Abstract
A comprehensive description of baseline characteristics, procedural features and outcomes related to the development of acute kidney injury (AKI) after transcatheter aortic valve implantation (TAVI) is reported in our research paper (Impact of contrast medium osmolality on the risk of acute kidney injury after transcatheter aortic valve implantation: insights from the Magna Graecia TAVI registry. Int J Cardiol. DOI: 10.1016/j.ijcard.2020.12.049). Three Italian heart centers were involved in this multicentric observational study. Between March 2011 and February 2019, a total of 888 patients underwent TAVI; according to the inclusion and exclusion criteria, 697 patients were included in the post-hoc analysis. This Data in Brief paper aims to report demographic, clinical, laboratory, echocardiographic, intraprocedural, periprocedural, postprocedural and follow-up data; all of them were prospectively collected from each patient's health record, whereas the analysis was performed retrospectively. Targets of this data analysis were: 1) to evaluate the impact of contrast medium (CM) osmolality on TAVI-related AKI; 2) to identify the most of risk factors involved in the development of such complication, and consequently in the occurrence of 1-year mortality; 3) to estimate the impact of CM osmolality on AKI in specific patient subgroups.
Keywords: Transcatheter aortic valve implantation, Acute kidney injury, Contrast medium, Osmolality, Risk factor, Mortality, Multivariate analysis
Specifications Table
| Subject | Cardiology and Cardiovascular Medicine |
| Specific subject area | Structural Interventional Cardiology, Valvular Heart Disease |
| Type of data | Table, Figure |
| How data were acquired | Each of the participating centers is maintaining a prospective database of all TAVI patients treated at that center, using the same dedicated archiving software. |
| Data format | Raw, analysed |
| Parameters for data collection | Among all consecutive patients undergoing TAVI, those ones died intraprocedurally, or with chronic kidney disease (CKD) requiring hemodialysis, or with recent pre-TAVI acute renal failure, or who did not received any CM during TAVI, or who received CM, nephrotoxic agents and/or n-acetylcysteine within 5 days prior and 72 h after TAVI as well as those ones whose sereum creatinine (SCr) level before TAVI was not available, were excluded. |
| Description of data collection | All baseline demographics, clinical, laboratory, electro- and echocardiographic, intra- and postprocedural data, and hospital complications and outcomes were prospectively collected from each patient's health record, whereas the analysis was performed retrospectively. Pre-TAVI mortality risk scores were retrospectively calculated online, using the official calculators. Data on events occurring after discharge and re-hospitalizations for all causes were derived from follow-up outpatient visits or by telephonic interview with the patient, the relatives or the responsible physicians. |
| Data source location |
|
| |
| |
| Data accessibility | With the article (raw data are available upon individual request) |
| Related research article | F. Iacovelli, A. Pignatelli, A. Cafaro, E. Stabile, L. Salemme, A. Cioppa, A. Pucciarelli, F. Spione, F. Loizzi, E. De Cillis, V. Pestrichella, A.S. Bortone, T. Tesorio, G. Contegiacomo. Impact of contrast medium osmolality on the risk of acute kidney injury after transcatheter aortic valve implantation: insights from the Magna Graecia TAVI registry. Int J Cardiol. DOI:10.1016/j.ijcard.2020.12.049 |
Value of the Data
