Highlights
-
•
Bacillus spp. are attractive for use as in-feed probiotics due to their robustness.
-
•
High spore density cultivations above 1 × 1010 CFU. mL−1 can be achieved.
-
•
Bacillus probiotics can be formulated into a powdered product with 100 % recovery.
-
•
Bacillus probiotics are capable of survival and proliferation in the simulated GIT conditions.
-
•
Bacillus probiotics can be easily incorporated into feed preparations at high concentrations.
Keywords: Bacillus, Probiotics, Production, Formulation, Feed-incorporation, Shelf life
Abstract
Probiotics can be effective alternatives to the prophylactic use of antibiotic growth promoters (AGPs) in response to industry and consumer concerns around their use in poultry. Studies on the suitability of Bacillus probiotics are emerging and showing benefits, but information on the production technology is limited. We developed the production process for a novel probiotic product previously shown to be effective in field trials. All strains were cultivated to a spore concentration exceeding 1 × 1010 CFU. mL−1. The spores of each strain were harvested, processed into a powder intermediate and formulated into an end product with 100 % recoveries and a shelf life stability >1 year. The probiotic was shown to be incorporated into broiler feed exceeding the desired concentration of 1 × 106 CFU. g−1. Using efficient process technology and lower cost materials, this study presents a commercially relevant case for the potential adoption of probiotic products by the poultry industry.
1. Introduction
Probiotics used in animal rearing benefit animal health and vigour by improving digestion and absorption, reducing the pathogen load, regulating the immune system and generally improving the gastrointestinal tract (GIT) microbiome [[1], [2], [3], [4]]. These benefits have a positive effect on feed conversion efficiency, growth rate and carcass quality, which consequently improves production efficiencies [5]. Probiotics therefore provide a cost-effective alternative to the prophylactic use of antibiotic growth promoters (AGP), thus addressing industry and consumer needs.
We recently developed a multi-strain Bacillus probiotic product, which showed excellent performance in a broiler production trial [6]. However, to ensure the commercial relevance of a probiotic product, the efficiency its production process, which includes high spore concentrations, productivities and high recoveries during the harvesting process at large scale must be assessed [7,8]. For a multi-strain product, it is imperative that the fermentation process requires high cell density cultivation (HCDC) for each strain typically exceeding 1 × 1010 spores. mL−1 to minimise production cost and account for losses in the downstream processes of cell harvesting and product formulation [9]. The cell harvesting process must also be efficient in terms of throughput and recovery of viable spores and should generally be greater than 80 % [7,10].
Formulation technology for probiotics is normally proprietary to industry, but the main requirement is that the probiotic product must be stable in a powdered form and generally at a spore concentration of 1 × 109 spores. g−1 [[11], [12], [13]]. The powder formulation process includes mechanically intensive drying and mixing steps, which have potential detrimental effects on probiotic viability [14]. Therefore the stability of the probiotic product during formulation and under industry relevant storage conditions remains a key requisite for successful commercial application, and the shelf life of such a product should ideally be no less than 2 years [15,16].
Before a probiotic product can be included in poultry feed, it is pertinent to test its stability (viability and growth) under simulated GIT conditions, because the harsh conditions of this environment can negatively affect its optimum functionality [17,18]. It is thus important to demonstrate that a probiotic can retain viability within the GIT and grow to a minimum concentration of 1 × 106 CFU. g−1 [19].
Lastly, in-feed probiotics must retain maximum viability during feed manufacturing because this process involves high pressures (138–552 kPa), high temperatures (76.7–93.3 °C) and intensive mechanically sheer [15,20]. Excessive losses in this process reduce the probiotic effect resulting in a negative impact on the cost of inclusion to benefit ratio, desired by the poultry industry. Literature dictates that probiotics must be delivered to the point of consumption at a minimum cell concentration of 1 × 106 CFU. g−1 to elicit the desired probiotic effect [21,22].
Bacillus spp. have been proven to be robust and can be cultivated at high cell densities with excellent sporulation efficiency at industrial scale [9]. The spores of Bacillus spp. are resistant to environmental conditions such as extreme temperature, radiation, pH, pressure and toxic chemical agents, thus offering several advantages in process viability and stability [23]. Thus most Bacillus based animal feed probiotics are manufactured, packaged, stored and administered as spores [24]. This paper shows the successful production, harvesting, product formulation and in-feed stability of a multi-strain probiotic product for poultry and further verifies the viability and growth under simulated poultry GIT conditions. The findings of this study are amongst the few to comprehensively quantify the efficiencies in each of the process steps and therefore facilitates the commercial adoption of in-feed Bacillus based probiotic products.
2. Materials and method
2.1. Culture storage and maintenance
Four Bacillus subtilis (CPB 011, CPB 029, D 014 and HP 1.6) and two Bacillus velezensis (CPB 020 and CPB 035) strains were individually cryopreserved as described by Acosta [25] and stored in a -80 °C ultra-freezer (Forma™ 80,000 series, Thermo scientific, USA) [26].
2.2. Probiotic biomass production using fermentation
2.2.1. Inoculum development
A cryo-culture of each isolate was thawed to ambient temperature and separately inoculated into 2 L Fern Bach flasks containing 700 mL of sterile Tryptone soy broth (TSB). Flasks were incubated at 37 °C and 180 rpm on a rotary platform shaker (Innova 2300 series, New Brunswick Scientific, USA) until the culture reached the desired optical density (OD660nm between 4.0–5.0), measured using a spectrophotometer (Pharo 300 Spectroquant®, Merck, Germany). The purity of the culture was verified by quadrant streaking onto Tryptone soy agar (TSA) plates, which were incubated at 37 °C for 24 h (which was the standard incubation conditions for all plate cultures, before use as an inoculum) and then the colony viewed under light microscopy.
