Abstract
An increasing number of studies show that listeners often have difficulty hearing in situations with background noise, despite normal tuning curves in quiet. One potential source of this difficulty could be sensorineural changes in the auditory periphery (the ear). Signal in noise detection deficits also arise in animals raised with developmental conductive hearing loss (CHL), a manipulation that induces acoustic attenuation to model how sound deprivation changes the central auditory system. This model attributes perceptual deficits to central changes by assuming that CHL does not affect sensorineural elements in the periphery that could raise masked thresholds. However, because of efferent feedback, altering the auditory system could affect cochlear elements. Indeed, recent studies show that adult-onset CHL can cause cochlear synapse loss, potentially calling into question the assumption of an intact periphery in early-onset CHL. To resolve this issue, we tested the long-term peripheral effects of CHL via developmental bilateral malleus displacement. Using forward masking tuning curves, we compared peripheral tuning in animals raised with CHL vs age-matched controls. Using compound action potential measurements from the round window, we assessed inner hair cell synapse integrity. Results indicate that developmental CHL can cause minor synaptopathy. However, developmental CHL does not appreciably alter peripheral frequency tuning.
Subject terms: Sensory processing, Neuronal development, Cochlea, Cortex
Introduction
Detection and identification of speech and other sounds in noisy environments is challenging, particularly for hearing-impaired listeners1,2. For those with hearing loss, much of this difficulty, known as masking, is assumed to arise from sensorineural damage in the auditory periphery (the cochlea)3–5. This is because under sensorineural loss, the acoustic mixture of target and masker abnormally activates the regions of the cochlea encoding the target. Specifically, elevated masking thresholds are ascribed to broadened frequency filters, temporally smeared encoding, and abnormal intensity perception due to cochlear damage, particularly of the outer hair cells1,3,6–8. However, even when sensorineural loss is small or absent, perceptual difficulty with masking can occur following conductive hearing loss (CHL). For instance, due to middle ear infections (otitis media), 5 out of 6 children experience periods of CHL during development9, an exposure predicting later problems with speech processing10,11. Even after peripheral hearing is restored, children with a history of CHL have increased difficulty with speech in noise perception12–18. Thus, there is a timely need to understand the mechanisms by which CHL increases vulnerability to masking.
CHL is broadly used as a model to understand how auditory deprivation affects the central auditory system as it has been assumed to leave the peripheral auditory region intact19,20. In addition to attenuating sound energy reaching the cochlea by disrupting sound transmission in the outer or middle ear, CHL can cause changes in the central auditory pathway21–26. We have previously suggested that long-term experience with bilateral developmental CHL impairs resilience to masking due to central changes under auditory deprivation27,28. A premise for this interpretation was that this CHL should not appreciably alter sensorineural elements in the periphery that could raise masked thresholds. However, this assumption has not been directly tested and is called into question by recent evidence of sensorineural changes to hair cell synapses as a result of extended CHL. Specifically, when tympanic membranes were disrupted in adult animals, cochlear histology one year later revealed inner hair cell (IHC) synapse loss, at high frequency regions of the basilar membrane above 5 kHz29. In adults, even one month of reversible CHL induced by earplugs was sufficient to induce lasting synapse loss30. Synaptic loss is purported to decrease the ability of auditory nerve fibers to encode a masked target29, which could have contributed to increased vulnerability to masking in our prior work27,28. In addition, when induced during development rather than in adulthood, CHL may further increase the likelihood of changes to the periphery that could impact tuning, as the cochlea is not fully mature prior to hearing onset and thus more susceptible to plastic changes31–34. For example, peripheral tuning is determined largely by outer hair cells (OHCs), which are innervated by efferent fibers (medial olivocochlear, MOCs)35. The auditory deprivation induced by CHL will change efferent feedback onto these OHCs, potentially altering tuning. Although CHL induced in adulthood did not affect OHCs29, peripheral tuning has not been examined after extended developmental CHL.
To examine how chronic bilateral developmental CHL alters peripheral processing, we tested gerbils raised with CHL induced prior to hearing onset. Cochlear compound action potentials (CAPs) are sound-evoked responses representing the summed activity of the auditory nerve to suprathreshold sounds36,37 that correlate with synaptic loss38,39. To assess the damage to afferent synapses between IHCs and auditory nerve fibers, also referred to as cochlear synaptopathy, we measured CAP amplitudes. To determine the sharpness of the cochlear filters, which would be expected if outer hair cell function was diminished by the CHL, we used forward masking tuning curves measured at the round window as a functional assay40. We then compared the sharpness of the peripheral masking tuning curves to a corollary of synaptic loss, CAP amplitudes. Our results show limited evidence of IHC synapse damage and confirm our key assumption that cochlear tuning is not appreciably broadened under CHL. This supports our prior hypothesis that perceptual masking deficits resulting from CHL arise primarily from central auditory changes.
Methods
Animals
All procedures relating to the maintenance and use of animals were performed in accordance with the relevant guidelines and approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University under protocol number 17–04-077. Seventeen Mongolian gerbils (Meriones unguiculatus) from multiple litters were raised by breeding pairs and housed in a 12/12 light/dark cycle. The Control and CHL groups contained 8 and 9 animals respectively (Controls: 4 males, 4 females; CHLs: 2 males, 7 females). CHL was induced at postnatal day (P) 11, and peripheral tuning was measured in adulthood, at ages ranging from P82–P243 (see Table 1; mean 152 ± 21 days).
Table 1.
Dissection-based confirmation of malleus removal or dislocation. Condition of the malleus, incus, stapes and the malleus/incus joint were determined following CAP recordings. The incus and stapes were intact in all animals. No infection occurred in any animal.