TAVI-related AKI is a common complication, and associated with adverse outcomes and mortality. The relationship between CM osmolality and AKI has not been established in patients undergoing TAVI yet. Our dataset aims to evaluate new predictors for both AKI and 1-year mortality after TAVI, as well as to identify the setting of patients that mostly benefits of a kind of CM according to its osmolality.
According to the progressive expansion of TAVI indication to low surgical risk patients too, these data are surely beneficial for the whole interventional cardiology community: choosing the CM based on its osmolality and tailoring such choice according to patient's TAVI-related AKI risk could be very important.
Our data might promote the development of larger, long-term, randomized clinical studies to confirm the correlation between CM osmolality, rather than other physicochemical properties, and the incidence of TAVI-related AKI, as well as the advantages of iso-osmolar CM (IOCM) administration in specific subgroups of patients.
1. Data Description
This dataset gives relevant details and explanations about the enrolled population/procedures and statistical analysis techniques. The data are expressed as figures and tables, and are available upon individual request.
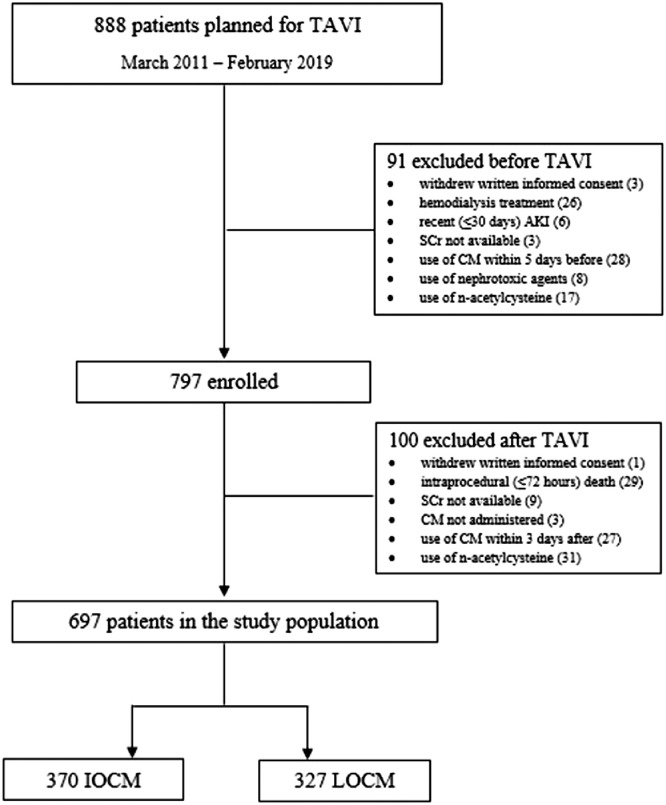
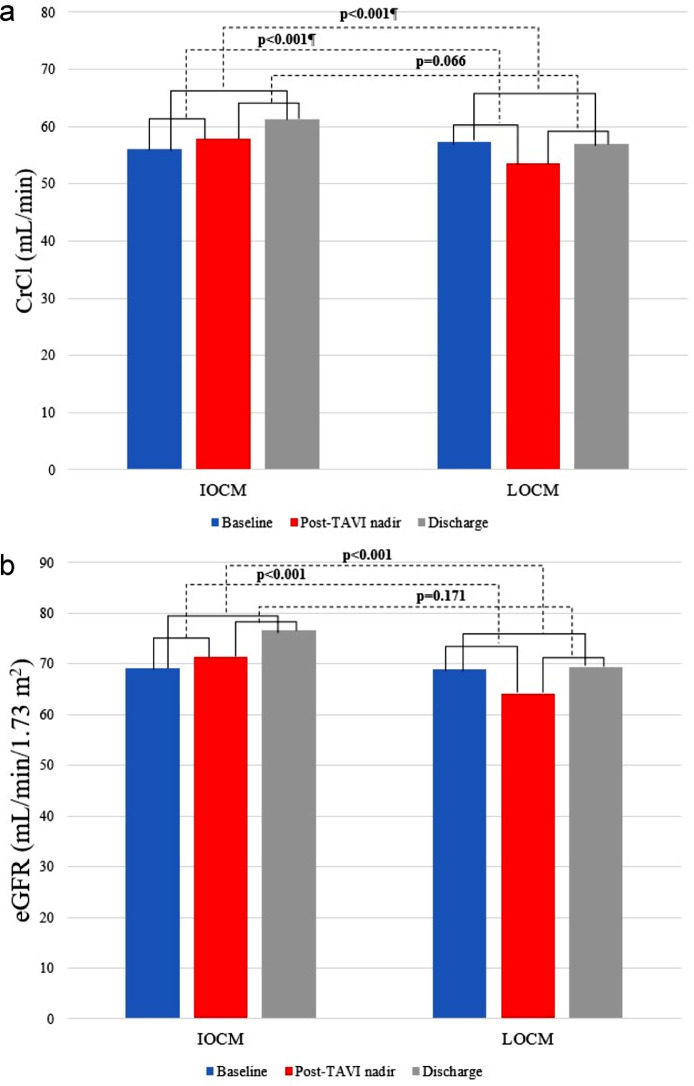
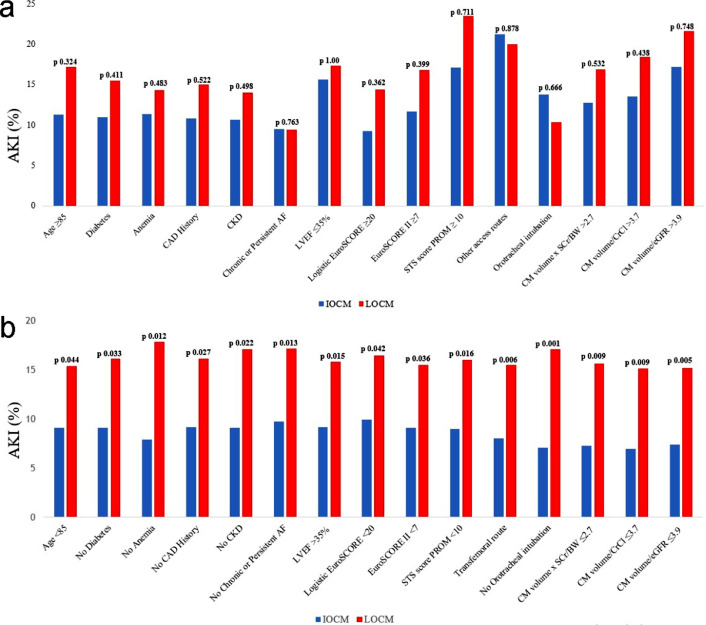
Fig. 1 shows the study flow-chart. Fig. 2 puts in evidence the variations of main renal function parameters, i.e. creatinine clearance (CrCl) and estimated glomerular filtration rate (eGFR), from baseline to both postprocedural and hospital discharge values, according to CM osmolality. After subgroup analysis, Fig. 3 illustrates the differences in the incidence of AKI between IOCM and low-osmolar CM (LOCM) in high and low AKI risk patients, stratifying them according to their age, operative mortality risk scores, amount of dye received as well as to the presence of diabetes, anemia, coronary artery disease (CAD) history, CKD, chronic or persistent atrial fibrillation and left ventricular ejection fraction (LVEF) ≤35%.
Fig. 1.
Study flowchart. TAVI = transcatheter aortic valve implantation; AKI = acute kidney injury; SCr = serum creatinine; CM = contrast medium; IOCM = iso-osmolar contrast medium; LOCM = low-osmolar contrast media.
Fig. 2.
Variations in CrCl and eGFR according to CM osmolality. CrCl = creatinine clearance; eGFR = estimated glomerular filtration rate; IOCM = iso-osmolar contrast medium; LOCM = low-osmolar contrast media; TAVI = transcatheter aortic valve implantation.
Fig. 3.
Subgroup analysis of differences in the incidence of AKI between IOCM and LOCM: high (a) and low (b) AKI risk patients. AF = atrial fibrillation; AKI = acute kidney injury; BW = body weight; CAD = coronary artery disease; CKD = chronic kidney disease; CrCl = creatinine clearance; eGFR = estimated glomerular filtration ratio; EuroSCORE = european system for cardiac operative risk evaluation; IOCM = iso-osmolar contrast medium; LOCM = low-osmolar contrast media; LVEF = left ventricular ejection fraction; SCr = serum creatinine; STS-PROM = Society of Thoracic Surgery predictive risk of mortality.
Table 1 describes the baseline characteristics (not included in the main paper) and procedural features of the study population according to AKI incidence and CM osmolality (t-test, Mann Whitney's U test, Fisher's exact test or χ2 test). Thanks to univariate and multivariate logistic regression (and logit interaction test), Table 2 points out AKI and 1-year mortality predictors, and their interactions. Table 3 highlights once again the differences in the incidence of TAVI-related AKI between IOCM and LOCM in the several patients’ subgroups: such analysis has been performed with t-test, Mann Whitney's U test, Fisher's exact test or χ2 test too.
Table 1.