2.2.2. Batch fermentation of strains
The batch fermentation process was performed according to method outlined by Lalloo et al., [7]. A proprietary growth media comprising a typical mixture of vitamins, salts, a carbon and nitrogen source were sterilized in situ in 30 L bioreactors (Braun, Biostat, Sartorius BBI systems, Germany) at 121 °C for 45 min prior to inoculation. The working volume of the reactor was ∼ 24 L and data points for all process parameters were captured on-line using MFCS software (Sartorius BBI systems, Germany).
For the B. subtilis strains the temperature was set to 37 °C, the pH to 6.80, the pressure to 500 mBar and the dissolved oxygen was maintained at a minimum of 30 % saturation. Aeration and agitation were ramped up from 1 to 2 v.v−1. m−1 and from 200 to 800 rpm respectively, over a period of five hours. The pH was controlled using 10 % (v/v) H2SO4 or 25 % (v/v) NH4OH. For the B. velezensis strains, modified methods reported by Gao et al., [27] were used. The temperature was maintained at 37 °C, the pH at 7.00, pressure at 100 mBar and dissolved oxygen was maintained above 50 % saturation. The aeration was controlled at 1 v.v−1. m−1 and agitation between 300 and 500 rpm. The pH was controlled using 10 % (v/v) HCL or 25 % (v/v) NaOH. The fermentation of each isolate was ended when the sporulation efficiency (SE) and target spore concentration exceeded 80 % and 1.0 × 1010 spores. mL−1, respectively. Fermentation broth was harvested into sterile 25 L drums and stored at 4 °C to be further processed within 24 h.
2.2.3. Sampling and analysis
Fermentation samples (50 mL) were collected from the bioreactor at 3 -h intervals for analysis of mono-septic status and calculations of cell concentration (Eq. 1) and SE (Eq. 2), all using a microscope (Olympus BX40, Olympus, Japan). Cell and spore counts were determined using a counting chamber (Thoma®, Hawksley, UK). Mono-septic status was confirmed by standard streak plating on TSA.
| (1) |
| (2) |
The final viable spore concentrations for each strain at the end of fermentation were determined using a standard TSA plate count method and resultant colonies were enumerated using a colony counter (Bibby, Stuart scientific UK).
2.3. Cell harvesting post fermentation
The spores for each strain were harvested from the fermentation broth as outlined by Lalloo et al., [10]. Briefly, the broth was mixed for 1 h and centrifuged using a disk stack centrifuge (SA1, Westfalia, Germany) operated in continuous mode at 11 000 × g with a bowl pressure of 80 kPa and a de-sludge time of 2 min. The OD660nm of the flow through material was monitored and the feed flow rate appropriately adjusted to maintain the supernatant OD660nm below 3.0, which equated to less than 5% of the spore concentration of the starting material. The resultant spore concentrate at the end of a single pass was re-suspended into stabilization buffer and then washed two more times using the same method. The volumes of the feed, supernatant and spore concentrate fractions were measured and the associated spore concentrations used to determine total spores for each of the three passes through the centrifuge. Viable spore concentration was used to calculate total spores using Eq. 3 and subsequent recoveries using Eq. 4. Statistical analysis was done using a simple two tailed t-test using equal variances and the 95 % confidence level.
| (4) |
| (5) |
2.4. Formulation of the multi-strain probiotic product
The resultant spore concentrates of each strain harvested by centrifugation were processed into a dried product intermediate by mixing the liquid pellet with an inert clay (Biocentric Technologies, South Africa) using a spiral dough mixer (Esmach ISE 30, Labotec, South Africa). This material was subsequently convection dried for 20 h at 60 °C in an oven (United Scientific, South Africa) and then pin milled (IKA, Germany) into a fine powder (particle size of ∼200 μm). The final multi-strain probiotic powder containing all six strains was produced by blending (Lenton, South Africa) the respective mass of each powder intermediate with a calcium carbonate- dextrose carrier (99:1), to achieve a normalised concentration of 1 × 109 CFU. g−1 in the final product, verified by plate counts. Total viable spores, recoveries and statistical analysis was done, as described in Section 2.2.
2.5. Stability analysis of the probiotic product
The packaging method for the probiotic product was guided by an industry partners requirement. The final probiotic product was filled into 1 kg plastic bags (Low density polyethylene, 70 μM), sealed and then 25 bags were packaged into a double walled corrugated cardboard box which was sealed using paper gum tape and stored under ambient warehouse conditions (OptimusBio (Pty) Ltd, South Africa). At production and 12 months later, viable spore concentrations from 3 different sealed bags were determined.
2.6. Survival and growth of the multi-strain probiotic product in a simulated gastrointestinal (GIT) model system
The final probiotic powder product was tested under simulated GIT conditions using filter sterilised simulated gastric fluid (SGF) prepared according to Lian et al., [28], adjusted to a pH of 2.5 with 12 N HCl, and sequentially simulated intestinal fluid (SIF), adjusted to pH 6.8, prepared according to US Pharmacopeial [29].
Finely crushed commercial broiler feed (50 g) (AFGRI, South Africa) was added to the SGF (50 mL) at a ratio of 1:1 and the mixture homogenized for 5 min using a T18 homogeniser (Ultra Turrex, IKA, Germany). Water (100 mL) was added to the resultant mixture at a ratio of 2:1, which represents the fed state within the broiler stomach. The mixture (150 mL) was added to a 250 mL Erlenmeyer flask and the test probiotic powder (0.05 g) was added to the reaction mixture to simulate a starting cell concentration of ∼ 1 × 105 spores. mL−1 in the stomach. The reaction mixture was then incubated for 4 h at 42 °C on a platform shaker (Innova 2300 series, New Brunswick, Canada) with agitation of 50 rpm. It was subsequently transferred to the Erlenmeyer flask (500 mL) containing 300 mL of SIF adjusted to a pH of 6.8 and agitated at 100 rpm, for a further 12 h. Samples (2 mL) were withdrawn from the flask at time points 0, 2, 4, 6, 9 and 12 h for analyses of viability and morphological (spores or vegetative) state of cells. This experiment was performed in triplicate.
