Abstract
This paper describes the spontaneous ovarian choriocarcinoma observed in a young female Crl:CD1 (ICR) mouse. The mouse was sacrificed at 8 weeks of age after oral administration of a compound for 2 weeks. The left ovary was found to be cystically enlarged with dark red hemorrhaging. The cystic mass contained abundant blood plasma and erythrocytes. At the peripheral regions of the mass, large pleomorphic tumor cells with bizarre shaped nuclei were detected. Tumor cells contained a single large nucleus and abundant eosinophilic to amphophilic cytoplasm. Histopathology of the tumor cells resembled that of trophoblastic giant cells. Therefore, the observed ovarian lesion was diagnosed as a choriocarcinoma. No microscopic lesions were observed in the right ovary or other reproductive organs. Ovarian choriocarcinoma was considered to be of non-gestational origin. This is the first report of ovarian choriocarcinoma in a young ICR mouse.
Keywords: ovarian choriocarcinoma, non-gestational origin, germ cell tumor, CrlCD1 (ICR) mouse, spontaneous lesion
Choriocarcinoma is a rare type of germ cell tumor found in organs of the reproductive system, such as the ovaries, uterus, and testes of animals and humans1, 2, 3, 4, 5, 6, 7, 8, 9. Eleven cases of spontaneous ovarian choriocarcinoma have been reported in B6C3F1 mice5, 6, 7. In all cases, tumors were observed in aged animals, except for one case observed in an 8-week-old B6C3F1 mouse. In this report, we describe a case of spontaneous ovarian choriocarcinoma in a young Crl:CD1 (ICR) mouse.
A female (ICR) mouse was purchased from Charles River Japan Inc. (Atsugi, Japan) at 5 weeks of age. The mouse was housed under controlled conditions (12-h light/dark cycle, temperature of 23 ± 3°C, relative humidity of 50 ± 20%) and permitted ad libitum access to food (autoclaved CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan), and tap water.
The mouse was orally administered a compound for 2 weeks according to the protocol of the toxicity study in which it was a test subject. At the end of the dosing period, blood samples were collected, and the mouse was euthanized by exsanguination via the abdominal aorta and post vena cava under deep isoflurane anesthesia at 8 weeks of age. Subsequently, necropsy and blood chemical examinations were performed. To perform histopathological examination, all organs were fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin sections (3–5 µm thick) were stained with hematoxylin and eosin. This study was approved by the Institutional Animal Care and Use Committee of Mitsubishi Tanabe Pharma Corporation, and all animal experiments were conducted in accordance with the guidelines of the Animal Care and Use of our division.
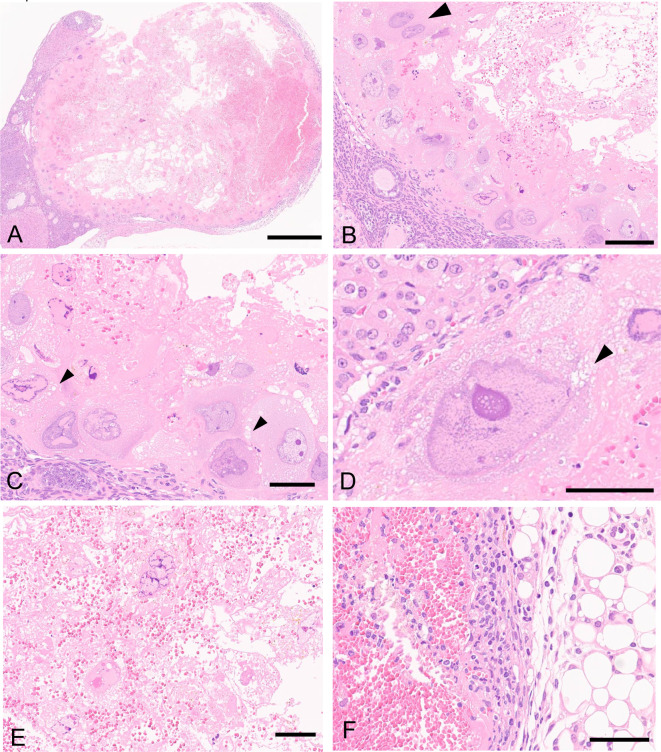
Macroscopically, an enlarged cystic mass was observed in the left ovary. The mass was 3 mm in diameter, dark red, and hemorrhagic. No macroscopic lesions were observed in the right ovary or other organs. Microscopically, the cystic mass contained abundant blood plasma and erythrocytes (Fig. 1A and B). Large pleomorphic tumor cells with bizarre shaped atypically shaped nuclei were arranged predominantly along the periphery of the mass, and binucleated cells were rarely observed (Fig. 1B). The tumor cells possessed abundant eosinophilic to amphophilic cytoplasm, which was occasionally found to be vacuolated in some cells. Additionally, each tumor cell possessed a single large nucleus (approximately 40–80 μm in diameter) containing a few clear nucleoli, with dark-stained chromatin occasionally observed near the nuclear membrane (Fig. 1C and D). Frequently degenerative or necrotic tumor cells were located in the center of the mass with abundant erythrocytes (Fig. 1E). No mitotic figures were observed in the tumor cells. These features were consistent with those of trophoblastic giant cells. The mass was well demarcated from normal ovarian tissues and peripheral adipose tissues, and no tumor cell metastasis was observed in other organs or tissues. Inflammatory cells infiltrated the edge of the mass and the peripheral adipose tissue (Fig. 1F).