Other baseline characteristics and procedural features of the study population according to AKI incidence and CM osmolality (n = 697).
| AKI |
Osmolality |
||||||
|---|---|---|---|---|---|---|---|
| Yes | No | IOCM | LOCM | ||||
| Variable | All | (n = 88) | (n = 609) | p | (n = 370) | (n = 327) | p |
| Anamnesis | |||||||
| Severe liver disease | 23 (3.30%) | 1 (1.14%) | 22 (3.61%) | 0.370 | 13 (4.05%) | 8 (2.45%) | 0.330 |
| Critical preoperative state | 38 (5.45%) | 6 (6.82%) | 32 (5.26%) | 0.724 | 16 (4.32%) | 22 (6.73%) | 0.220 |
| Prior myocardial revascularization | 172 (24.68%) | 20 (22.73%) | 152 (24.96%) | 0.748 | 105 (28.38%) | 67 (20.49%) | 0.020 |
| PCI | 96 (13.77%) | 14 (15.91%) | 82 (13.47%) | 0.648 | 57 (15.41%) | 38 (11.93%) | 0.223 |
| CABG | 44 (6.31%) | 2 (2.27%) | 42 (6.90%) | 0.152 | 26 (7.03%) | 18 (5.51%) | 0.504 |
| PCI + CABG | 32 (4.59%) | 4 (4.55%) | 28 (4.60%) | 0.802 | 22 (5.95%) | 10 (3.06%) | 0.102 |
| Myocardial revascularization for TAVI | 87 (12.48%) | 15 (17.05%) | 72 (11.82%) | 0.225 | 45 (12.16%) | 42 (12.84%) | 0.875 |
| PCI | 84 (12.05%) | 15 (17.05%) | 69 (11.33%) | 0.172 | 43 (11.62%) | 41 (12.54%) | 0.799 |
| CABG | 2 (0.29%) | 0 (0.00%) | 2 (0.33%) | 0.598 | 2 (0.54%) | 0 (0.00%) | 0.534 |
| PCI + CABG | 1 (0.14%) | 0 (0.00%) | 1 (0.16%) | 0.260 | 0 (0.00%) | 1 (0.31%) | 0.951 |
| Prior PM/ICD/CRT implantation | 81 (11.62%) | 17 (19.32%) | 64 (10.51%) | 0.026 | 41 (11.08%) | 40 (12.23%) | 0.723 |
| Baseline renal function assessment | |||||||
| SCr (mg/dL) | 1.08±0.42 | 1.11±0.54 | 1.07±0.40 | 0.621 | 1.09±0.50 | 1.07±0.39 | 0.907 |
| CrCl (mL/min) | 56.53±22.81 | 56.50±24.81 | 56.66±22.54 | 0.972 | 56.08±23.29 | 57.28±22.29 | 0.394 |
| eGFR (mL/min/1.73 m2) | 69.03±25.63 | 70.06±30.32 | 68.88±24.90 | 0.878 | 69.14±26.17 | 68.91±25.03 | 0.939 |
| Electrocardiography | |||||||
| Sinus rhythm | 533 (76.47%) | 67 (76.14%) | 466 (76.52%) | 0.956 | 287 (77.57%) | 246 (75.23%) | 0.524 |
| Atrial fibrillation / flutter | 116 (16.64%) | 11 (12.50%) | 107 (17.57%) | 0.335 | 63 (17.03%) | 53 (16.21%) | 0.851 |
| PM-induced rhythm | 48 (6.89%) | 10 (11.36%) | 38 (6.24%) | 0.121 | 20 (5.41%) | 28 (8.56%) | 0.135 |
| Echocardiography | |||||||
| LVEF (%) | 52.86±11.13 | 52.09±11.91 | 52.97±11.02 | 0.502 | 53.24±12.16 | 52.44±9.83 | 0.932 |
| Maximum aortic gradient (mmHg) | 76.20±20.76 | 76.41±22.05 | 76.17±20.60 | 0.959 | 72.41±19.91 | 80.23±20.93 | <0.001 |
| Mean aortic gradient (mmHg) | 46.92±14.39 | 45.76±14.66 | 47.09±14.35 | 0.547 | 45.43±13.88 | 48.58±14.78 | 0.004 |
| Moderate-to-severe mitral regurgitation | 162 (23.24%) | 23 (26.14%) | 139 (22.82%) | 0.581 | 105 (28.38%) | 57 (17.43%) | <0.001 |
| Pulmonary arterial systolic pressure (mmHg) | 40.01±12.76 | 40.53±12.38 | 39.93±12.83 | 0.678 | 39.42±12.35 | 40.59±13.15 | 0.419 |
| CT-guided procedure | 652 (93.54%) | 81 (92.05%) | 571 (93.76%) | 0.704 | 353 (95.41%) | 299 (91.44%) | 0.049 |
| Procedural details | |||||||
| Transfemoral access route | 620 (88.95%) | 71 (81.82%) | 548 (89.98%) | 0.036 | 323 (87.30%) | 297 (90.82%) | 0.173 |
| Other access routes | 77 (11.05%) | 16 (18.18%) | 61 (10.02%) | 0.036 | 47 (12.70%) | 30 (9.17%) | 0.173 |
| transsubclavian | 21 (3.01%) | 1 (1.14%) | 20 (3.28%) | 0.442 | 4 (1.08%) | 17 (5.20%) | 0.003 |
| transapical | 50 (7.17%) | 11 (12.50%) | 39 (6.40%) | 0.064 | 40 (10.81%) | 10 (3.06%) | <0.001 |
| direct aortic | 6 (0.86%) | 4 (4.45%) | 2 (0.33%) | <0.001 | 3 (0.81%) | 3 (0.92%) | 0.796 |
| Orotracheal intubation | 203 (29.12%) | 26 (29.55%) | 177 (29.06%) | 0.974 | 145 (39.19%) | 58 (17.74%) | <0.001 |
| Valve-in-valve | 19 (2.73%) | 0 (0.00%) | 19 (3.12%) | 0.184 | 10 (2.70%) | 9 (2.75%) | 0.847 |
| Predilation | 425 (60.98%) | 39 (44.32%) | 233 (38.26%) | 0.331 | 253 (68.38%) | 172 (52.59%) | <0.001 |
| Valve kind | |||||||
| balloon-expandable | 436 (62.55%) | 59 (67.05%) | 377 (61.90%) | 0.416 | 209 (56.49%) | 227 (69.42%) | <0.001 |
| self-expandable | 228 (32.71%) | 28 (32.82%) | 200 (32.84%) | 0.945 | 139 (37.57%) | 89 (27.22%) | 0.005 |