2.7. Survival of the multi-strain probiotic product through the feed manufacturing process
Broiler feeds for different growth phases were produced in eight ton batches, whereby the probiotic powder (1 kg/ton) was added to the feed ingredients of composition described by Ramlucken et al., [6]. The components were mixed for 30 min, extruded into pellets at temperatures ranging from 75 to 80 °C and then crumbled to different sizes depending on the feed type. A composite sample of each feed type including associated probiotic negative control feeds was treated by adding 1 g of crushed feed into 9 mL of sterile saline, and homogenised for 5 min. The homogenate was heat treated at 60 °C for 15 min in a water bath (Labotec, South Africa). Viable spore concentration was determined by the standard plate count method on TSA plates. The negative controls were used to correct for background microbial load from the feed ingredients.
3. Results
3.1. Probiotic biomass produced through batch fermentation
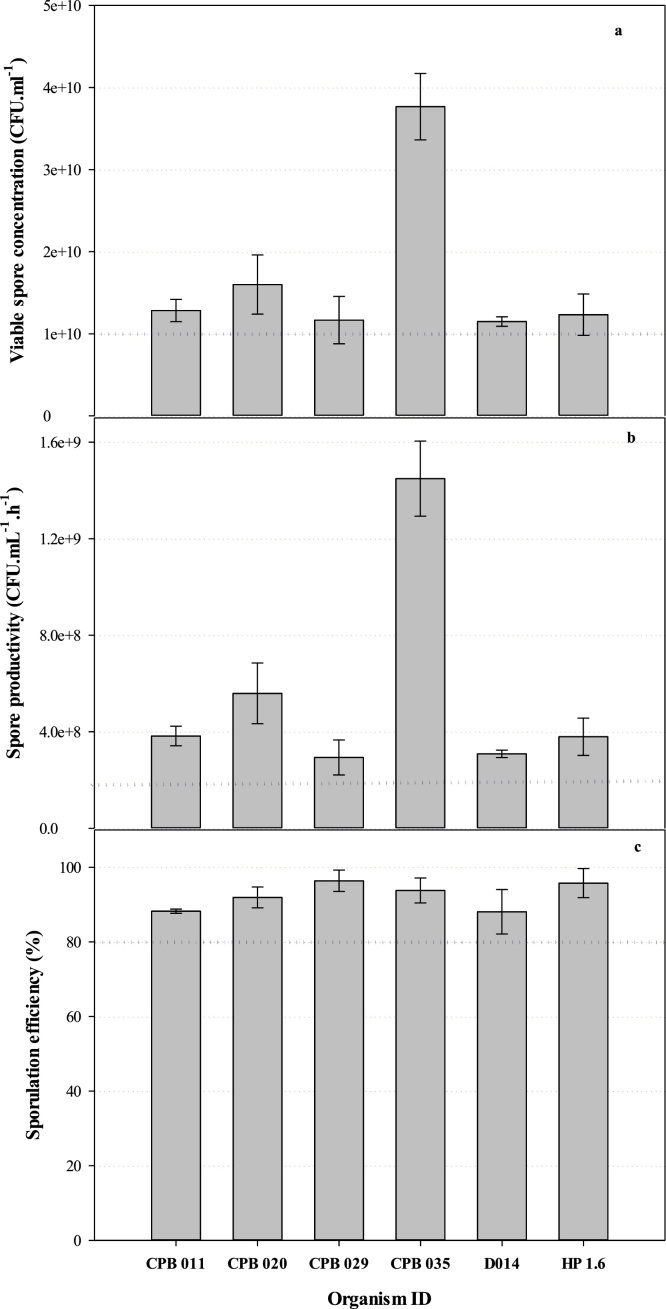
The overall fermentation performance of each strain is illustrated in Fig. 1. All the isolates exceeded the target viable spore concentration of 1 × 1010 CFU. mL−1 (based on commercial requirements) (Fig. 1a). In addition, the cell productivity of each isolate was greater than the target productivity of 1 × 108 CFU. mL−1 per hour (Fig. 1b). The target sporulation efficiency (> 80 %) was also exceeded for all six isolates produced (Fig. 1c).
Fig. 1.
Performance parameters of the Bacillus strains during fermentation; (a) viable spore concentration, (b) spore productivity (c) sporulation efficiency. The dotted line represents the target performance of each parameter. Error bars expressed as ± the standard deviation (n = 3).
3.2. Cell harvesting post fermentation
All strains except CPB 020 displayed total cell recoveries above 90 % through the cell harvesting process unit operation, which resulted in the lowest recovery (88 %) (Table 1). Nevertheless, this strain still exceeded the minimum threshold recovery of 80 %. There were no significant differences (p > 0.05) between the total spores into the process and the final spores recovered for all strains during the harvesting process.
Table 1.
Total viable spore count and recovery of each strain.
| Strain designation | Total spores in (CFU) | Total spores out (CFU) | p value | Recovery (%) |
|---|---|---|---|---|
| CPB 011 | 3.11 × 1014 | 3.11 × 1014 | 0.11 | 100 |
| CPB 020 | 3.40 × 1014 | 2.98 × 1014 | 0.26 | 88 |
| CPB 029 | 3.23 × 1014 | 3.19 × 1014 | 0.17 | 99 |
| CPB 035 | 9.14 × 1015 | 8.23 × 1015 | 0.49 | 90 |
| HP 1.6 | 3.40 × 1014 | 3.32 × 1014 | 0.12 | 98 |
| D014 | 2.79 × 1014 | 2.62 × 1014 | 0.17 | 94 |
Statistical analysis was done using a simple two tailed t-test using equal variances and the 95 % confidence level (n = 5).