| Animal | Age | CAP Threshold (dB SPL) | Malleus intact? | Joint of incus/malleus | Incus intact? | Stapes intact? | Infection? | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 k | 2 K | 4 K | 8 K | 16 K | |||||||
| M1501 | P151 | 60 | 65 | 60 | 65 | 60 |
Long arm removed Head dislocated |
Intact | + | + | − |
| F1502 | P152 | 60 | 70 | 55 | 60 | 55 | Head dislocated | Partially missing | + | + | − |
| F1528 | P139 | 60 | 55 | 60 | 70 | 55 |
Long arm removed Head dislocated |
Partially missing | + | + | − |
| F1627 | P82 | 60 | 65 | 45 | 60 | 50 | Head removed | Intact | + | + | − |
| F1629 | P82 | 70 | 70 | 50 | 65 | 55 | Head removed | Intact | + | + | − |
| M1626 | P83 | 65 | 60 | 45 | 60 | 50 | Head removed | Intact | + | + | − |
| F2150 | P196 | 70 | 70 | 70 | 75 | 60 |
Long arm removed Head dislocated |
Intact | + | + | − |
| F2074 | P242 | 50 | 45 | 40 | 50 | 45 |
Long arm broken Head partly removed |
Intact | + | + | − |
| F2073 | P243 | 50 | 55 | 35 | 60 | 65 |
Long arm broken Head intact |
Dislocated | + | + | − |
Malleus surgery
To induce CHL, bilateral malleus surgery was performed on gerbil pups at P11, as previously described23,27. This surgery resulted in either complete removal or dislocation of the malleus (see Table 1). Gerbil pups were anesthetized with Metofane (methoxyflurane; Medical Development International Ltd, Australia) until the pedal reflex disappeared. A pre-auricular skin incision was made to expose the ear canals, and pars flaccida of the tympanic membrane was visualized and torn. The malleus was removed or dislocated with forceps, and the incision was sealed with cyanoacrylate. The rest of the tympanic membrane (pars tensa) and the ossicles (incus and stapes) remained intact, as verified by later dissection (see Table 1). Control animals were anesthetized with Metofane, a sham incision was made and sealed with cyanoacrylate. Immediately following surgery, both Control and CHL pups received prophylactic injections of Baytril (enrofloxacin; Bayer, USA) to prevent infections (0.45 mg/ml, 5 mg/kg), and were returned to their parents. Pups were weaned at P30 into unisex groups of siblings. Malleus dislocation and intactness of stapes was verified bilaterally via dissection in adulthood, after CAPs were recorded (see Table 1). Animals that developed middle ear infections were excluded from this study (verified upon dissection).
CAP recordings
CAPs were recorded from the round window in Control and CHL animals in adulthood (> P80). Gerbils were anesthetized with pentobarbital (60 mg/kg, IP), and given supplementary injections (50 mg/kg, IM) hourly to maintain sedation. A single dose of atropine (1 mg/kg, IM) was injected to avoid airway blockage. A 10 mm right ear post auricular incision41 was made after a local injection of lidocaine. The neck muscles were bluntly separated and retracted to expose the middle ear space (bulla). The bulla was opened with forceps and the round window niche was exposed. A silver Teflon-insulated ball electrode was positioned at the round window niche, a stainless steel reference needle electrode was placed subcutaneously at the vertex of the head, and a ground electrode was placed in the right leg muscle. Gerbils were positioned on a homeothermic blanket (Harvard Apparatus, MA, USA) and temperature was maintained at 37 °C. CAP recordings were used to determine thresholds at multiple frequencies, assess likely IHC loss and/or synaptopathy, and measure frequency tuning curves, as described below.
Stimuli
Stimuli were presented in free field, from a calibrated MF1 multi-field magnetic speaker (Tucker-Davis Technologies (TDT), Alachua FL) positioned 3 cm from the right ear canal. To calibrate the speaker output, a ¼-inch free-field microphone (Brüel and Kjær (B&K) 4939, Denmark) was positioned 3 cm from the center of the MF1 speaker, facing the speaker. The microphone output was amplified (B&K 2690-A Nexus conditioning amplifier) and digitized at a sampling frequency of 195.3 kHz (TDT RZ6 auditory signal processor). A 1–30 kHz frequency sweep was presented and recorded using BioSig RZ software (v5.7.2, TDT) to create a calibration file. These calibration files were applied to spectrally flatten the speaker output, ensuring similar presentation levels at all tested frequencies, across a range of output levels from 0 to 115 dB SPL. Calibrated output levels were verified with custom-written Matlab scripts (Mathworks, Natick MA; scripts by S.J. Shanbhag).
Probe and masker stimuli were created using SigGen software (v5.7.2, TDT); a schematic is shown in Fig. 1. Probe tones were 10 ms in duration (2 ms rise/fall cosine-squared ramps) with frequencies at 1, 2, 4, 8, and 16 kHz; levels varied from 5 to 105 dB SPL. Masking tones were 70 ms in duration (1 ms rise/fall) presented at varied frequencies and levels. Stimuli were presented at a rate of 8.33/second. All stimuli were presented with BioSigRZ software with alternating polarity to minimize the presence of cochlear microphonics. Traces were averaged across 510 repetitions. Voltage responses were amplified (20 × gain, RA16LI preamplifier, TDT), bandpass filtered (300 Hz to 3 kHz), digitized at 24.4 kHz and 24 bits (RZ6, TDT) and recorded using BioSigRZ on a Dell PC running Microsoft Windows 7.
Figure 1.
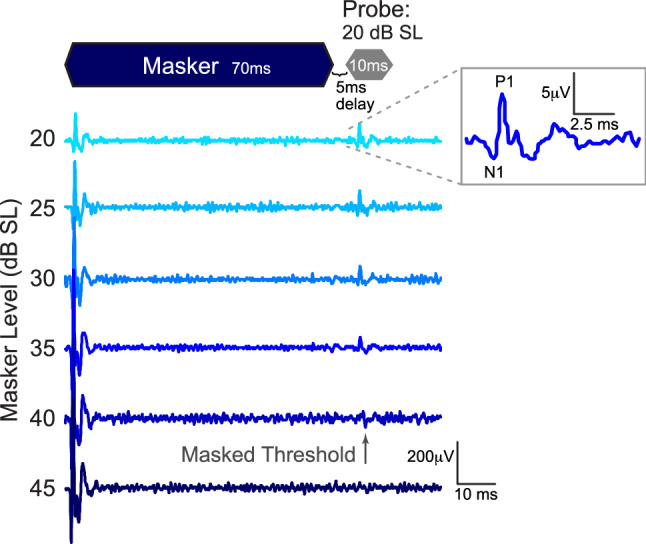
Example of forward masking response used to obtain tuning curves measured at the round window. A two-tone suppression paradigm measured the response to a 10 ms probe tone presented at 20 dB SL. The probe tone was presented 5 ms after the offset of a 70 ms masker tone of a specific frequency (± 1/10 octave steps surrounding the probe tone frequency; here, probe and masker were 4 and 4.8 kHz, respectively). The masker level increased in 5 dB steps; masking threshold was determined as the last visible response to the probe tone. Inset shows the masked response and identifies N1 and P1 peaks.