| others | 33 (4.73) | 1 (1.14%) | 32 (5.26%) | 0.152 | 22 (5.94%) | 11 (3.36%) | 0.155 |
| Valve size | |||||||
| ≤26 mm | 533 (76.47%) | 65 (73.86%) | 468 (76.85%) | 0.630 | 270 (72.97%) | 263 (80.43%) | 0.026 |
| >26 mm | 164 (23.53%) | 23 (26.14%) | 141 (23.15%) | 0.630 | 100 (27.03%) | 64 (19.57%) | 0.026 |
| Postdilation | 86 (12.34%) | 11 (12.50%) | 75 (12.32%) | 0.901 | 61 (16.49%) | 25 (7.64%) | <0.001 |
| CM volume (mL) | 166.10±60.61 | 171.60±72.44 | 165.30±70.36 | 0.475 | 185.34±71.83 | 144.32±62.52 | <0.001 |
| CM volume x SCr/BW | 2.50±1.39 | 2.70±1.69 | 2.47±1.34 | 0.602 | 2.81±1.42 | 2.15±1.26 | <0.001 |
| CM volume x SCr/BW >2.7 | 235 (33.72%) | 33 (37.50%) | 202 (33.17%) | 0.495 | 164 (44.32%) | 71 (21.71%) | <0.001 |
| CM volume/CrCl | 3.40±2.03 | 3.77±2.54 | 3.34±1.94 | 0.292 | 3.83±2.12 | 2.91±1.79 | <0.001 |
| CM volume/CrCl >3.7 | 231 (33.14%) | 35 (39.77%) | 196 (32.18%) | 0.196 | 155 (41.89%) | 76 (23.24%) | <0.001 |
| CM volume/eGFR | 2.74±1.58 | 3.05±1.96 | 2.70±1.51 | 0.359 | 3.05±1.58 | 2.40±1.50 | <0.001 |
| CM volume/eGFR >3.9 | 124 (17.79%) | 23 (26.13%) | 101 (16.58%) | 0.041 | 87 (23.51%) | 37 (11.31%) | <0.001 |
| Mehran score ≥11 | 482 (69.15%) | 62 (70.45%) | 420 (68.97%) | 0.873 | 280 (75.68%) | 202 (61.77%) | <0.001 |
| IOCM | 370 (53.08%) | 36 (40.91%) | 334 (54.84%) | 0.020 | |||
| LOCM | 327 (46.92%) | 52 (59.09%) | 275 (45.16%) | 0.020 | |||
| iopromide | 130 (18.65%) | 11 (12.50%) | 119 (19.54%) | 0.005 | |||
| iobitridol | 92 (13.20%) | 16 (18.18%) | 76 (12.48%) | 0.770 | |||
| iohexol | 80 (11.48%) | 18 (20.45%) | 62 (10.18%) | 0.093 | |||
| iomeprol | 25 (3.59%) | 7 (7.95%) | 18 (2.96%) | 0.151 | |||
| Post-TAVI and discharge renal function assessment | |||||||
| Post-TAVI peak SCr (mg/dL) | 1.12±0.51 | 1.72±0.87 | 1.03±0.35 | <0.001 | 1.08±0.51 | 1.16±0.50 | 0.001 |
| Post-TAVI peak – basal ΔSCr (mg/dL) | 0.04±0.35 | 0.61±0.63 | −0.04±0.18 | <0.001 | −0.01±0.33 | 0.09±0.37 | <0.001 |
| Post-TAVI nadir CrCl (mL/min) | 55.80±24.01 | 38.09±19.13 | 58.37±23.56 | <0.001 | 57.80±26.28 | 53.51±20.92 | 0.115 |
| Basal – post-TAVI nadir ΔCrCl (mL/min) | 0.84±12.30 | 18.40±14.67 | −1.70±9.55 | <0.001 | −1.72±13.17 | 3.76±10.52 | <0.001 |
| Post-TAVI nadir eGFR (mL/min/1.73 m2) | 67.94±27.31 | 44.64±22.73 | 71.31±26.26 | <0.001 | 71.37±29.63 | 64.05±23.88 | 0.002 |
| Basal – post-TAVI nadir ΔeGFR (mL/min/1.73 m2) | 1.09±16.83 | 25.41±19.62 | −2.42±13.08 | <0.001 | −2.24±18.17 | 4.86±14.29 | <0.001 |
| Discharge SCr (mg/dL) | 1.06±0.47 | 1.37±0.83 | 1.01±0.38 | <0.001 | 1.03±0.49 | 1.07±0.46 | 0.008 |
| Discharge – basal ΔSCr (mg/dL) | −0.35±0.35 | 0.26±0.68 | −0.07±0.24 | <0.001 | −0.06±0.35 | 0.00±0.34 | <0.001 |
| Discharge – post-TAVI peak ΔSCr (mg/dL) | −0.07±0.31 | −0.35±0.64 | −0.03±0.19 | <0.001 | −0.06±0.30 | −0.09±0.32 | 0.045 |
| Discharge CrCl (mL/min) | 59.32±25.67 | 49.42±25.43 | 60.75±25.41 | <0.001 | 61.36±28.27 | 57.02±22.19 | 0.240 |
| Basal – discharge ΔCrCl (mL/min) | −2.92±12.55 | 7.08±16.92 | −4.38±11.07 | <0.001 | −5.27±13.93 | 0.27±10.16 | <0.001 |
| Discharge – post-TAVI nadir ΔCrCl (mL/min) | 3.76±10.68 | 11.33±14.48 | 2.67±9.55 | <0.001 | 3.55±12.01 | 4.00±8.98 | 0.066 |
| Discharge eGFR (mL/min/1.73 m2) | 73.24±29.42 | 60.67±33.41 | 75.06±28.37 | <0.001 | 76.61±32.58 | 69.42±24.87 | 0.007 |
| Basal – discharge ΔeGFR (mL/min/1.73 m2) | −4.21±17.42 | 9.38±23.99 | −6.17±15.37 | <0.001 | −7.47±19.65 | −0.52±13.73 | <0.001 |
| Discharge – post-TAVI nadir ΔeGFR (mL/min/1.73 m2) | 5.30±15.09 | 16.03±20.42 | 3.75±13.49 | <0.001 | 5.24±16.83 | 5.37±12.86 | 0.171 |
AKI = acute kidney injury; CM = contrast medium; IOCM = iso-osmolar contrast medium; LOCM = low-osmolar contrast media; PCI = percutaneous coronary intervention; CABG = coronary artery by-pass grafting; TAVI = transcatheter aortic valve implantation; PM = pacemaker; ICD = implantable cardioverter-defibrillator; CRT = cardiac resynchronization therapy; SCr = serum creatinine; CrCl = creatinine clearance; eGFR = estimated glomerular filtration ratio; LVEF = left ventricular ejection fraction; CT = computed tomography; BW = body weight.
Table 2.