3.3. Formulation of the product
3.3.1. Formulation of powder intermediates for each strain
No significant differences (p > 0.05) were observed between the total spores into and out for each of the intermediate powders produced (Table 2). Spore recoveries of all strains through the process exceeded 100 %.
Table 2.
Recovery of the viable spores of each strain during powder product intermediate process.
| Strain designation | Total spores in (CFU) | Total spores out (CFU) | p value | Recovery (%) |
|---|---|---|---|---|
| CPB 011 | 3.11 × 1014 | 3.26 × 1014 | 0.64 | 105 |
| CPB 020 | 2.98 × 1014 | 3.50 × 1014 | 0.17 | 118 |
| CPB 029 | 3.19 × 1014 | 3.40 × 1014 | 0.16 | 106 |
| CPB 035 | 8.23 × 1015 | 9.61 × 1015 | 0.99 | 117 |
| D 014 | 3.32 × 1014 | 3.39 × 1014 | 0.81 | 102 |
| HP 1.6 | 2.62 × 1014 | 3.13 × 1014 | 0.17 | 119 |
Statistical analysis was done using a simple two tailed t-test using equal variances and the 95 % confidence level (n = 5).
3.3.2. Blending of the powder intermediates of each strain into a final probiotic product
The final probiotic product containing the powder intermediates of all the six strains had a spore concentration of 1.45 × 109 CFU. g−1, which exceeded the minimum threshold target concentration of 1 × 109 CFU. g−1.
3.4. Stability of the multi-strain probiotic product
The probiotic product retained a cell concentration of 1.9 × 109 ± 8.12 × 108 CFU. g−1 after a period of 12 months post production, which was not significantly different (p > 0.05, n = 9) to the concentration of the fresh product which had a viable spore concentration of 1.45 × 109 CFU. g−1.
3.5. Survival of the multi-strain probiotic product in a simulated GIT model system
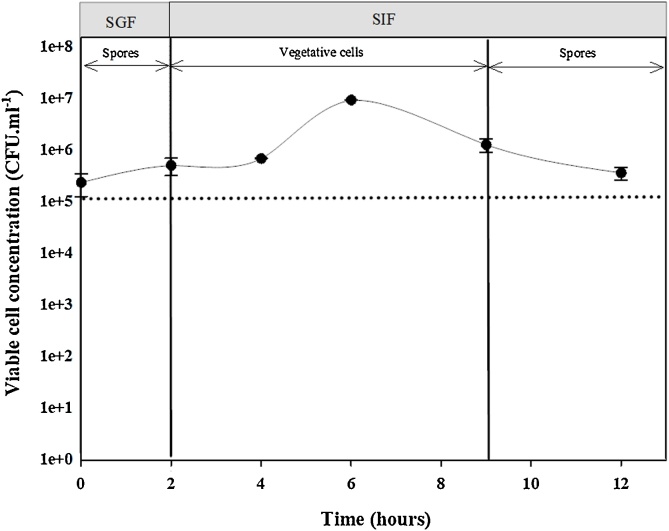
The initial cell concentration of the product at T0 was ∼1 × 105 CFU. mL−1 to simulate the approximate concentration present in the stomach of the chicken. Microscopic evaluation revealed that the probiotic remained dormant and in a spore state in the simulated gastric environment (0–2 hours), followed by rapid growth in the intestinal environment between 4 and 6 h (Fig. 2). In the initial stage of the intestine, the cells were confirmed to be actively vegetative, followed by a transition from vegetative cells back into spores (6–12 hours). The maximum viable cell concentration reached was ∼ 1 × 107 CFU. mL-1 resulting in an approximate 100-fold increase in viable cells from the initial probiotic dose.
Fig. 2.
The survivability of the probiotic product under simulated GIT conditions. Time period 1 to 2 h indicated simulated stomach conditions and 2 to 12 h indicated simulated intestinal conditions. Error bars represent the standard deviations (n = 5).
3.6. Survival of the multi-strain probiotic product through the feed manufacturing process
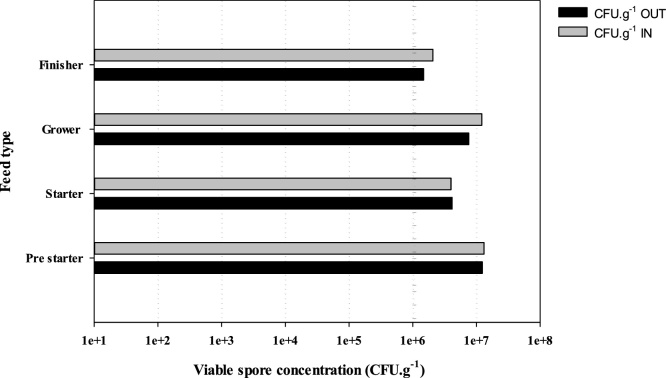
The initial and final viable spore count during the feed manufacturing process did not indicate any major loss in viability in any of the feed types produced. The resultant final viable spore concentration in all the feed produced exceeded the minimum threshold concentration of 1 × 106 CFU. g−1 (Fig. 3).
Fig. 3.
The viable spore concentration (n = 5) of the probiotic product during the manufacturing of different feed types (pre starter, starter, grower and finisher). The dotted line represents the target cell concentration (1 × 106 CFU. g−1) of the probiotic in the feed.
4. Discussion
4.1. Production of the probiotic strains in fermentation
The six-strains used in the product were selected based on rigorous screening against probiotic criteria of relevance to the broiler industry. Six strains were chosen based on holistic performance for a multi-mode product which was described comprehensively in our previous study [30].
The spore concentration, productivity and SE for each individual strain achieved at the end of the fermentation process exceeded the targeted minimum threshold of 1 × 1010 CFU. mL−1, 2.5 × 108 CFU. mL−1. h−1 and 80 % respectively (Fig. 1). The performance thresholds were based on previously reported production of Bacillus spp. [7,9], together with consideration of the techno-economic targets for acceptance by the poultry industry.