To determine thresholds and indirectly assess cochlear synaptopathy, we measured the amplitude of wave 1, also known as the N1P1 response, which closely predicts synaptopathy38,39. To determine thresholds, we recorded responses to each of the probe tone frequencies without the masker present, with probe tones beginning at loud sound levels (90 and 105 dB SPL for Control and CHL animals, respectively) and decreasing in 5 dB steps until the N1P1 response was no longer visible, as determined by an experienced observer. This point was defined as probe tone threshold for each animal (0 dB SL; Fig. 1). This normalization accounted for a ~ 37 dB shift in probe tone thresholds in CHL animals. We measured the peak-to-peak amplitude of the N1P1 responses to generate CAP input–output functions (measuring response amplitudes to tones varying in level). For 5 animals (n = 2 Control and 3 CHL), 10 dB steps were used, so these animals did not contribute to the averaged functions. Functions were collected at each probe frequency except 1 kHz, where cochlear microphonics prevented clear measurement of peak-to-peak amplitudes. To compare across groups, levels included in the input–output function ranged from each animal’s 0 dB SL threshold to a maximum of 35 dB SL, as the system could not present sounds louder than 35 dB SL for most CHL animals. All animals were tested at least up to 15 dB SL at all frequencies, and most animals were tested up to 35 dB SL at most frequencies.
To determine whether here, CHL compromised masked cochlear frequency tuning in adulthood, we employed a two-tone suppression forward masking paradigm. The probe tone was always presented at 20 dB SL, and 5 ms following masking tone offset. The masking tone frequency was adjusted in ± 1/10 octave steps from the probe tone frequency, and the level was varied until the N1P1 response to the probe tone disappeared42,43. The masker level required to eliminate the probe tone response was considered the masked threshold. Masked thresholds were used to create frequency tuning curves.
Tuning curves
To compare overall shapes of the tuning curves between Control and CHL groups, a linear mixed-effects regression model fitted probe tone thresholds across all masker and probe frequencies. Probe frequency was nested in masker frequency. To adjust for possible sampling variation owing to idiosyncratic effects of individual animals, probe threshold was included as a random effect, in addition to being a covariate. Correlated residual errors accounted for repeated measures within each animal.
Separately, in order to estimate the sharpness of cochlear tuning in a way that allows direct comparison with the human psychophysical literature, raw masked threshold data W were fitted using a traditional data fitting approach with a rounded exponential (roex) filter shape44:
The term p is an exponential parameter that defines the passband of the filter shape. The term r defines the shallow tail portion of the filter outside of the passband. The term g is the normalized distance from the center frequency fC of the filter: g =|f − fC|/fC. Using a nonlinear least square solver, r and p were estimated from the measured W as a function of g (lsqcurvefit command in Matlab). To quantify the sharpness of the masked tuning curves, at each center probe frequency, Q factors were calculated at the 10 dB down points of the fitted roex functions (e.g., Q10 = fc/(fhi(10) − flow(10))).
Statistics
IBM SPSS Statistics 22 (IBM, USA) was used for statistical analysis. CAP thresholds between groups were compared with a two-way repeated-measures mixed analysis of variance (rANOVA), testing for between-subject effects of treatment and within-subject effects of probe frequency. Q values comparing the sharpness of tuning curves were analyzed with a mixed rANOVA. CAP input–output functions were analyzed with a generalized linear mixed regression model, with treatment as a between-subject effect and probe frequency as a within-subject repeated-measures effect. This analysis is robust to missing data, although reduced power due to missing data may increase the chance of a type II error. All posthoc tests were adjusted for multiple comparisons using Sidak corrections.
Experimental design
In summary, CAPs assessed the frequency selectivity of the cochlear response. To measure the threshold shift created by bilateral malleus removal prior to hearing onset, we first recorded CAPs in adulthood at multiple probe tone frequencies. The amplitudes of these responses were used to assess possible synaptopathy, and to determine probe tone thresholds at each frequency. Then, to resolve whether developmental CHL alters peripheral frequency selectivity, we acquired tuning curves using a forward masking paradigm. We fit these curves with an auditory filter model for tonal maskers (a roex function) and used the Q10 factor to quantify and compare the sharpness of tuning. To confirm surgical accuracy and to exclude animals with middle ear infections, a postmortem middle ear dissection was performed in adulthood after measuring CAP thresholds and tuning curves. Malleus absence or dislocation was identified bilaterally, and the intactness of incus and stapes was confirmed. Postmortem dissections were also performed on control animals, to exclude any animals with pathology in the ear canal or middle ear (see Table 1 for details).
Results
Threshold shift introduced by CHL
To quantify the CHL introduced by malleus surgery at P11, in adulthood (> P80), CAP thresholds were measured in both Control (n = 8) and CHL animals (n = 9). Thresholds at probe frequencies of 1, 2, 4, 8 and 16 kHz were determined as the lowest sound level that yielded a visible N1P1 response, and are plotted as a function of probe frequency (Fig. 2, open symbols). A two-way ANOVA between Controls and CHLs confirmed that the malleus surgery raised thresholds by 30–40 dB at all tested frequencies (main effect of treatment: mean threshold shift across frequencies = 36.77 ± 5.71 dB; F(1,15) = 188.06, p < 0.0001; Partial ɳ2 = 0.93). Threshold shifts were significant at all individual tested frequencies (Table 2).
Figure 2.
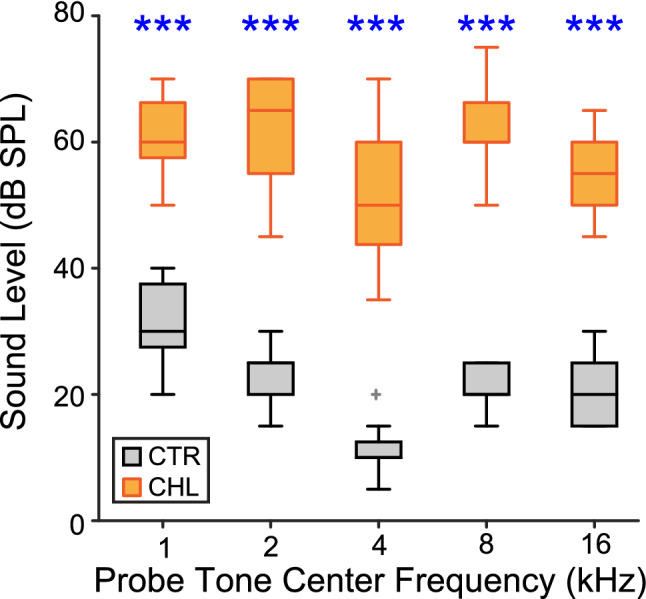
Early malleus displacement induces a hearing loss of ~ 37 dB across a frequency range of 1–16 kHz. CAP thresholds are plotted for each probe frequency, for Controls (CTR) and CHLs (black and orange, respectively). Boxplot centers are medians; edges are 25th and 75th percentiles; whiskers extend to the most extreme data points excluding outliers. ***p < 0.0001.