AKI and 1-year mortality predictors.
| Univariate | p value | Multivariate | p value | p-interaction | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | (LOCM) | |||
| AKI predictors | |||||
| PAD | 1.25 (0.77–2.01) | 0.362 | 1.03 (0.60–1.77) | 0.911 | 0.046 |
| STS-PROM score | 1.03 (1.00–1.05) | 0.018 | 1.00 (0.99–1.01) | 0.793 | 0.022 |
| Non-transfemoral access route | 2.00 (1.09–3.65) | 0.025 | |||
| CM volume/eGFR >3.9 | 1.78 (1.06–3.00) | 0.030 | 2.01 (1.20–3.67) | 0.010 | 0.445 |
| LOCM | 1.75 (1.11–2.76) | 0.015 | 1.97 (1.21–3.21) | 0.006 | |
| Any bleeding | 3.41 (2.15–5.42) | <0.001 | |||
| Any transfusion | 4.19 (2.49–7.06) | <0.001 | 4.22 (2.49–7.34) | <0.001 | 0.991 |
| New-onset AF/flutter | 2.39 (1.18–4.82) | 0.015 | 1.97 (0.94–4.14) | 0.072 | 0.419 |
| 1-year mortality predictors | |||||
| Anemia | 2.62 (1.45–4.74) | 0.001 | 2.28 (1.24–4.21) | 0.008 | 0.789 |
| LVEF ≤35% | 2.51 (1.19–5.27) | 0.015 | 2.47 (1.14–5.33) | 0.021 | 0.485 |
| EuroSCORE II | 1.05 (1.02–1.09) | 0.003 | |||
| LOCM | 2.51 (1.44–4.38) | 0.001 | 2.62 (1.48–4.62) | 0.001 | |
| AKI | 2.52 (1.34–4.73) | 0.004 | |||
| Any transfusion | 2.03 (1.05–3.93) | 0.035 | 1.60 (0.80–3.21) | 0.179 | 0.928 |
AKI = acute kidney injury; OR = odds ratio; CI = confidence interval; LOCM = low-osmolar contrast medium; PAD = peripheral artery disease; STS-PROM = Society of Thoracic Surgery predictive risk of mortality; CM = contrast medium; eGFR = estimated glomerular filtration ratio; AF = atrial fibrillation; LVEF = left ventricular ejection fraction; EuroSCORE = european system for cardiac operative risk evaluation.
Table 3.
Subgroup analysis of differences in the incidence of TAVI-related AKI between IOCM and LOCM.
| Subgroup | IOCM | LOCM | p |
|---|---|---|---|
| Age ≥85 years (n) | 106 | 93 | |
| basal SCr (SD) | 1.06±0.42 | 1.04±0.38 | 0.888 |
| AKI, n (%) | 12 (11.32%) | 16 (17.20%) | 0.324 |
| Age <85 years (n) | 264 | 234 | |
| basal SCr (SD) | 1.16±0.50 | 1.13±0.42 | 0.990 |
| AKI, n (%) | 24 (9.09%) | 36 (15.38%) | 0.044 |
| Diabetes (n) | 118 | 116 | |
| basal SCr (SD) | 1.11±0.47 | 1.06±0.38 | 0.697 |
| AKI, n (%) | 13 (11.02%) | 18 (15.52%) | 0.411 |
| No diabetes (n) | 252 | 211 | |
| basal SCr (SD) | 1.08±0.44 | 1.07±0.40 | 0.832 |
| AKI, n (%) | 23 (9.13%) | 34 (16.11%) | 0.033 |
| Anemia (n) | 193 | 181 | |
| basal SCr (SD) | 1.19±0.52 | 1.15±0.44 | 0.570 |
| AKI, n (%) | 22 (11.40%) | 26 (14.37%) | 0.483 |
| No anemia (n) | 177 | 146 | |
| basal SCr (SD) | 0.98±0.33 | 0.97±0.29 | 0.839 |
| AKI, n (%) | 14 (7.91%) | 26 (17.81%) | 0.012 |
| COPD (n) | 136 | 98 | |
| basal SCr (SD) | 1.13±0.47 | 1.10±0.44 | 0.461 |
| AKI, n (%) | 12 (8.82%) | 14 (14.29%) | 0.271 |
| No COPD (n) | 234 | 229 | |
| basal SCr (SD) | 1.06±0.43 | 1.05±0.37 | 0.621 |
| AKI, n (%) | 24 (10.26%) | 38 (16.59%) | 0.062 |
| PAD (n) | 118 | 63 | |
| basal SCr (SD) | 1.22±0.56 | 1.12±0.42 | 0.315 |
| AKI, n (%) | 10 (8.48%) | 12 (19.05%) | 0.067 |
| No PAD (n) | 252 | 264 | |
| basal SCr (SD) | 1.03±0.37 | 1.05±0.38 | 0.290 |
| AKI, n (%) | 26 (10.32%) | 40 (15.15%) | 0.131 |
| CAD history (n) | 120 | 73 | |
| basal SCr (SD) | 1.13±0.37 | 1.01±0.31 | 0.019 |
| AKI, n (%) | 13 (10.83%) | 11 (15.07%) | 0.522 |
| No CAD history (n) | 250 | 254 | |
| basal SCr (SD) | 1.07±0.49 | 1.08±0.41 | 0.171 |
| AKI, n (%) | 23 (9.20%) | 41 (16.14%) | 0.027 |
| NYHA functional class III-IV (n) | 348 | 272 | |
| basal SCr (SD) | 1.09±0.45 | 1.06±0.40 | 0.450 |
| AKI, n (%) | 32 (9.20%) | 44 (16.18%) | 0.012 |
| NYHA functional class I-II (n) | 22 | 55 | |
| basal SCr (SD) | 1.00±0.43 | 1.09±0.34 | 0.056 |
| AKI, n (%) | 4 (18.18%) | 8 (14.55%) | 0.734 |
| CKD (n) | 150 | 128 | |
| basal SCr (SD) | 1.45±0.49 | 1.41±0.38 | 0.753 |
| AKI, n (%) | 16 (10.67%) | 18 (14.06%) | 0.498 |
| No CKD (n) | 220 | 199 | |
| basal SCr (SD) | 0.84±0.18 | 0.85±0.18 | 0.744 |
| AKI, n (%) | 20 (9.09%) | 34 (17.09%) | 0.022 |
| Chronic or persistent AF (n) | 63 | 53 | |
| basal SCr (SD) | 1.09±0.37 | 1.09±0.41 | 0.799 |
| AKI, n (%) | 6 (9.52%) | 5 (9.43%) | 0.763 |
| No chronic or persistent AF (n) | 307 | 274 | |
| basal SCr (SD) | 1.09±0.47 | 1.06±0.39 | 0.986 |
| AKI, n (%) | 30 (9.77%) | 47 (17.15%) | 0.013 |
| LVEF ≤35% (n) | 32 | 23 | |
| basal SCr (SD) | 1.16±0.35 | 1.35±0.51 | 0.103 |
| AKI, n (%) | 5 (15.63%) | 4 (17.39%) | 1.00 |
| LVEF >35% (n) | 338 | 304 | |
| basal SCr (SD) | 1.08±0.46 | 1.05±0.37 | 0.742 |
| AKI, n (%) | 31 (9.17%) | 48 (15.79%) | 0.015 |
| Logistic EuroSCORE ≥20% | 108 | 90 | |
| basal SCr (SD) | 1.08±0.85 | 1.11±0.82 | 0.591 |
