine eligibility and stratification factors for randomization are entered into a web-based application, minimizing the possibility of enrolling an ineligible person, while allowing US-based collaborators real-time access to enrollment data.
2.16. Analytic methods
2.16.1. Major hypotheses and analytic plans
The study aims to test the hypothesis that 1) varenicline will have greater effects than NRT for reducing alcohol consumption, smoking, inflammation, CHD, and mortality risk; 2) cytisine will have greater effects than NRT for these outcomes and; 3) varenicline will have greater effects than cytisine for these outcomes. The overall primary outcome of the study is percentage of heavy drinking days (HDD) in the past month measured at 3 months using self-reported past 30-day alcohol consumption obtained via the Timeline Follow Back (TLFB) method [43]. The primary smoking-related outcome is self-reported cigarettes per day in the past week measured at 3 months. Secondary outcomes are alcohol craving; percentage of HDD in the past month measured at 1, 6, and 12 months; biochemically-confirmed 7-day point prevalence abstinence from smoking at 3 months (exhaled carbon monoxide, threshold of <10 parts per million) [83]; and biomarkers of inflammation, Reynolds Risk Score and VACS Index Score at 3 months.
The main analysis evaluating the impact of each intervention on the primary study outcome (i.e., the continuous variable percentage of HDD at 3 months) will use multiple regression models. The models will include indicator variables to represent study arm. We will test 3 pairwise comparisons (i.e., varenicline + NRT placebo vs. NRT + varenicline placebo; cytisine + NRT placebo vs. NRT + cytisine placebo; varenicline vs. cytisine) and will adjust for the multiple comparisons using the Hochberg sequential test procedure. To improve efficiency, the regression analyses will control for stratification factors: alcohol consumption, average daily cigarettes, and current ART use. If the data are normally distributed, multiple linear regression models will be used. However, if the distribution of percentage of HDD is skewed, transformations of the data will be performed (e.g., log transformation). If an appropriate transformation is not identified, a median regression model will be used. Other continuous outcomes will be analyzed using the same approach described above. If count data are not approximately normally distributed, they will be analyzed using Poisson or negative binomial (to account for overdispersion) regression models. Binary outcomes will be analyzed using logistic regression models. Models will control for stratification factors as described above for percentage of HDD. A secondary analysis will be conducted using a per protocol approach that includes only those participants who were adherent to their assigned intervention (i.e., taking study medications or placebos 80% of the time).
2.16.2. Sample size and power calculations
To define the limits of the study, we present power calculations to assess the differences we will be able to detect with reasonably high power for the primary study outcome: percentage of HDD. It is expected that 400 participants will be enrolled into the study and we expect to have ~320 participants (80 participants in each of the 4 randomized groups) completing the 3-month follow-up, conservatively assuming 20% loss to follow-up. The following calculations assume 2-sided tests, with an overall significance level of 0.05. For the purposes of power calculations, we consider a simple, conservative setting based on a Bonferroni adjustment for multiple comparisons, although in our analyses we will use the Hochberg sequential correction method, an approach that will result in higher power than the Bonferroni method. To maintain an overall type I error rate of 5%, we assume each of the 3 pairwise comparisons will be conducted at an alpha level of 0.0167 for the following power calculations. Based on the Russia ARCH study participants who had ≥5 HDD and were regular smokers, the mean number of HDD in the past month was 14.7 and the standard deviation of percentage of HDD was 27.2%. Given this assumption, the minimum detectable difference in percentage of HDD at 3 months that the study can detect with 80% power is 14.1 for any of the 3 comparisons of interest (i.e., varenicline vs. cytisine; varenicline vs. NRT; cytisine vs. NRT), (e.g., 50% vs. 64.1% for varenicline vs. cytisine, respectively) using a 2-sided t-test. The study, therefore, has sufficient power to test for clinically important differences between any of the comparisons.
2.16.3. Protection of study participants and their data
The St PETER study was approved by the Institutional Review Boards of Boston University Medical Campus and Pavlov University. All study participants will complete the informed consent process and provide written informed consent.