Table 2.
Threshold differences for CHL vs CTR (ANOVA).
| Freq (Hz) | Mean diff (dB) | F(1,15) | p | Partial ɳ2 |
|---|---|---|---|---|
| 1000 | 29.3 | 71.816 | 0.000* | .827 |
| 2000 | 38.5 | 126.354 | 0.000* | .894 |
| 4000 | 39.9 | 89.616 | 0.000* | .857 |
| 8000 | 41.5 | 222.206 | 0.000* | .937 |
| 16,000 | 34.4 | 143.845 | 0.000* | .906 |
Significance indicated by asterisks.
Activity-based assessment of synaptopathy
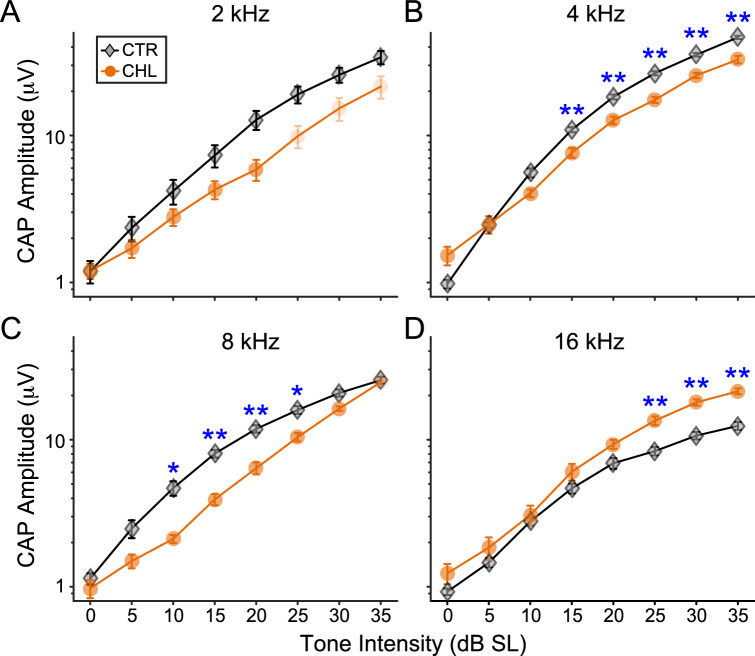
Figure 3 plots wave I peak-to-peak amplitude as a function of SL. CHL affected CAP suprathreshold amplitudes, but did so differently across frequencies (two-way ANOVA between Controls and CHLs, main effect of treatment: F(1,35) = 29.19, p < 0.0001; interaction between treatment and probe frequency: F(1,11) = 74.73, p < 0.0001). Pairwise comparisons revealed significant differences between the groups at higher signal levels for 4, 8 and 16 kHz (Fig. 3B–D, blue asterisks). At 4 and 8 kHz, CHL wave 1 amplitudes were smaller than those of Controls, consistent with cochlear synaptopathy. The same may hold true at 2 kHz, where a lack of significance was potentially due to speaker output limitations which prevented us from collecting more data points at the higher levels (data points where not all animals contributed are depicted by pale orange symbols). Similarly, at 8 kHz, only three data points were available at the loudest level tested. At 16 kHz, an unexpected reversal was seen, where CHL wave 1 amplitudes were larger than those of Controls at higher sound levels; no data points were excluded at this frequency.
Figure 3.
Frequency–response functions at suprathreshold sound levels show evidence for cochlear synaptopathy at some frequencies. (A–D) Response functions in response to 2, 4, 8 or 16 kHz tones, normalized to threshold for each animal (0 dB SL). Significantly higher or lower responses are indicated by blue asterisks (** p < 0.008, * p < 0.02). Pale symbols indicate levels with low n’s. Error bars are SEM.
Forward masking and tuning curves
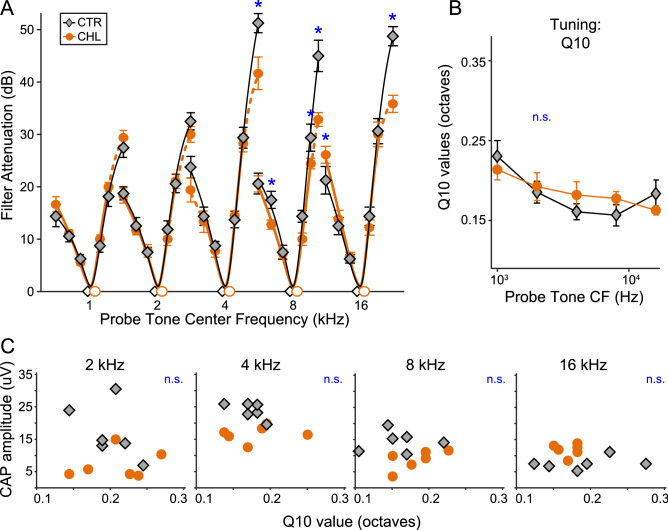
To determine whether early-induced CHL compromised cochlear frequency tuning in adulthood, we employed a two-tone suppression forward masking paradigm, measuring masked tuning curves. Boxplots of unnormalized raw values used to create tuning curves are depicted in Fig. 2, and roex-fitted averaged tuning curves, normalized to the center probe tone frequency, are depicted in Fig. 4A. Mean masked tone levels (closed symbols) are plotted as a function of their frequencies surrounding each probe tone center frequency (open symbols). The curves in Fig. 4A are derived from roex filter fits, allowing comparison of our fits to an established literature44,45. This fit assumes that the auditory filter is well fit by a rounded exponential function – i.e., a pair of back-to-back exponentials with a rounded tip, and shallow skirts in the frequency region beyond ∆f/fC = 0.4.
Figure 4.
Frequency tuning is not altered by early malleus displacement. (A) Forward masked tuning curves were obtained with the probe at 20 dB above threshold, and are depicted normalized to sensation level to allow comparison across treatment groups. Normalized tuning curves were fit using a roex filter function. Masked thresholds are plotted for 1/10 octave frequency steps of maskers surrounding each center probe frequency at 1, 2, 4, 8 and 16 kHz. (B) Q10 values derived from roex filter functions indicate that conductive hearing loss did not significantly broaden tuning at 10 dB above threshold. (C) There are no significant correlations between CAP amplitude and Q10 values for either group at any probe frequency, indicating that synaptopathy does not correlate with cochlear tuning. Error bars are SEM.