| AKI, n (%) | 10 (9.26%) | 13 (14.44%) | 0.362 |
| Logistic EuroSCORE <20% | 262 | 237 | |
| basal SCr (SD) | 1.04±0.40 | 1.01±0.33 | 0.764 |
| AKI, n (%) | 26 (9.92%) | 39 (16.46%) | 0.042 |
| EuroSCORE II ≥7% | 103 | 95 | |
| basal SCr (SD) | 1.30±0.56 | 1.22±0.48 | 0.204 |
| AKI, n (%) | 12 (11.65%) | 16 (16.84%) | 0.399 |
| EuroSCORE II <7% | 267 | 232 | |
| basal SCr (SD) | 1.01±0.37 | 1.01±0.33 | 0.495 |
| AKI, n (%) | 24 (9.10%) | 36 (15.52%) | 0.036 |
| STS-PROM score ≥10% | 35 | 17 | |
| basal SCr (SD) | 1.33±0.96 | 1.35±1.00 | 0.961 |
| AKI, n (%) | 6 (17.14%) | 4 (23.53%) | 0.711 |
| STS-PROM score <10% | 335 | 310 | |
| basal SCr (SD) | 1.05±0.40 | 1.05±0.36 | 0.731 |
| AKI, n (%) | 30 (8.96%) | 48 (15.98%) | 0.016 |
| Other access routes (n) | 47 | 30 | |
| basal SCr (SD) | 1.17±0.47 | 1.17±0.35 | 0.904 |
| AKI, n (%) | 10 (21.28%) | 6 (20.00%) | 0.878 |
| Transfemoral route (n) | 323 | 297 | |
| basal SCr (SD) | 1.08±0.45 | 1.06±0.39 | 0.872 |
| AKI, n (%) | 26 (8.05%) | 46 (15.49%) | 0.006 |
| Orotracheal intubation (n) | 145 | 58 | |
| basal SCr (SD) | 1.13±0.48 | 1.15±0.41 | 0.453 |
| AKI, n (%) | 20 (13.79%) | 6 (10.35%) | 0.666 |
| No orotracheal intubation (n) | 225 | 269 | |
| basal SCr (SD) | 1.06±0.43 | 1.05±0.38 | 0.939 |
| AKI, n (%) | 16 (7.11%) | 46 (17.10%) | 0.001 |
| CM volume x SCr/BW >2.7 (n) | 164 | 71 | |
| basal SCr (SD) | 1.24±0.44 | 1.45±0.47 | <0.001 |
| AKI, n (%) | 21 (12.81%) | 12 (16.90%) | 0.532 |
| CM volume x SCr/BW ≤2.7 (n) | 206 | 256 | |
| basal SCr (SD) | 0.96±0.42 | 0.96±0.28 | 0.213 |
| AKI, n (%) | 15 (7.28%) | 40 (15.63%) | 0.009 |
| CM volume/CrCl >3.7 (n) | 155 | 76 | |
| basal SCr (SD) | 1.24±0.44 | 1.41±0.47 | 0.004 |
| AKI, n (%) | 21 (13.55%) | 14 (18.42%) | 0.438 |
| CM volume/CrCl ≤3.7 (n) | 215 | 251 | |
| basal SCr (SD) | 0.97±0.42 | 0.96±0.29 | 0.362 |
| AKI, n (%) | 15 (6.98%) | 38 (15.14%) | 0.009 |
| CM volume/eGFR >3.9 (n) | 87 | 37 | |
| basal SCr (SD) | 1.37±0.48 | 1.65±0.52 | 0.004 |
| AKI, n (%) | 15 (17.24%) | 8 (21.62%) | 0.748 |
| CM volume/eGFR ≤3.9 (n) | 283 | 290 | |
| basal SCr (SD) | 1.00±0.40 | 0.99±0.30 | 0.390 |
| AKI, n (%) | 21 (7.42%) | 44 (15.17%) | 0.005 |
| Mehran score ≥11 | 244 | 191 | |
| basal SCr (SD) | 1.20±0.50 | 1.17±0.43 | 0.771 |
| AKI, n (%) | 27 (11.07%) | 29 (15.18%) | 0.259 |
| Mehran score <11 | 126 | 136 | |
| basal SCr (SD) | 0.87±0.21 | 0.92±0.27 | 0.182 |
| AKI, n (%) | 9 (7.14%) | 23 (16.91%) | 0.026 |
| Any bleeding (n) | 99 | 66 | |
| basal SCr (SD) | 1.11±0.59 | 1.06±0.41 | 0.829 |
| AKI, n (%) | 20 (20.20%) | 21 (31.82%) | 0.132 |
| No bleedings (n) | 271 | 261 | |
| basal SCr (SD) | 1.08±0.39 | 1.07±0.39 | 0.713 |
| AKI, n (%) | 16 (5.90%) | 31 (11.88%) | 0.023 |
| Any transfusion (n) | 52 | 39 | |
| basal SCr (SD) | 1.19±0.61 | 1.12±0.47 | 0.776 |
| AKI, n (%) | 16 (30.77%) | 12 (30.77%) | 0.818 |
| No transfusions (n) | 318 | 288 | |
| basal SCr (SD) | 1.07±0.42 | 1.06±0.38 | 0.990 |
| AKI, n (%) | 20 (6.29%) | 40 (13.89%) | 0.003 |
| Any vascular complication (n) | 64 | 38 | |
| basal SCr (SD) | 1.11±0.58 | 1.16±0.41 | 0.183 |
| AKI, n (%) | 7 (10.94%) | 7 (18.42%) | 0.445 |
| No vascular complications (n) | 306 | 289 | |
| basal SCr (SD) | 1.08±0.42 | 1.06±0.39 | 0.529 |
| AKI, n (%) | 29 (9.48%) | 45 (15.57%) | 0.033 |
| Post-TAVI moderate-to-severe residual AR (n) | 105 | 57 | |
| basal SCr (SD) | 1.21±0.59 | 1.10±0.49 | 0.275 |
| AKI, n (%) | 15 (14.29%) | 8 (14.04%) | 0.848 |
| Post-TAVI trivial-to-mild residual AR (n) | 265 | 270 | |
| basal SCr (SD) | 1.04±0.37 | 1.06±0.37 | 0.410 |
| AKI, n (%) | 21 (7.93%) | 44 (16.30%) | 0.005 |
| New-onset AF/flutter (n) | 17 | 33 | |
| basal SCr (SD) | 0.97±0.33 | 1.06±0.39 | 0.396 |
| AKI, n (%) | 2 (11.77%) | 10 (30.30%) | 0.175 |
| No new-onset AF/flutter (n) | 257 | 200 | |
| basal SCr (SD) | 1.06±0.39 | 1.10±0.48 | 0.518 |
| AKI, n (%) | 27 (9.51%) | 35 (14.89%) | 0.081 |
TAVI = transcatheter aortic valve implantation; AKI = acute kidney injury; IOCM = iso-osmolar contrast medium; LOCM = low-osmolar contrast media; SCr = serum creatinine; SD = standard deviation; COPD = chronic obstructive pulmonary disease; PAD = peripheral artery disease; CAD = coronary artery disease; NYHA = New York Heart Association; CKD = chronic kidney disease; eGFR = estimated glomerular filtration ratio; AF = atrial fibrillation; LVEF = left ventricular ejection fraction; EuroSCORE = european system for cardiac operative risk evaluation; STS-PROM = Society of Thoracic Surgery predictive risk of mortality; BW = body weight; CrCl = creatinine clearance; AR = aortic regurgitation.