All study data are captured electronically on computers via a secure, web-based data capture system. Access to the system is protected via secure logins and all data transmissions are encrypted using secure socket layering (SSL). The project website and study data are located on a secure server within the Boston University Medical Center (BUMC) domain. All web-forms are protected using SSL encryption technology and files are protected by electronic firewalls that restrict access to designated users. Identifiers needed to track participants are kept separate from research data.
2.16.4. Data Safety Monitoring Board (DSMB)
To ensure the safety of the participants and the validity and integrity of the data, an external DSMB was established to assume oversight of the entire URBAN ARCH Consortium and any studies originating from the URBAN ARCH cohorts, including the St PETER HIV study. The Board meets every 6 months and is charged with evaluating the quality of trial administration, monitoring safety issues, and providing guidance on scientific, methodological, and ethical issues. Specifically, the Board reviews investigators' plans and processes for identifying individual or patterns of adverse events and reviews accumulating safety data.
3. Discussion
The St PETER HIV study establishes a cohort of HIV-positive smokers and heavy drinkers to compare the effects of two nicotinic acetylcholine receptor partial agonist medications, varenicline and cytisine, on alcohol consumption, alcohol craving, smoking, inflammation, CHD risk, and mortality risk. St PETER HIV further addresses the paucity of randomized controlled trial data to guide treatment of heavy alcohol consumption and smoking in PLWH. If the study hypotheses are correct, St PETER HIV could make nicotinic acetylcholine receptor partial agonists standard care for HIV-positive heavy drinking smokers, and lead to reduced inflammation, CHD and mortality risk through this “one drug, two diseases” approach.
Clinical trial registration details
This study was registered with ClinicalTrials.gov through the National Institutes of Health - Studying Partial agonists for Ethanol and Tobacco Elimination in Russians with HIV (St PETER HIV): NCT02797587.
Ethics approval and consent to participate
The St PETER study was approved by the Institutional Review Boards of Boston University Medical Campus, Vanderbilt University Medical Center, and Pavlov University.
Funding
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in support of URBAN ARCH: U01AA020780, U24AA020779, U24AA020778; and by the Providence/Boston Center for AIDS Research (P30AI042853) and Tennessee Center for AIDS Research (P30AI110527). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Hilary A. Tindle: Conceptualization, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition. Matthew S. Freiberg: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Natalia Gnatienko: Writing - original draft, Visualization, Project administration, Supervision. Elena Blokhina: Investigation, Resources, Writing - review & editing, Project administration, Supervision. Debbie M. Cheng: Methodology, Formal analysis, Writing - review & editing, Funding acquisition. Tatiana Yaroslavtseva: Investigation, Resources, Writing - review & editing, Project administration. Sally Bendiks: Writing - original draft, Visualization, Project administration. Michael Winter: Software, Resources, Data curation, Writing - review & editing. Evgeny Krupitsky: Conceptualization, Resources, Writing - review & editing, Supervision. Jeffrey H. Samet: Conceptualization, Writing - review & editing, Supervision, Funding acquisition, Supervision.
Declaration of competing interest
Dr. Tindle has provided scientific input into the design of a phase III trial for cytisine to be considered for approval by the United States Food and Drug Administration to aid in smoking cessation. In this capacity she has served as an unpaid consultant to Achieve Life Sciences. She is also the principal investigator of an NIH-supported study that has received donated varenicline medication from the manufacturer (Pfizer) for smokers with cancer. The remaining authors report no declarations of interest.
Acknowledgements
Dr. Tindle is supported in part by the William Anderson Spickard Jr., M.D. Chair in Medicine endowment from the Department of Medicine, Vanderbilt University School of Medicine. Dr. Freiberg is supported in part by the Dorothy and Laurence Grossman Chair in Cardiology endowment from the Division of Cardiology, Vanderbilt University School of Medicine. The authors acknowledge Stephen King for editorial assistance.