A generalized linear mixed regression on the raw data (normalized to 0 dB SL for each individual) tested the overall shape of tuning, and found no significant effect of treatment group (F(1,12.58) = 0.56, p = 0.47). However, there was a significant effect of masking frequency with nested probe frequency, indicating that tuning curve shapes were not identical across probe frequencies (F(34,495.4) = 138.2, p < 0.0001). Furthermore, there was a significant interaction between treatment group and masking frequency with nested probe frequency, indicating that the curve shapes were altered by CHL treatment (F(34,495.4) = 3.3, p < 0.0001). However, posthoc analyses identified that these differences were confined to the flanks of the tuning curves for the center frequencies 4, 8, and 16 kHz (Fig. 4A, asterisks; Table 3).
Table 3.
Forward masking tuning curve statistics (generalized linear mixed regression).
| Probe freq (Hz) | Masking freq (Hz) | Mean diff (dB) | Std error | Df | F | p |
|---|---|---|---|---|---|---|
| 1000 | 700 | 2.8 | 2.193 | 206.45 | 1.573 | 0.211 |
| 800 | 1.6 | 2.193 | 206.45 | 0.235 | 0.628 | |
| 900 | 0.2 | 2.193 | 206.45 | 0.008 | 0.931 | |
| 1000 | 1.3 | 2.193 | 206.45 | 0.353 | 0.553 | |
| 1100 | 2.4 | 2.193 | 206.45 | 1.176 | 0.280 | |
| 1200 | 2.8 | 2.193 | 206.45 | 1.624 | 0.204 | |
| 1400 | 2.0 | 2.246 | 218.90 | 0.777 | 0.379 | |
| 2000 | 1400 | 0.9 | 2.172 | 230.95 | 0.174 | 0.677 |
| 1600 | 0.1 | 2.172 | 230.95 | 0.001 | 0.972 | |
| 1800 | 0.9 | 2.172 | 230.95 | 0.168 | 0.682 | |
| 2000 | 1.0 | 2.172 | 230.95 | 0.205 | 0.651 | |
| 2200 | 1.4 | 2.172 | 230.95 | 0.440 | 0.508 | |
| 2400 | 1.0 | 2.172 | 230.95 | 0.233 | 0.630 | |
| 2800 | 2.0 | 2.409 | 290.99 | 0.666 | 0.415 | |
| 4000 | 2800 | 0.1 | 2.102 | 351.82 | 2.331 | 0.128 |
| 3200 | 0.2 | 2.102 | 351.82 | 0.008 | 0.930 | |
| 3600 | 0.0 | 2.102 | 351.82 | 0.019 | 0.890 | |
| 4000 | 0.9 | 2.102 | 351.82 | 0.178 | 0.673 | |
| 4400 | 0.6 | 2.102 | 351.82 | 0.087 | 0.768 | |
| 4800 | 0.6 | 2.102 | 351.82 | 0.072 | 0.788 | |
| 5600 | 8.5 | 2.329 | 428.90 | 13.289 | 0.000* | |
| 8000 | 5600 | 1.3 | 2.175 | 227.00 | 0.381 | 0.538 |
| 6400 | 4.4 | 2.175 | 227.00 | 4.006 | 0.047* | |
| 7200 | 0.6 | 2.175 | 227.00 | 0.083 | 0.774 | |
| 8000 | 1.0 | 2.175 | 227.00 | 0.215 | 0.643 | |
| 8800 | 3.4 | 2.175 | 227.00 | 2.453 | 0.119 | |
| 9600 | 5.0 | 2.175 | 227.00 | 5.204 | 0.023* | |
| 10,400 | 12.2 | 2.283 | 259.62 | 28.409 | 0.000* | |
| 16,000 | 11,200 | − 5.4 | 2.139 | 281.91 | 6.472 | 0.011* |
| 12,800 | − 1.8 | 2.139 | 281.91 | 0.677 | 0.411 | |
| 14,400 | − 1.5 | 2.139 | 281.91 | 0.490 | 0.484 | |
| 16,000 | − 1.0 | 2.139 | 281.91 | 0.221 | 0.638 | |
| 17,600 | 0.9 | 2.139 | 281.91 | 0.181 | 0.671 | |
| 19,200 | 0.4 | 2.139 | 281.91 | 0.032 | 0.859 | |
| 22,400 | 12.4 | 2.362 | 359.58 | 27.695 | 0.000* |
Significance indicated by asterisks.
Q values and correlations
In addition to comparing the shapes of tuning curves between groups, we calculated the width of individual tuning curves using Q values at 10 dB above the peak of each roex-fitted curve for each subject (mean ± SEM of Q10 depicted in Fig. 4B for the two groups). This Q value, a standard measure of neural and perceptual frequency selectivity, indicates the width of tuning curves at 10 dB above threshold. A mixed ANOVA found no treatment effect, indicating that developmental CHL did not appreciably change functional frequency selectivity of the auditory nerve when measured in adulthood (Q10: F(1,15) = 0.045, p = 0.824).
Finally, to test whether putative synaptopathy is related to cochlear filter width in either group, we correlated suprathreshold CAP amplitudes (means for each animal from 10 to 35 dB SL) with Q10 filter width, across individual animals. There were no significant correlations at any probe frequency for either group (Fig. 4C; Spearman’s rank correlations, p > 0.12 for all comparisons).
Discussion
Many studies show how reduced peripheral frequency resolution impairs the ability to identify target sound, both in quiet and background noise46–48. A hallmark of sensorineural hearing loss, widened peripheral frequency tuning, is considered a key reason for why individuals with sensorineural damage often struggle to understand speech in noisy backgrounds49. In contrast, CHL has been assumed to leave peripheral frequency tuning intact, based on assessments of both bottom-up input to the auditory nerve measured via bone conduction thresholds and hair cell counts19,20. Despite this, converging evidence shows that CHL (occurring either during development or adulthood) impairs speech comprehension in noise12–17. Moreover, using a common animal model that mimics sound deprivation in children with otitis media (chronic bilateral CHL, induced prior to hearing onset), our own work shows worsened behavioral tone detection thresholds in both stationary and temporally fluctuating background noise27,28. The mechanisms by which CHL increases vulnerability to masking are incompletely understood. We here sought to test the hypothesis that this type of developmental CHL widens peripheral frequency tuning.