High spore concentrations and productivities are important as they impact on the cost of production, capital utilization efficiency and furthermore, accommodates for any losses in the subsequent downstream processes such as cell harvesting, probiotic product formulation and feed production. Strain CPB 035 resulted in the highest spore concentration and productivity, followed by CPB 020 (both B. velezensis strains), in contrast to the other four strains which were all B. subtilis, due to slower growth (longer time to end of exponential vegetative growth) of the latter species [31].
It is important to achieve high SE in the fermentation process as the spore-form is the desired state regarding product stability and survival through the GIT. All strains in our study exceeded 80 % SE (Fig. 1c) under production conditions, indicating minimum loss of viability in the transition phase from vegetative cells to spores [32]. We were able to achieve high production performance in a batch process, which is simpler and less capital intensive than fed-batch processes typically used to attain such high cell densities [33]. All strains were successfully produced under intensive industry conditions which influences the uptake of the technology [34,35], especially in the poultry sector, which operates at a high volume and low cost margin. Furthermore, our batch process was able to achieve this performance using lower cost media, thus increasing its attractiveness for industry adoption [36].
Information regarding the production processes for Bacillus spp. as poultry probiotics is limited, however the production performance achieved in our study correlates well with other reports of high density cultivation of Bacillus spp. [7,9,37].
4.2. Cell harvesting post fermentation
There were no significant differences (p > 0.05) between the total spores into the centrifugation process and the total spores recovered in the spore concentrate, for any of the strains. Furthermore, spore recovery exceeded 80 % for all strains (Table 1). Although cell harvesting by continuous flow centrifugation is the most preferred method by industry [38], there is still a degree of physical stress, such as pressure and mechanical sheer exerted on the probiotic bacteria, due to the centrifugal force and pressure drop during desludging of the spore concentrate. These can have a negative effect on viability [39]. However, the results obtained during this study shows the robustness of the Bacillus spores as loss in viability was minimal. The high recovery of Bacillus spp. spores could be attributed for its ability to withstand pressures up to ∼50 mPa [40] and sheer stress up to 500 Pa [41] A similarly high recovery of B. cereus spores was previously demonstrated by Lalloo et al., [10].
Downstream product processing is a key factor to consider during the production of biological products and it is essential to ensure that recovery steps are developed to minimize losses and process time [38]. The efficient harvesting of cells has a major effect on economic feasibility of implementation of probiotic products [42]. With regards to production of probiotics for poultry production, there is limited information available on their survivability during the cell harvesting process but our study shows that this can be done successfully.
4.3. Formulation of the product
Each of the strains that were processed from the liquid spore concentrates into powder intermediates, resulted in no significant differences (p > 0.05) between the total spores added and the total spores in the dry powder product intermediates (Table 2). This is attributable to the robustness of Bacillus spores, which offers protection from the heat and mechanical shear, conferred by the spore exosporium and small acid-soluble DNA binding proteins, which are characteristic of this genus [10,43]. The retained viability of spores during the powder intermediate manufacturing process lead to a viable spore recovery marginally exceeding 100 % for each strain as the recovery was calculated on an as is basis (Table 2).
The final blended probiotic product was produced by incorporating the normalised concentration corrected mass of each of the individual probiotics powder intermediates to achieve a theoretical target of 1.0 × 109 CFU. g−1, which was based on input from the feed and premix industry as well as specifications from other commercial probiotic products [11,13,44]. The final viable spore concentration achieved in our probiotic product was 1.45 × 109 CFU. g−1, which correlated well with the theoretical target concentration. A slightly higher final product spore concentration is desirable to circumvent the losses that occur during storage and eventual incorporation into the feed manufacturing process [42]. Due to the scale at which our demonstration studies were conducted, the use of the preferred large scale industrial processes was not feasible.
A powder form of poultry probiotics is preferred by end users as it has advantages such as uniformity, stability, longer shelf life, ease of packaging and transportation, and easy incorporation into current feed manufacturing processes [10,45,46]. In our study, inert clay was used as a carrier to covert each individual probiotic liquid into a powder intermediate. This carrier has no deleterious effects on chickens when consumed, and in fact, it has been shown to have beneficial effects against gastrointestinal disorders [47]. The final product was formulated using calcium carbonate and dextrose as carriers. All ingredients are food grade and typically used in other commercial probiotic products. Sugars such as dextrose have been known to be an effective protectant against oxidation damage [48] whilst calcium carbonate helps the probiotic bacteria adhere to the intestinal wall and assists in lowering the acid content, thus increasing probiotic activity in the GIT [49,50].
The formulation method developed in this study involved lower drying temperatures (∼60 °C) and low sheer blending, which preserved viability loss, in contrast to the higher losses using spray drying which causes more severe heat stress and dehydration [14,51]. The high temperatures used in spray drying result in high mortality and inactivation of microorganisms, thus damaging cytoplasmatic membranes, cell walls, ribosomes and DNA [52,53], which can also occur even with heat resistant Bacilli spores [54]. The use of our simple processing technique circumvents the conventional challenges associated with more costly carriers, protective agents and capital. There is limited information regarding the formulation of feed probiotic products as this is usually proprietary to industry, thus our findings contribute to this knowledge gap.
4.4. Stability of the multi-strain probiotic product
A shelf life study of the six-strain probiotic product, stored for 12 months under warehouse conditions, advised by an industry partner, did not result in any measurable decline in spore concentration. However, for wider scale commercial supply, alternative means of packaging such as 25 kg paper bags and 1-ton bulk bags would be more appropriate. The change in spore concentration between the initial and final samples showed no significant difference (1.45 × 109 vs 1.9 × 109 CFU. mL−1, p > 0.05, n = 9) indicating the stability of the spore product. These results show that since the probiotic product was stored at ambient temperature, it mitigates the use of storage at low temperatures which adds cost to the logistics [24]. One of the technological requisites for feed probiotics, and a serious limiting factor of most conventional probiotics [16], is the ability to be stored under warehouse conditions [46]. Since our study mimicked typical industrial storage conditions, the stability of the probiotic product was verified and we could predict a shelf life of up to 5 years, using accelerated stability kinetics for Bacillus spp. (data not shown). It has been reported that free spores have a lower shelf life [55], but our product formulation approach successfully addressed shelf life constraints. Furthermore Mizak et al. [56], stated that probiotics in feed and premixes must be active for at least 4 months, a criteria that our probiotic has substantially exceeded.