Contributor Information
Hilary A. Tindle, Email: hilary.tindle@vumc.org.
Matthew S. Freiberg, Email: matthew.s.freiberg@vumc.org.
Natalia Gnatienko, Email: natalia.gnatienko@bmc.org.
Elena Blokhina, Email: blokhinaelena@gmail.com.
Debbie M. Cheng, Email: dmcheng@bu.edu.
Tatiana Yaroslavtseva, Email: tatianayaroslavtseva@gmail.com.
Sally Bendiks, Email: sally.bendiks@bmc.org.
Michael Winter, Email: mwinter@bu.edu.
Evgeny Krupitsky, Email: kruenator@gmail.com.
Jeffrey H. Samet, Email: jsamet@bu.edu.
References
- 1.Freiberg M.S., Chang C.C., Kuller L.H. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant V.A. HIV infection and coronary heart disease: an intersection of epidemics. J. Infect. Dis. 2012;205(Suppl 3):S355–S361. doi: 10.1093/infdis/jis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller L., SMART Study Group . Paper Presented at: 15th Conference on Retroviruses and Opportunistic Infections. 2008. Elevated levels of interleukin-6 and D-dimer are associated with an increased risk of death in patients with HIV. [Google Scholar]
- 4.Kuller L.H., Tracy R., Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10) doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker J.V., Neuhaus J., Duprez D. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J. Acquir. Immune Defic. Syndr. 2011;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triant V.A., Meigs J.B., Grinspoon S.K. Association of C-reactive protein and HIV infection with acute myocardial infarction. J. Acquir. Immune Defic. Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niaura R., Shadel W.G., Morrow K., Tashima K., Flanigan T., Abrams D.B. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin. Infect. Dis. 2000;31(3):808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 8.Armah K.A., McGinnis K., Baker J. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin. Infect. Dis. 2012;55(1):126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg M.S., Bebu I., Tracy R. D-dimer levels before HIV seroconversion remain elevated even after viral suppression and are associated with an increased risk of non-AIDS events. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson B.A., Rosenthal N., Capece J.A. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch. Intern. Med. 2008;168(11):1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- 11.Cahill K., Lindson-Hawley N., Thomas K.H., Fanshawe T.R., Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2016;(5) doi: 10.1002/14651858.CD006103.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West R., Zatonski W., Cedzynska M. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 2011;365(13):1193–1200. doi: 10.1056/NEJMoa1102035. [DOI] [PubMed] [Google Scholar]
- 13.Walker N., Howe C., Glover M. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 2014;371(25):2353–2362. doi: 10.1056/NEJMoa1407764. [DOI] [PubMed] [Google Scholar]
- 14.Hajek P., McRobbie H., Myers K. Efficacy of cytisine in helping smokers quit: systematic review and meta-analysis. Thorax. 2013;68(11):1037–1042. doi: 10.1136/thoraxjnl-2012-203035. [DOI] [PubMed] [Google Scholar]
- 15.Leaviss J., Sullivan W., Ren S. A Systematic Review and Economic Evaluation. 2014. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? pp. 2046–4924. (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthenelli R.M., Benowitz N.L., West R. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 17.Walker N., Smith B., Barnes J. Cytisine versus varenicline for smoking cessation for Māori (the indigenous people of New Zealand) and their extended family: protocol for a randomized non-inferiority trial. Addiction. 2019;114(2):344–352. doi: 10.1111/add.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas D., Farrell M., McRobbie H. The effectiveness, safety and cost-effectiveness of cytisine versus varenicline for smoking cessation in an Australian population: a study protocol for a randomised controlled non-inferiority trial. Addiction. 2019;114(5):923–933. doi: 10.1111/add.14541. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell J.M., Teague C.H., Kayser A.S., Bartlett S.E., Fields H.L. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223(3):299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schacht J.P., Rf Fau Anton, Randall P.K., Pk Fau Randall, Li X., Fau Li X., Henderson S., Fau Henderson S., Myrick H., Myrick H. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology. 2014;231(18):3799–3807. doi: 10.1007/s00213-014-3518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurt R.T., Ebbert J.O., Croghan I.T., Schroeder D.R., Hurt R.D., Hays J.T. Varenicline for tobacco-dependence treatment in alcohol-dependent smokers: a randomized controlled trial. Drug Alcohol Depend. 2018;184:12–17. doi: 10.1016/j.drugalcdep.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litten R.Z., Ryan M.L., Fertig J.B. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J. Addiction Med. 2013;7(4):277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fucito L.M., Toll B.A., Wu R., Romano D.M., Tek E., O'Malley S.S. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215(4):655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee S.A., Harrison E.L., O'Malley S.S. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol. Psychiatr. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plebani J.G., Lynch K.G., Rennert L., Pettinati H.M., O'Brien C.P., Kampman K.M. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 2013;133(2):754–758. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Childs E., D J.R., King A.C., de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin. Exp. Res. 2012;36(5):906–914. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meszaros Z.S., Abdul-Malak Y., Dimmock J.A., Wang D., Ajagbe T.O., Batki S.L. Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo-controlled pilot trial. J. Clin. Psychopharmacol. 2013;33(2):243–247. doi: 10.1097/JCP.0b013e3182870551. [DOI] [PubMed] [Google Scholar]
- 28.de Bejczy A., Lof E., Walther L. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin. Exp. Res. 2015;39(11):2189–2199. doi: 10.1111/acer.12854. [DOI] [PubMed] [Google Scholar]
- 29.O’Malley S.S., Zweben A., Fucito L.M. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):129–138. doi: 10.1001/jamapsychiatry.2017.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verplaetse T.L., Pittman B.P., Shi J.M., Tetrault J.M., Coppola S., McKee S.A. Effect of varenicline combined with high-dose alcohol on craving, subjective intoxication, perceptual motor response, and executive cognitive function in adults with alcohol use disorders: preliminary findings. Alcohol Clin. Exp. Res. 2016;40(7):1567–1576. doi: 10.1111/acer.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verplaetse T.L., Pittman B.P., Shi J.M., Tetrault J.M., Coppola S., McKee S.A. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J. Addiction Med. 2016;10(3):166–173. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oon-Arom A., Likhitsathain S., Srisurapanont M. Efficacy and acceptability of varenicline for alcoholism: a systematic review and meta-analysis of randomized-controlled trials. Drug Alcohol Depend. 2019;205 doi: 10.1016/j.drugalcdep.2019.107631. [DOI] [PubMed] [Google Scholar]
- 33.Sajja R.K., Rahman S. Cytisine modulates chronic voluntary ethanol consumption and ethanol-induced striatal up-regulation of DeltaFosB in mice. Alcohol. 2013;47(4):299–307. doi: 10.1016/j.alcohol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Sajja R.K., Rahman S. Neuronal nicotinic receptor ligands modulate chronic nicotine-induced ethanol consumption in C57BL/6J mice. Pharmacol. Biochem. Behav. 2012;102(1):36–43. doi: 10.1016/j.pbb.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Bell R.L., BJn Eiler, Cook J.B., Rahman S. Nicotinic receptor ligands reduce ethanol intake by high alcohol-drinking HAD-2 rats. Alcohol. 2009;43(8) doi: 10.1016/j.alcohol.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tutka P., Vinnikov D., Courtney R.J., Benowitz N.L. Cytisine for nicotine addiction treatment: a review of pharmacology, therapeutics and an update of clinical trial evidence for smoking cessation. Addiction. 2019;114(11):1951–1969. doi: 10.1111/add.14721. [DOI] [PubMed] [Google Scholar]
- 37.Five Major Steps to Intervention (The "5 A's") Agency for Healthcare Research and Quality; 2012. http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html Updated December. Accessed March 20, 2019. [Google Scholar]
- 38.National Institutes on Alcohol Abuse and Alcoholism . National Institutes of Health; Rockville, MD: 2007. A Pocket Guide for Alcohol Screening and Brief Intervention: Updated 2005 Edition. [Google Scholar]