The rationale for this study was that the current CHL model may induce synaptopathy and/or broaden tuning, potentially contributing to behavioral deficits that we had previously attributed to changes in the central auditory system. For instance, recent data from aged mice shows loss of IHC synapses following a year of adult-induced CHL29, and even following 4 weeks of adult CHL induced by earplugs30. This was surprising, because cochlear synaptopathy has been reliably demonstrated in response to excessive sound from noise exposure, not to reduced sound resulting from CHL38. Such IHC synaptopathy typically is confined to high threshold fibers which are presumed to support signal in noise processing, as they are resilient to masking by continuous noise50–52. Specifically, as background noise increases, these high-threshold fibers are not saturated, so can carry information about relevant sounds51. Yet across studies there is inconsistent evidence supporting this idea39,53, and a model of > 50% IHC synapse loss does not affect signal in noise processing54. Furthermore, peripheral tuning is determined largely by outer hair cells, which are innervated by efferent medial olivocochlear fibers (MOCs)35. Efferent activity is reduced during the auditory deprivation induced by CHL, which could affect peripheral tuning. We here induced developmental CHL at hearing onset via malleus dislocation or removal, raising pure tone thresholds by 30–40 dB. Here, reduced CAP N1P1 amplitudes are consistent with mild IHC synaptopathy at 4 and 8 kHz, for sounds above 10 dB SL. However, at 2 kHz, as well as below 15 dB SL, the effect of CHL was too small to reach statistical significance, suggesting that at the lowest tested frequency, CHL does not meaningfully increase synaptopathy. Further at 16 kHz, CAP amplitudes were actually increased by developmental CHL. In addition, the spectral sharpness of peripheral tuning, as assessed by Q10, was not affected by CHL at any frequency. The CAP N1P1 amplitudes did not predict filter width at any of the tested frequencies, consistent with a dissociation between functional tuning of the cochlea and synaptopathy. These results are consistent with a milder and transient form of developmental CHL (earplugs inducing ~ 25 dB attenuation), which does not affect peripheral masked tuning curves yet affects perceptual temporal detection tasks55,56. Thus, the current data help rule out peripheral confounds to an extensive literature that uses bilateral CHL to study central perceptual effects of auditory deprivation24,26–28,55,57,58.
Although CHL did not change the overall shape or sharpness of tuning curves, CHL attenuated the flanks at higher center frequencies (4 – 16 kHz) by 5–10 dB (Fig. 4A, where orange symbols fall below gray symbols only at the edges of the tuning curves). This could be due to ceiling effects, as the maximum output of our system was 115 dB SPL, corresponding to less than 25 dB SL in some of our CHL animals. An acoustically louder stimulus is needed to robustly measure tuning at the flanks in CHL animals, which have a threshold shift of ~ 40 dB. Another possibility is that CHL during development may induce changes in frequency tuning as the cochlea matures, producing an effect due to abnormal development rather than synaptopathy. Prior to maturation, both central and peripheral auditory elements are affected by auditory experience59–61. Frequency tuning of the rodent cochlea is still immature at hearing onset, the age when we removed the malleus31,32,34,62,63. This immaturity suggests that developmental auditory deprivation could induce plasticity in frequency tuning.
Our data indicate mild IHC synaptopathy at 4 and 8 kHz (Fig. 3). To reconcile the current results with the seemingly contrasting finding of clear synaptopathy from the prior work29, two major differences are worth noting across the studies. First, the studies use different species. Unlike mice, gerbils have low frequency hearing, with equivalent low-frequency sensitivity to 250 Hz in gerbils compared with ~ 1000 Hz in mice64,65. The previous work tested tones across the range of the most sensitive frequencies in mouse65 (~ 5 to 70 kHz) and found synaptopathy only at 8 kHz and higher29. Here we similarly tested frequencies across the most sensitive range in the gerbil (from 1 to 16 kHz)64, finding modest evidence for synaptopathy at only 4 and 8 kHz. The current data does not address whether CHL may affect tuning or synaptopathy at higher frequencies in gerbils. However, based on mice we would have expected to see an effect across most of the hearing range excluding only the lowest frequencies. Second the previous work tested aged animals, showing that the loss of acoustic drive reduced efferent innervation, a phenomenon thought to exacerbate age-related IHC synaptic loss29. With an age range of 2.8 to 8 months, our animals were too young to show age-related hearing loss. In summary, the lack of strong evidence suggesting synaptopathy in our model of developmental CHL as compared to the mouse model with adult-onset CHL may arise from a difference in tested frequencies outside the midrange as compared to the prior work, and/or synaptopathy may be less likely to occur for younger animals, or there could be species differences. It is worth noting that in chinchillas, > 50% loss of the number of IHCs broadened perceptual masked tuning despite leaving tuning in quiet intact66. However, CHL in adulthood did not reduce IHC number despite causing IHC synaptopathy29. Further studies are needed to assess the effect of extended developmental CHL on IHC number.
Implications and summary
The current results extend prior work suggesting that central auditory deficits can lead to behavioral hearing deficits, including increased vulnerability to masking, reduced sensitivity to amplitude and frequency modulations, and impaired gap detection24,26–28,56,57,67. Long-term CHL weakens inhibitory responses and alters cellular properties throughout the central auditory system68,69. In animals with transient developmental CHL induced by earplugs from P11–24, peripheral tuning recovers two weeks after removing the earplugs55. However, animals with permanent developmental malleus removal induced at P11 (as in the current study) display raised peripheral thresholds, amplitudes, and response latencies, as assessed via auditory brainstem responses24,26. Importantly, these peripheral effects of CHL do not predict degraded behavioral performance on several perceptual tasks in adulthood, including signal-in-noise detection24,26,27. Moreover, in a behavioral assay on a mechanism of masking called modulation masking release, CHL mostly reduced masking mechanisms thought to rely on central rather than peripheral processing28. In conjunction, these data indicate that behavioral deficits following CHL arise primarily from central rather than peripheral changes.
In summary, this study addressed whether altered peripheral function introduced a possible confound in prior work suggesting that central changes can degrade behavioral resilience to masking24,26,27. The current data show no evidence of appreciable developmental CHL-induced cochlear dysfunction.
Acknowledgements
This work was supported by NIDCD R01 DC013314 to M.J.R and NIDCD R01 DC019126 to A.I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Amanda Lauer for comments on an earlier version of the manuscript.
Author contributions
M.J.R and A.I. designed the research concepts; M.J.R. designed the experiments; Y.Y. collected the data; Y.Y., A.I., and M.J.R. analyzed the data; M.J.R., A.I. and Y.Y. wrote the manuscript.