2. Experimental Design, Materials and Methods
2.1. Study population
This prospective multicentric observational study [1] assessed all consecutive patients who underwent TAVI at 3 Italian heart centers (Policlinico University Hospital of Bari, “Santa Maria” Clinic of Bari and “Montevergine” Clinic of Mercogliano) involved into the “Magna Graecia” TAVI registry. Such all-comers study protocol was firstly approved by the Independent Ethical Committee (study number 6244) of the Policlinico University Hospital of Bari, Italy, in accordance with the Declaration of Helsinki.
Between March 2011 and February 2019, a total of 888 patients underwent TAVI; according to the inclusion and exclusion criteria from such post-hoc analysis, the final study population consisted of 697 patients (Fig. 1).
All patients referred for consideration of TAVI underwent a systematic assessment including transthoracic echocardiography, coronary angiography, computed tomography scan of the heart, aorta, and peripheral vasculature, pulmonary function testing, carotid artery ultrasonography and multidisciplinary evaluation by both a cardiac surgeon and an interventional cardiologist.
Details on the TAVI procedure are provided elsewhere [2]. The majority of procedures were performed under local anesthesia and analgesia, under fluoroscopic guidance in a standard cardiac catheterization laboratory with surgical back-up by a dedicated team of experienced operators. Type of TAVI device implanted was defined as balloon-expandable (Edwards Sapien XT and Sapien 3; Meril Myval), self-expandable (Medtronic CoreValve, Engager, Evolut R and Evolut PRO; Boston Acurate and Acurate neo; Abbott Portico; JenaValve) and others (Boston Lotus; Direct Flow Medical).
In diabetic patients on metformin treatment, this drug was suspended 48 h before and re-administered 48 h after TAVI. All patients had intravenous hydration therapy for 24 h before the procedure, and continued 48 h after TAVI: 1 mL/kg/h of 0.9% NaCl solution, at a rate of 40 to 100 mL/h (according to the individual left ventricular function, pulmonary artery pressure, and combined valvular disease). The decision to give or hold diuretics preoperatively, was individualized to each patient aiming for an euvolemic state.
Each of the participating centers is maintaining a prospective database of all TAVI patients treated at that center, using the same dedicated archiving software. All baseline demographics, clinical, laboratory, echocardiographic, intraprocedural and postprocedural data, and hospital outcomes were prospectively collected from each patient's health record, whereas the analysis was performed retrospectively. Pre-TAVI Logistic european system for cardiac operative risk evaluation (EuroSCORE) mortality risk, EuroSCORE II mortality risk and STS-PROM score were retrospectively calculated online using the official websites and calculators based on previously published data. The number of rapid pacing runs, the occurrence of any complication leading to severe sustained hypotension, and/or the need for hemodynamic support (e.g. pharmacological, aortic counterpulsation balloon and extracorporeal circulation) were recorded.
Data on follow-up echocardiography were extracted from each center's echocardiography database, while data on events occurring after discharge and re-hospitalizations for all causes were derived from follow-up outpatient visits or by telephonic interview. Physicians responsible for the patients were contacted and/or medical charts were reviewed to determine the causes of re-hospitalization and/or death when necessary.
In order to assemble a unified database, all data required for the study were sent back to the first author (FI), who compiled the final database used for the statistical analysis. No extramural funding was used to support the study. The authors wrote the manuscript and are responsible for the completeness and accuracy of data gathering and analysis.
2.2. CM and renal function assessment, and definitions
The choice of the type of CM to be used for the procedure was institution- and physician-dependent; the CM were: (1) iodixanol, iodinated non-ionic iso-osmolar, dimeric, (2) iopromide, (3) iobitridol, (4) iohexol and (5) iomeprol, all iodinated non-ionic low-osmolar, monomeric. According to CM osmolality, the population was retrospectively divided in 2 groups: IOCM group (n = 370) and LOCM group (n = 327).
The amount of CM was recorded during all TAVI procedures. According to the previous investigations, the CM volume x SCr/body weight, CM volume/CrCl and CM volume/eGFR ratios were used to evaluate the degree of CM dose in individual patients [3], [4], [5].
Isotope diluition mass spectroscopy was used to measure SCr level at the admission (at least 1 day before the procedure), on the procedure day (after continuing the overnight hydration), and then daily until the discharge. Baseline SCr was defined as the SCr measured before and closest to the time of TAVI procedure. If there was >1 measurement post-TAVI available, the greater SCr value within 48 h was included in the analysis. Via a Foley catheter or an external collection device, urine output (UO) was evaluated through at least 72 h after TAVI or until hospital discharge if that occurred earlier than 72 h after TAVI. eGFR was calculated with the simplified Modification of Diet in Renal Disease formula [6], while CrCl rate using Cockcroft-Gault formula. For the present analysis, CKD was defined as baseline eGFR of <60 mL/min/1.73 m2.