- 39.Herron A.J., Mariani J.J., Pavlicova M. Assessment of riboflavin as a tracer substance: comparison of a qualitative to a quantitative method of riboflavin measurement. Drug Alcohol Depend. 2013;128(1–2):77–82. doi: 10.1016/j.drugalcdep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes R.B. A critical review of the “determinants” of patient compliance with therapeutic regimens. In: Sackett D.L., Haynes R.B., editors. Compliance with Therapeutic Regimens. Johns Hopkins University Press; Baltimore, MD: 1976. pp. 26–39. [Google Scholar]
- 41.McLellan A.T., Luborsky L., Cacciola J. New data from the Addiction Severity Index. Reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 42.United States Department of Health and Human Services, National Center for Health Statistics . (ICPSR 2954); 1997. National Health Interview Survey. 2006. [Google Scholar]
- 43.Sobell L.C., Sobell M.B. Alcohol Research Foundation; Toronto, Canada: 1995. Alcohol Timeline Followback (TLFB) Users' Manual. [Google Scholar]
- 44.Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. 2001. Audit the Alcohol Use Disorders Identification Test (AUDIT): Guidelines for Use in Primary Care. [Google Scholar]
- 45.National Institute on Alcohol Abuse and Alcoholism . NIH Publication No; 2016. Alcohol Use Disorder: A Comparison between DSM–IV and DSM–5. 13-7999, Reviewed July. [Google Scholar]
- 46.Miller W.R., Tonigan J.S., Longabaugh R. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. The Drinker Inventory of Consequences (DrInC) [Google Scholar]
- 47.Alterman A.I., Cacciola J.S., Ivey M.A., Habing B., Lynch K.G. Reliability and validity of the alcohol short index of problems and a newly constructed drug short index of problems. J. Stud. Alcohol Drugs. 2009;70(2):304–307. doi: 10.15288/jsad.2009.70.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flannery B.A., Volpicelli J.R., Pettinati H.M. Psychometric properties of the penn alcohol craving scale. Alcohol Clin. Exp. Res. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- 49.LaBrie J.W., Quinlan T., Schiffman J.E., Earleywine M.E. Performance of alcohol and safer sex change rulers compared with readiness to change questionnaires. Psychol. Addict. Behav. 2005;19(1):112–115. doi: 10.1037/0893-164X.19.1.112. [DOI] [PubMed] [Google Scholar]
- 50.Sobell L., Sobell M., Buchan G. 1996. Timeline followback method (drugs, cigarettes, and marijuana) [Google Scholar]
- 51.Robinson S.M., Sobell L.C., Sobell M.B., Leo G.I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 52.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 53.Meneses-Gaya I.C., Zuardi A.W., Loureiro S.R., Crippa J.A. Psychometric properties of the fagerstrom test for nicotine dependence. J. Bras. Pneumol. 2009;35(1):73–82. doi: 10.1590/s1806-37132009000100011. [DOI] [PubMed] [Google Scholar]
- 54.Inter-University Consortium for Political and Social Research (ICPSR) United States Department of Health and Human Services. National Institute of Health. National Institute on Drug Abuse; 2016. Population Assessment of Tobacco and Health (PATH Study) [Google Scholar]
- 55.Tiffany S.T., Drobes D.J. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 56.Cox L.S., Tiffany S.T., Christen A.G. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 57.West R., Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology. 2010;208(3):427–432. doi: 10.1007/s00213-009-1742-x. [DOI] [PubMed] [Google Scholar]
- 58.Biener L., Abrams D.B. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 59.Navaline H.A., Snider E.C., Petro C.J. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res. Hum. Retrovir. 1994;10(Suppl 2):S281–S283. [PubMed] [Google Scholar]
- 60.Chishinga N., Kinyanda E., Weiss H.A., Patel V., Ayles H., Seedat S. Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia. BMC Psychiatr. 2011;11:75. doi: 10.1186/1471-244X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. [Google Scholar]
- 62.Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 63.Chesney M.A., Ickovics J.R., Chambers D.B. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 64.Adapted from the HIV Cost and Services Utilization Study (HCSUS) Implemented by the Agency for Health Care Policy Research (AHCPR) and RAND.