Data availability
The data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kidd G, Jr, Arbogast TL, Mason CR, Walsh M. Informational masking in listeners with sensorineural hearing loss. J. Assoc. Res. Otolaryngol. 2002;3:107–119. doi: 10.1007/s101620010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed CM, Braida LD, Zurek PM. Review article: review of the literature on temporal resolution in listeners with cochlear hearing impairment: A critical assessment of the role of suprathreshold deficits. Trends Amplif. 2009;13:4–43. doi: 10.1177/1084713808325412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasberg BR, Moore BC, Bacon SP. Gap detection and masking in hearing-impaired and normal-hearing subjects. J. Acoust. Soc. Am. 1987;81:1546–1556. doi: 10.1121/1.394507. [DOI] [PubMed] [Google Scholar]
- 4.Souza PE, Turner CW. Masking of speech in young and elderly listeners with hearing loss. J. Speech Hear Res. 1994;37:655–661. doi: 10.1044/jshr.3703.655. [DOI] [PubMed] [Google Scholar]
- 5.Desloge JG, Reed CM, Braida LD, Perez ZD, Delhorne LA. Speech reception by listeners with real and simulated hearing impairment: Effects of continuous and interrupted noise. J. Acoust. Soc. Am. 2010;128:342–359. doi: 10.1121/1.3436522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florentine M. Effects of cochlear impairment and equivalent-threshold masking on psychoacoustic tuning curves. Audiology. 1992;31:241–253. doi: 10.3109/00206099209072913. [DOI] [PubMed] [Google Scholar]
- 7.Moore BC, Glasberg BR. Comparisons of frequency selectivity in simultaneous and forward masking for subjects with unilateral cochlear impairments. J. Acoust. Soc. Am. 1986;80:93–107. doi: 10.1121/1.394087. [DOI] [PubMed] [Google Scholar]
- 8.Oxenham AJ, Bacon SP. Cochlear compression: Perceptual measures and implications for normal and impaired hearing. Ear Hear. 2003;24:352–366. doi: 10.1097/01.AUD.0000090470.73934.7800003446-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study. J. Infect. Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Deggouj N, et al. Functional consequences of chronic ENT inflammation on the development of hearing and communicative abilities. B-ENT. 2012;8(Suppl 19):105–115. [PubMed] [Google Scholar]
- 11.Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: A comparison of basic and clinical studies. J. Assoc. Res. Otolaryngol. 2011;12:535–547. doi: 10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravel JS, Wallace IF. Listening and language at 4 years of age: Effects of early otitis media. J. Speech Hear Res. 1992;35:588–595. doi: 10.1044/jshr.3503.588. [DOI] [PubMed] [Google Scholar]
- 13.Schilder AG, Snik AF, Straatman H, van den Broek P. The effect of otitis media with effusion at preschool age on some aspects of auditory perception at school age. Ear Hear. 1994;15:224–231. doi: 10.1097/00003446-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hall JW, et al. The effect of otitis media with effusion on perceptual masking. Arch. Otolaryngol. Head Neck Surg. 2003;129:1056–1062. doi: 10.1001/archotol.129.10.1056129/10/1056. [DOI] [PubMed] [Google Scholar]
- 15.Eapen RJ, et al. The development of frequency weighting for speech in children with a history of otitis media with effusion. Ear Hear. 2008;29:718–724. doi: 10.1097/AUD.0b013e31817a98cb00003446-200810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh DL, Lin KN, Ho JH, Liu TC. Hearing in noise test in subjects with conductive hearing loss. J. Formos. Med. Assoc. 2009;108:937–942. doi: 10.1016/S0929-6646(10)60006-X. [DOI] [PubMed] [Google Scholar]
- 17.Keogh T, Kei J, Driscoll C, Khan A. Children with minimal conductive hearing impairment: Speech comprehension in noise. Audiol. Neurootol. 2010;15:27–35. doi: 10.1159/000218360. [DOI] [PubMed] [Google Scholar]
- 18.Hall JW, Grose JH. Effect of otitis media with effusion on comodulation masking release in children. J. Speech Hear. Res. 1994;37:1441–1449. doi: 10.1044/jshr.3706.1441. [DOI] [PubMed] [Google Scholar]
- 19.Tucci DL, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: effects of conductive and sensorineural hearing loss on N. magnocellularis. J. Comp. Neurol. 1985;238:371–381. doi: 10.1002/cne.902380402. [DOI] [PubMed] [Google Scholar]
- 20.Chhan D, McKinnon ML, Rosowski JJ. Identification of induced and naturally occurring conductive hearing loss in mice using bone conduction. Hear Res. 2017;346:45–54. doi: 10.1016/j.heares.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuermer IW, Scheich H. Early unilateral auditory deprivation increases 2-deoxyglucose uptake in contralateral auditory cortex of juvenile Mongolian gerbils. Hear Res. 2000;146:185–199. doi: 10.1016/s0378-5955(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 22.Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res. 2001;155:124–132. doi: 10.1016/S0378-5955(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J. Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen MJ, Sarro EC, Kelly JB, Sanes DH. Diminished behavioral and neural sensitivity to sound modulation is associated with moderate developmental hearing loss. PLoS ONE. 2012;7:e41514. doi: 10.1371/journal.pone.0041514PONE-D-11-08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J. Neurophysiol. 2012;107:937–947. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao JD, Sanes DH. Developmental deprivation-induced perceptual and cortical processing deficits in awake-behaving animals. Elife. 2018 doi: 10.7554/eLife.33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay JD, Voytenko SV, Galazyuk AV, Rosen MJ. Developmental hearing loss impairs signal detection in noise: Putative central mechanisms. Front. Syst. Neurosci. 2014;8:162. doi: 10.3389/fnsys.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihlefeld A, Chen YW, Sanes DH. Developmental conductive hearing loss reduces modulation masking release. Trends Hear. 2016;20:2331216516676255. doi: 10.1177/2331216516676255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberman MC, Liberman LD, Maison SF. Chronic conductive hearing loss leads to cochlear degeneration. PLoS ONE. 2015;10:e0142341. doi: 10.1371/journal.pone.0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurioka T, Mogi S, Tanaka M, Yamashita T. Activity-dependent neurodegeneration and neuroplasticity of auditory neurons following conductive hearing loss in adult mice. Cell Mol. Neurobiol. 2020 doi: 10.1007/s10571-020-00829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubsamen R, Lippe W. In: Springer Handbook of Auditory Research: Development of the mammalian auditory system. Popper AN, Rubel EW, Fay RR, editors. Berlin: Springer-Verlag; 1998. [Google Scholar]
- 32.Abdala C, Keefe DH. In: Springer Handbook of Auditory Research: Human Auditory Development. Werner L, Popper AN, Fay RR, editors. Berlin: Springer-Verlag; 2012. [Google Scholar]