4.5. Survival of the multi-strain probiotic product in a simulated GIT model system
The novel multi-strain probiotic product (in a spore state) was introduced to the simulated GIT model at a concertation of 1 × 10−5 CFU.mL-1 to simulate the actual concentration of the probiotic when it reaches the stomach (Fig. 2). The probiotic did not grow within the simulated stomach conditions (SGF), mainly due to low pH, but the spores retained full viability. When the spores were transferred into simulated intestinal conditions (SIF), the spores germinated at high efficiency into vegetative cells, which replicated rapidly and resulted in a ∼2 log increase (Fig. 2). The germination of spores could be attributable to both the favourable conditions of the SIF and the rich nutrients from the feed.
The simulated gastric study was designed to mimic the environment of the chicken GIT as closely as possible. The first phase of the experiment was meant to simulate the fed state of the stomach conditions at low pH. Mean retention time in the stomach has been estimated to vary between half an hour and an hour [57], and the natural pH of the stomach is approximately 2−3. However, in a fed state the pH varies between pH 3 and 4.5 [57]. For the purposes of the study we decided to test the extreme pH as well as the longest retention time, to subject the probiotic to the most extreme conditions. The second phase of the study was meant to mimic the chicken intestine which has a much more neutral pH, thereby more favourable, but consists of the digestive enzymes, trypsin and pepsin, which could potentially affect the proliferation of the probiotic. Furthermore, Svihus [57] reported that with variances in feed makeup, age, sex and environmental conditions, the retention time of feed in the gut could increase up to 24 h (especially in the ceca). In an attempt to accommodate the extreme situations, we opted for a 24 h total retention time. However, there was no change in the viability trend (data not shown), therefore we reported on a 12 h retention time. These results obtained from this study infer that the probiotic product could survive the stomach conditions within the host and grow in the intestine which is where probiotic needs to be metabolically active to elicit its beneficial effect [18,58].
Two hours after peak growth, the vegetative cells progressively reverted into the spore state, possibly due to nutrient depletion since the SIF was only dosed once with feed. The trend also showed a loss in viability, which is typical when vegetative cells transition into sporulation. Additional factors that could have contributed to decrease in viability include the antibacterial effect of bile salts or the enzymes (trypsin and pepsin) present in the SIF [59,60]. The spore viability however did not decline to lower than the initial dosage concentration of 1 × 105 CFU. mL−1 (Fig. 2). The results obtained in this study are consistent with the gastrointestinal life cycle of Bacillus as reported by Bernardeau et al., [18]. This suggests that the probiotic needs to be continuously administered within the feed to confer maximum benefit [61].
4.6. The survival of the multi-mode probiotic product through the feed manufacturing process
The probiotic was successfully incorporated into the different types of feed, and results show that all feeds exceeded the target cell concentration of 1 × 106 CFU. g−1 (Fig. 3). This target is based on literature which states that in order to be effective, probiotics must be delivered to the point of consumption at a minimum cell concentration of 1 × 106 CFU. g−1 [21,22] but higher dosages normally result in excessive costs of inclusion [62]. Furthermore, spore recovery in the feed manufacturing process exceeded 70 %, indicating the survivability of Bacillus spp., attributable to the thermostability, sheer and pressure resistance of spores [63]. Because of the intensive physical stress involved in poultry feed manufacturing, some degree of loss is inevitable, as shown in our data where there was a less than 30 % loss, but we were still able to meet the minimum threshold of 1 × 106 CFU. g−1 [15,45,64]. The feed manufacturing process is a critical limiting factor for most conventional in-feed probiotics, thus hampering implementation in the poultry industry, but out results show potential for the feasible inclusion of Bacillus based probiotics in feed as was also previously reported by Simon et al., [65].
5. Conclusion
The results of this study endorse the use of Bacillus based in-feed probiotics as it confirms that all strains could be produced at high efficiency, were stable with minimal losses during the various processing steps and were successfully incorporated into various types of broiler feed. The study furthermore concluded the survival and growth of the strains under GIT simulated conditions. In a separate broiler field study the actual functionality of the probiotic was verified, showing improved feed efficiency, weight gain and gut morphology [6]. We further demonstrated excellent product stability under industry relevant storage conditions, therefore comprehensively showing the suitability for commercial adoption by the poultry industry, of this novel multi-strain probiotic product.
CRediT authorship contribution statement
Uraisha Ramlucken: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing - original draft. Santosh O. Ramchuran: Conceptualization, Methodology, Project administration, Resources, Visualization, Writing - review & editing. Ghaneshree Moonsamy: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing - original draft. Christine Jansen van Rensburg: Project administration, Supervision, Writing - review & editing. Mapitsi S. Thantsha: Conceptualization, Project administration, Supervision, Writing - review & editing. Rajesh Lalloo: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors thank the Department of Science and Technology (South Africa) and the Council for Scientific and Industrial Research (CSIR) for financial support, OptimusBio (PTY) Ltd for assistance with the probiotic production and SimpleGrow Agricultural services for the production of the poultry feeds.