AKI was defined as stage 1, 2 or 3 by AKI Network from the SCr- and UO-based criteria; according to such system [7]:
-
•
stage 1: increase in SCr of 150–199% (1.5–1.99 × increase compared with baseline) or increase of ≥0.3 mg/dL (≥26.4 mmol/L) or UO <0.5 mL/kg/h for >6 h but <12 h;
-
•
stage 2: increase in SCr of 200–299% (2.0–2.99 × increase compared with baseline) or UO <0.5 mL/kg/h for >12 h but <24 h;
-
•
stage 3: increase in SCr of ≥300% (>3 × increase compared with baseline) or SCrof ≥4.0 mg/dL (≥354 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L) or UO <0.3 mL/kg/h for >24 h or anuria for >12 h.
Patients receiving renal replacement therapy were considered to meet stage 3 criteria irrespective of other criteria. The indications for renal replacement therapy included fluid overload with heart failure, hyperkalemia, hypercalcemia, metabolic acidosis, uremic symptoms, and oliguria or anuria (UO <200 mL/12 h or UO <50 mL/12 h, respectively).
Preprocedural anemia was defined by the World Health Organization definition of anemia: hemoglobin <12 g/dL for women and <13 g/dL for men [8]. Nadir hemoglobin was defined as the lowest hemoglobin measured after TAVI until discharge.
All other complications as well as device success and early safety were defined according to the VARC-2 standardized criteria [9].
2.3. Statistical analysis
Statistical analyses were performed using SigmaStat 3.5 and STATA 13.1. Continuous variables are expressed as mean ± standard deviation or median (interquartile range) of absolute numbers. Categorical variables are reported as frequencies and percentages. The data reported in Table 1 were analysed by t-test, Mann Whitney's U test, Fisher's exact test or χ2 test, as appropriate [10,11]. For all regression analyses (Table 2), the most of variables with a p-value of <0.05 in univariable analysis were incorporated in the multivariable model, if not covariates [12]. Odds ratios with 95% confidence intervals were estimated, and then tests for interaction were performed, as appropriate [13]. All statistical tests were two-sided. For all tests, a p-value <0.05 was considered statistically significant. About subgroup analysis, odds ratios with 95% confidence intervals were first calculated and plotted in a Forest graph with effects sizes, and then tests for interaction were performed too, as appropriate. A p-value <0.025 was considered statistically significant for interaction. The subgroup analysis in Table 3 was performed with t-test, Mann Whitney's U test, Fisher's exact test or χ2 test, as appropriate.
Ethics Statement
The study protocol was in accordance with the institutional ethics committee of each participating center as well as the Declaration of Helsinki, and all patients gave informed written consent for the procedures.
CRediT Author Statement
Fortunato Iacovelli, Vincenzo Pestrichella and Gaetano Contegiacomo: Conceptualization, Methodology, Software; Francesco Spione, Eugenio Stabile and Antonio Pignatelli: Data curation; Francesco Loizzi and Emanuela De Cillis: Writing- Original draft preparation; Angelo Cioppa and Armando Pucciarelli: Visualization, Investigation; Tullio Tesorio: Supervision; Alessandro Cafaro: Software, Validation; Luigi Salemme and Alessandro Santo Bortone: Writing- Reviewing and Editing.
Declaration of Competing Interest
Gaetano Contegiacomo serves as transcatheter heart valve proctor for Abbott and Meril; the remaining authors have no conflicts of interest to declare. This research received no specific grants from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgments
None.
References
- 1.Iacovelli F., Pignatelli A., Cafaro A., Stabile E., Salemme L., Cioppa A., Pucciarelli A., Spione F., Loizzi F., De Cillis E., Pestrichella V., Bortone A.S., Tesorio T., Contegiacomo G. Impact of contrast medium osmolality on the risk of acute kidney injury after transcatheter aortic valve implantation: insights from the Magna Graecia TAVI registry. Int. J. Cardiol. 2021 doi: 10.1016/j.ijcard.2020.12.049. In Press. [DOI] [PubMed] [Google Scholar]
- 2.Rodes-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat. Rev. Cardiol. 2011;9:15–29. doi: 10.1038/nrcardio.2011.164. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M., Hayashida K., Mouillet G., Chevalier B., Meguro K., Watanabe Y., Dubois-Rande J.L., Morice M.C., Lefèvre T., Teiger E. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2013;6:479–486. doi: 10.1016/j.jcin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Gül I., Zungur M., Tastan A., Okur F.F., Damar E., Uyar S., Sahin V., Tavli T. The importance of contrast volume/glomerular filtration rate ratio in contrast-induced nephropathy patients after transcatheter aortic valve implantation. Cardiorenal Med. 2015;5(1):31–39. doi: 10.1159/000369943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannini F., Latib A., Jabbour R.J., Slavich M., Benincasa S., Chieffo A., Montorfano M., Stella S., Buzzatti N., Alfieri O., Colombo A. The ratio of contrast volume to glomerular filtration rate predicts acute kidney injury and mortality after transcatheter aortic valve implantation. Cardiovasc. Revasc. Med. 2017;18:349–355. doi: 10.1016/j.carrev.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G., Levin A. Acute kidney injury N. acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc B., Finch C.A., Hallberg L., Herbert V., Lawkowicz W., Layrisse M., Miollin D.L., Rachmilewitz M., Ramalingaswami V., Sanchez-Medal L. World Health Organization; 1968. Nutritional Anemias: Report of a WHO Scientific Group; pp. 1–40. Tech Rep Ser. [Google Scholar]
- 9.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.A., Hahn R.T., Kirtane A.J., Krucoff M.W., Kodali S., Mack M.J., Mehran R., Rodés-Cabau J., Vranckx P., Webb J.G., Windecker S., Serruys P.W., Leon M.B. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 10.Mishra P., Singh U., Pandey C.M., Mishra P., Pandey G. Application of student's t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019;22:407–411. doi: 10.4103/aca.ACA_94_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y., Huang Y. Modified wilcoxon-Mann-Whitney test and power against strong null. Am. Stat. 2019;73:43–49. doi: 10.1080/00031305.2017.1328375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nick T.G., Campbell K.M. Logistic regression. Methods Mol. Biol. 2007;404:273–301. doi: 10.1007/978-1-59745-530-5_14. [DOI] [PubMed] [Google Scholar]
- 13.Ganju J., Julie Ma G. The potential for increased power from combining P-values testing the same hypothesis. Stat. Methods Med. Res. 2017;26:64–74. doi: 10.1177/0962280214538016. [DOI] [PubMed] [Google Scholar]