- 33.Jeng JY, et al. Hair cell maturation is differentially regulated along the tonotopic axis of the mammalian cochlea. J. Physiol. 2020;598:151–170. doi: 10.1113/JP279012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills DM, Rubel EW. Development of the cochlear amplifier. J. Acoust. Soc. Am. 1996;100:428–441. doi: 10.1121/1.415857. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs PA, Lauer AM. Efferent inhibition of the cochlea. Cold Spring Harb. Perspect. Med. 2018 doi: 10.1101/cshperspect.a033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchwald JS, Huang C. Far-field acoustic response: origins in the cat. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- 37.Antoli-Candela F, Kiang NY. Evoked Electrical Activity in the Auditory Nervous System. Cambridge: Academic Press; 1978. pp. 165–191. [Google Scholar]
- 38.Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after "temporary" noise-induced hearing loss. J. Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bramhall N, et al. The search for noise-induced cochlear synaptopathy in humans: Mission impossible? Hear Res. 2019;377:88–103. doi: 10.1016/j.heares.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Hellstrom LI, Schmiedt RA. Measures of tuning and suppression in single-fiber and whole-nerve responses in young and quiet-aged gerbils. J. Acoust. Soc. Am. 1996;100:3275–3285. doi: 10.1121/1.417211. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Ding D, Gao K, Salvi R. Standardized surgical approaches to ear surgery in rats. J. Otol. 2015;10:72–77. doi: 10.1016/j.joto.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dallos P, Cheatham MA. Compound action potential (AP) tuning curves. J. Acoust. Soc. Am. 1976;59:591–597. doi: 10.1121/1.380903. [DOI] [PubMed] [Google Scholar]
- 43.Earl BR, Chertoff ME. Mapping auditory nerve firing density using high-level compound action potentials and high-pass noise masking. J. Acoust. Soc. Am. 2012;131:337–352. doi: 10.1121/1.3664052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson RD, Nimmo-Smith I, Weber DL, Milroy R. The deterioration of hearing with age: Frequency selectivity, the critical ratio, the audiogram, and speech threshold. J. Acoust. Soc. Am. 1982;72:1788–1803. doi: 10.1121/1.388652. [DOI] [PubMed] [Google Scholar]
- 45.Moore BC, Glasberg BR, Simpson A. Evaluation of a method of simulating reduced frequency selectivity. J. Acoust. Soc. Am. 1992;91:3402–3423. doi: 10.1121/1.402830. [DOI] [PubMed] [Google Scholar]
- 46.Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J. Assoc. Res. Otolaryngol. 2005;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. J. Acoust. Soc. Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- 48.Gnansia D, Pean V, Meyer B, Lorenzi C. Effects of spectral smearing and temporal fine structure degradation on speech masking release. J. Acoust. Soc. Am. 2009;125:4023–4033. doi: 10.1121/1.3126344. [DOI] [PubMed] [Google Scholar]
- 49.Baer T, Moore BC. Effects of spectral smearing on the intelligibility of sentences in the presence of interfering speech. J. Acoust. Soc. Am. 1994;95:2277–2280. doi: 10.1121/1.408640. [DOI] [PubMed] [Google Scholar]
- 50.Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J. Neurophysiol. 1984;51:1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- 53.Lobarinas E, Spankovich C, Le Prell CG. Evidence of "hidden hearing loss" following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res. 2017;349:155–163. doi: 10.1016/j.heares.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Oxenham AJ. Predicting the perceptual consequences of hidden hearing loss. Trends Hear. 2016;20:1–6. doi: 10.1177/2331216516686768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caras ML, Sanes DH. Sustained perceptual deficits from transient sensory deprivation. J. Neurosci. 2015;35:10831–10842. doi: 10.1523/JNEUROSCI.0837-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DB, Mattingly MM, Ye Y, Gay JD, Rosen MJ. Brief Stimulus exposure fully remediates temporal processing deficits induced by early hearing loss. J. Neurosci. 2017;37:7759–7771. doi: 10.1523/JNEUROSCI.0916-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buran BN, et al. A sensitive period for the impact of hearing loss on auditory perception. J. Neurosci. 2014;34:2276–2284. doi: 10.1523/JNEUROSCI.0647-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Trapp G, Aloni I, Young S, Semple MN, Sanes DH. Developmental hearing loss impedes auditory task learning and performance in gerbils. Hear Res. 2017;347:3–10. doi: 10.1016/j.heares.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanes DH, Woolley SM. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011;72:912–929. doi: 10.1016/j.neuron.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knipper M, Panford-Walsh R, Singer W, Ruttiger L, Zimmermann U. Specific synaptopathies diversify brain responses and hearing disorders: you lose the gain from early life. Cell Tissue Res. 2015;361:77–93. doi: 10.1007/s00441-015-2168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coate TM, Scott MK, Gurjar M. Current concepts in cochlear ribbon synapse formation. Synapse. 2019;73:e22087. doi: 10.1002/syn.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puel JL, Uziel A. Correlative development of cochlear action potential sensitivity, latency, and frequency selectivity. Brain Res. 1987;465:179–188. doi: 10.1016/0165-3806(87)90239-2. [DOI] [PubMed] [Google Scholar]
- 63.Arjmand E, Harris D, Dallos P. Developmental changes in frequency mapping of the gerbil cochlea: Comparison of two cochlear locations. Hear Res. 1988;32:93–96. doi: 10.1016/0378-5955(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 64.Ryan A. Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis. J. Acoust. Soc. Am. 1976;59:1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- 65.Koay G, Heffner R, Heffner H. Behavioral audiograms of homozygous med(J) mutant mice with sodium channel deficiency and unaffected controls. Hear Res. 2002;171:111–118. doi: 10.1016/s0378-5955(02)00492-6. [DOI] [PubMed] [Google Scholar]
- 66.Lobarinas E, Salvi R, Ding D. Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. J. Assoc. Res. Otolaryngol. 2016;17:89–101. doi: 10.1007/s10162-015-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chambers AR, et al. Central gain restores auditory processing following near-complete cochlear denervation. Neuron. 2016;89:867–879. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr. Opin. Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mowery TM, Kotak VC, Sanes DH. Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cereb. Cortex. 2015;25:2083–2094. doi: 10.1093/cercor/bhu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.