- 65.Kazis L.E., Miller D.R., Clark J. Health-related quality of life in patients served by the department of veterans affairs: results from the veterans health study. Arch. Intern. Med. 1998;158(6):626–632. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 66.Landmark T., Romundstad P., Dale O., Borchgrevink P.C., Kaasa S. Estimating the prevalence of chronic pain: validation of recall against longitudinal reporting (the HUNT pain study) Pain. 2012;153(7):1368–1373. doi: 10.1016/j.pain.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Merlin J.S., Walcott M.M., Herbey I. Qualitative investigation of a brief chronic pain screening tool in HIV-infected patients. AIDS Patient Care STDS. 2014;28(4):176–182. doi: 10.1089/apc.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Justice A.C., Holmes W., Gifford A.L. Development and validation of a self-completed HIV symptom index. J. Clin. Epidemiol. 2001;54(Suppl 1):S77–S90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 69.Ferris B.G. Epidemiology standardization project (American thoracic society) Am. Rev. Respir. Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 70.Helsing K.J., Comstock G.W., Speizer F.E. Comparison of three standardized questionnaires on respiratory symptoms. Am. Rev. Respir. Dis. 1979;120(6):1221–1231. doi: 10.1164/arrd.1979.120.6.1221. [DOI] [PubMed] [Google Scholar]
- 71.Stein M.D., Weinstock M.C., Herman D.S., Anderson B.J. Respiratory symptom relief related to reduction in cigarette use. J. Gen. Intern. Med. 2005;20(10):889–894. doi: 10.1111/j.1525-1497.2005.0190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.NIAID AIDS Clinical Trials Group ACTG A5322 fall history questionnaire. Review date January. 2014;31 [Google Scholar]
- 73.Miller W.R. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1996. Form 90: A Structured Assessment Interview for Drinking and Related Behaviors: Test Manual. [Google Scholar]
- 74.Kalyadina S.A., Ionova T.I., Ivanova M.O. Russian Brief Pain Inventory: validation and application in cancer pain. J. Pain Symptom Manag. 2008;35(1):95–102. doi: 10.1016/j.jpainsymman.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 75.Weatherby N.L., Needle R., Cesari H. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Eval. Progr. Plann. 1994;17(4):347–355. [Google Scholar]
- 76.Needle R., Fisher D.G., Weatherby N. Reliability of self-reported HIV risk behaviors of drug users. Psychol. Addict. Behav. 1995;9(4):242. [Google Scholar]
- 77.Britton P.C., Bohnert A.S., Wines J.D., Jr., Conner K.R. A procedure that differentiates unintentional from intentional overdose in opioid abusers. Addict. Behav. 2012;37(1):127–130. doi: 10.1016/j.addbeh.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antelman G., Smith Fawzi M.C., Kaaya S. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15(14):1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Broadhead W.E., Gehlbach S.H., de Gruy F.V., Kaplan B.H. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med. Care. 1988;26(7):709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Kazis L.E., Miller D.R., Clark J.A. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J. Ambul. Care Manag. 2004;27(3):263–280. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Wu A.W., Revicki D.A., Jacobson D., Malitz F.E. Evidence for reliability, validity and usefulness of the medical outcomes study HIV health survey (MOS-HIV) Qual. Life Res. 1997;6(6):481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 82.Atkinson M.J., Sinha A., Hass S.L. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual. Life Outcome. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benowitz N.L., Bernert J.T., Foulds J. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res. 2020;22(7):1086–1097. doi: 10.1093/ntr/ntz132. [DOI] [PMC free article] [PubMed] [Google Scholar]