References
- 1.Chapman C., Gibson G.R., Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur. J. Nutr. 2011;v50:1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 2.Fijan S. InTech; 2016. Antimicrobial Effect of Probiotics against Common Pathogens in Probiotics and Prebiotics in Human Nutrition and Health. [Google Scholar]
- 3.Chaucheyras-Durand F., Durand H. Probiotics in animal nutrition and health. Benef. Microbes. 2009;1:3–9. doi: 10.3920/BM2008.1002. [DOI] [PubMed] [Google Scholar]
- 4.Grant A.Q., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- 5.Bajagai Y.S., Klieve A.V., Dart P.J., Bryden W.L. FAO; 2016. Probiotics in Animal Nutrition: Production, Impact and Regulation. [Google Scholar]
- 6.Ramlucken U., Ramchuran S.O. A novel Bacillus based multi-strain probiotic improves growth performance and intestinal properties of Clostridium perfringens challenged broilers. Poult. Sci. 2019 doi: 10.3382/ps/pez496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalloo R., Maharajh D., Görgens J., Gardiner N., Görgens J. High-density spore production of a B. Cereus aquaculture biological agent by nutrient supplementation. Appl. Microbiol. Biotechnol. 2009;83:59–66. doi: 10.1007/s00253-008-1845-z. [DOI] [PubMed] [Google Scholar]
- 8.Lacroix C., Yildirim S. Fermentation technologies for the production of probiotics with high viability and functionality. Curr. Opin. Biotechnol. 2007;18:176–183. doi: 10.1016/j.copbio.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro S.M., Clemente J.J. A procedure for high‐yield spore production by Bacillus s ubtilis. Biotechnol. Progr. 2005;21:1026–1031. doi: 10.1021/bp050062z. [DOI] [PubMed] [Google Scholar]
- 10.Lalloo R., Maharajh D., Görgens J., Gardiner N. A downstream process for production of a viable and stable Bacillus cereus aquaculture biological agent. Appl. Microbiol. Biotechnol. 2010;86:499–508. doi: 10.1007/s00253-009-2294-z. [DOI] [PubMed] [Google Scholar]
- 11.Teo A., Tan H.-M. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT) J. Appl. Poult. Res. 2007;16:296–303. [Google Scholar]
- 12.Kim H.-W., Miller D.K. Probiotic supplementation and fast freezing to improve quality attributes and oxidation stability of frozen chicken breast muscle. LWT. 2017;75:34–41. [Google Scholar]
- 13.Jeong J., Kim I. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- 14.Chávez B., Ledeboer A. Drying of probiotics: optimization of formulation and process to enhance storage survival. Drying Technol. 2007;25:1193–1201. [Google Scholar]
- 15.Kosin B., Rakshit S.K. Microbial and processing criteria for production of probiotics: a review. Food Technol. Biotechnol. 2006;44:371–379. [Google Scholar]
- 16.Mattila-Sandholm T., Myllärinen P. Technological challenges for future probiotic foods. Int. Dairy J. 2002;12:173–182. [Google Scholar]
- 17.Reuter G. Probiotics--possibilities and limitations of their application in food, animal feed, and in pharmaceutical preparations for men and animals. Berl. Munch. Tierarztl. Wochenschr. 2001;114:410–419. [PubMed] [Google Scholar]
- 18.Bernardeau M., Lehtinen M., Forssten S., Nurminen P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017;54:2570–2584. doi: 10.1007/s13197-017-2688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millette M., Nguyen A., Amine K.M., Lacroix M. Gastrointestinal survival of bacteria in commercial probiotic products. Int. J. Probiotics Prebiotics. 2013;8:149. [Google Scholar]
- 20.Cutlip S.E., Hott J. The effect of steam-conditioning practices on pellet quality and growing broiler nutritional value. J. Appl. Poult. Res. 2008;17:249–261. [Google Scholar]
- 21.Tripathi M.K., Giri S.K. Probiotic functional foods: survival of probiotics during processing and storage. J. Funct. Foods. 2014;9:225–241. [Google Scholar]
- 22.Haffner F.B., Diab R., Pasc A. Encapsulation of probiotics: insights into academic and industrial approaches. Aims Mater. Sci. 2016;3:114–136. [Google Scholar]
- 23.Abel-Santos E. Elsevier; 2015. Endospores, Sporulation and Germination in Molecular Medical Microbiology; pp. 163–178. [Google Scholar]
- 24.Vasquez A. Bacillus species are superior probiotic feed-additives for poultry. J Bacteriol Mycol Open Access. 2016;2:00023. [Google Scholar]
- 25.Acosta A. Study of the stability in real time of cryopreserved strain banks. Univ. Sci. 2004;9:35–42. [Google Scholar]
- 26.Monroy A.F., Mercado M., Poutou R.A., Rodríguez P., Pedroza A.M. Study of the stability in real time of cryopreserved strain banks. Univ. Sci. 2004;9:35–42. [Google Scholar]
- 27.Gao W., Kim H.-J., Chung C.-H., Lee J.-W. Enhanced production of carboxymethylcellulase by a marine bacterium, Bacillus velezensis A-68, by using rice hulls in pilot-scale bioreactor under optimized conditions for dissolved oxygen. J. Microbiol. 2014;52:755–761. doi: 10.1007/s12275-014-4156-3. [DOI] [PubMed] [Google Scholar]
- 28.Lian W.-C., Hsiao H.-C., Chou C.-C. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int. J. Food Microbiol. 2003;86:293–301. doi: 10.1016/s0168-1605(02)00563-9. [DOI] [PubMed] [Google Scholar]
- 29.U.S.P. Convention. United States Phamacopeia. 2005.
- 30.Ramlucken U., Roets Y. Isolation, selection and evaluation of Bacillus spp. As potential multi-mode probiotics for poultry. J. Gen. Appl. Microbiol. 2019 doi: 10.2323/jgam.2019.11.002. 11. 002. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Sueoka N. Elsevier; 1997. Cell Membrane and Chromosome Replication in Bacillus subtilis in Progress in Nucleic Acid Research and Molecular Biology; pp. 35–53. [DOI] [PubMed] [Google Scholar]
- 32.Posada-Uribe L.F., Romero-Tabarez M., Villegas-Escobar V. Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst. Eng. 2015;38:1879–1888. doi: 10.1007/s00449-015-1428-1. [DOI] [PubMed] [Google Scholar]
- 33.Elisashvili V., Kachlishvili E., Chikindas M.L. Recent advances in the physiology of spore formation for Bacillus Probiotic production. Probiotics Antimicrob. Proteins. 2019;11:731–747. doi: 10.1007/s12602-018-9492-x. [DOI] [PubMed] [Google Scholar]
- 34.Amer G., Utkhede R. Development of formulations of biological agents for management of root rot of lettuce and cucumber. Can. J. Microbiol. 2000;46:809–816. doi: 10.1139/w00-063. [DOI] [PubMed] [Google Scholar]
- 35.Keller K., Friedmann T., Boxman A. The bioseparation needs for tomorrow. Trends Biotechnol. 2001;19:438–441. doi: 10.1016/s0167-7799(01)01803-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh V., Haque S. Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 2017;7:2087. doi: 10.3389/fmicb.2016.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z.-M., Li Q. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl. Microbiol. Biotechnol. 2010;85:1353–1360. doi: 10.1007/s00253-009-2162-x. [DOI] [PubMed] [Google Scholar]
- 38.Moodley G., Mashigo L., Lalloo R., Singh S. IntechOpen; 2014. Application of Biological Agents in Abalone Aquaculture: a South African Perspective in Sustainable Aquaculture Techniques. [Google Scholar]
- 39.Lange H., Taillandier P., Riba J.P. Effect of high shear stress on microbial viability. J. Chem. Technol. Biotechnol. Int. Res. process, Environ. Clean Technol. 2001;76:501–505. [Google Scholar]
- 40.Aoyama Y., Shigeta Y., Okazaki T., Hagura Y., Suzuki K. Non-thermal inactivation of Bacillus spores by pressure-holding. Food Sci. Technol. Res. 2005;11:324–327. [Google Scholar]
- 41.Faille C., Benezech T. Role of mechanical vs. chemical action in the removal of adherent Bacillus spores during CIP procedures. Food Microbiol. 2013;33:149–157. doi: 10.1016/j.fm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Brar S.K., Verma M., Tyagi R., Valéro J. Recent advances in downstream processing and formulations of Bacillus thuringiensis based biopesticides. Process. Biochem. 2006;41:323–342. [Google Scholar]
- 43.Setlow B., Setlow P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl. Environ. Microbiol. 1995;61:2787–2790. doi: 10.1128/aem.61.7.2787-2790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y., Xu S. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12:e0182426. doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhat A.R., Irorere V.U. Bacillus subtilis natto: a non-toxic source of poly-γ-glutamic acid that could be used as a cryoprotectant for probiotic bacteria. AMB Express. 2013;3:36. doi: 10.1186/2191-0855-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markowiak P., Śliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slamova R., Trckova M., Vondruskova H., Zraly Z., Pavlik I. Clay minerals in animal nutrition. Appl. Clay Sci. 2011;51:395–398. [Google Scholar]
- 48.Schutyser M.A., Perdana J., Boom R.M. Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci. Technol. 2012;27:73–82. [Google Scholar]
- 49.Zhao X., Zhang Y., Li D. Elimination of acidic or oxidative stress for four probiotics with some chemicals in vitro. Afr. J. Microbiol. Res. 2009;3:353–357. [Google Scholar]
- 50.Trivedi M.K., Tallapragada R.M. Physicochemical characterization of biofield energy treated calcium carbonate powder. Am. J. Health Res. 2015;3:368–375. [Google Scholar]
- 51.Yadav A., Chaudhari A., Kothari R. 2009. Enhanced Viability of Bacillus coagulans After Spray Drying with Calcium Lactate, Storage and Re-hydration. [Google Scholar]
- 52.Chávarri M., Marañón I., Villarán M.C. IntechOpen; 2012. Encapsulation Technology to Protect Probiotic Bacteria in Probiotics. [Google Scholar]
- 53.Dong Q.Y., Chen M.Y. Alginate‐based and protein‐based materials for probiotics encapsulation: a review. Int. J. Food Sci. Tech. 2013;48:1339–1351. [Google Scholar]
- 54.Zhou X., Dong J., Gao J., Yu Z. Activity-loss characteristics of spores of Bacillus thuringiensis during spray drying. Food Bioprod. Process. 2008;86:37–42. [Google Scholar]
- 55.Sorokulova I.B., Pinchuk I.V. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008;53:954–963. doi: 10.1007/s10620-007-9959-1. [DOI] [PubMed] [Google Scholar]
- 56.Mizak L., Gryko R., Kwiatek M., Parasion S. Probiotyki w żywieniu zwierząt. Życie Weterynaryjne. 2012;87:736–741. [Google Scholar]
- 57.Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- 58.Clavel T., Carlin F., Lairon D., Nguyen‐The C., Schmitt P. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J. Appl. Microbiol. 2004;97:214–219. doi: 10.1111/j.1365-2672.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- 59.Tam N.K., Uyen N.Q. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006;188:2692–2700. doi: 10.1128/JB.188.7.2692-2700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Begley M., Gahan C.G., Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Latorre J.D., Hernandez-Velasco X. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014;93:1793–1800. doi: 10.3382/ps.2013-03809. [DOI] [PubMed] [Google Scholar]
- 62.Gomes A.M., Malcata F.X. Bifidobacterium spp. And Lactobacillus acidophilus: biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999;10:139–157. [Google Scholar]
- 63.Cutting S.M. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Bora P., Puri V., BANSAL A. Physicochemical properties and excipient compatibility studies of probiotic Bacillus coagulans spores. Sci. Pharm. 2009;77:625–638. [Google Scholar]
- 65.Simon O., Vahjen W., Scharek L. Micro-organisms as feed additives-probiotics. Adv. pork Production. 2005;16:161. [Google Scholar]