Fig. 1.
Histopathology the ovary of a young female Crl:CD1 (ICR) mouse stained with hematoxylin and eosin. (A) A cystic mass containing abundant blood plasma and erythrocytes. The mass is well defined from normal ovarian tissues and peripheral adipose tissues. Bar = 500 μm. (B) Large pleomorphic tumor cells with bizarre shaped abnormally shaped nuclei are arranged at the edges of the mass. Note: binucleated cell (arrowhead), blood plasma, and erythrocytes are observed. Bar = 100 μm. (C, D) The tumor cells possess abundant eosinophilic to amphophilic cytoplasm, which is occasionally vacuolated (arrowheads). The tumor cells contain a single large nucleus, and dark-stained chromatin is occasionally observed near the nuclear membrane. Bar = 50 μm. (E) Tumor cells located in the center of the mass are frequently degenerative or necrotic. Bar = 50 μm. (F) Inflammatory cells have infiltrated the edge of the mass and in the peripheral adipose tissue. Bar = 50 μm.
Based on the above histological features, the ovarian lesion was diagnosed as a choriocarcinoma. The tumor was considered to be a spontaneous lesion, as the duration of treatment with the test compound was short (2 weeks). In addition, no other preneoplastic or proliferative lesions were observed in the ovaries of other mice treated with the compound.
Ovarian choriocarcinoma is one of the rarest tumors in the ovary in humans1, 2 and animals3, 4, 5, 6, 7, 8, 9. In mice, the incidence of choriocarcinoma in the ovary is less than 0.02%, according to a report from the National Cancer Institute (NCI) and National Toxicology Program (NTP) Carcinogenesis Testing Programs in B6C3F1 mice4, 5.
In humans, the development of ovarian choriocarcinoma can be divided into the following three types: gestational choriocarcinoma associated with ovarian pregnancy; metastatic gestational choriocarcinoma spread from other parts of the genital tract, predominantly the uterus; and non-gestational choriocarcinoma, which could arise from trophoblastic differentiation of germ cells2. The occurrence of pure ovarian choriocarcinoma of non-gestational origin in humans is exceedingly rare compared with that of gestational origin. Pure ovarian choriocarcinoma of non-gestational origin is highly malignant, and frequently metastatic compared to that of gestational origin1, 10. Additionally, tumor cells in choriocarcinoma of non-gestational origin are associated with those of hemorrhage and necrosis2. In animals, ovarian choriocarcinoma of non-gestational origin has been reported in mice, rats, and cynomolgus monkeys4, 5, 6, 7, 8. In the present study, the affected female mouse had no contact with a male mouse, and no prior pregnancies. No macroscopic lesions were observed in the right ovary or other reproductive organs, such as the uterus and vagina. Therefore, this case of ovarian choriocarcinoma was considered to be of non-gestational origin.
Macroscopically, choriocarcinoma is a necrotic and hemorrhagic mass in humans. In mice, Alison et al. reported that choriocarcinoma is often a dark red, hemorrhagic cyst, and the cystic component is observed in 65–95% of the area of each mass. The macroscopic features in this case were consistent with those of this previous report.
Microscopically, choriocarcinoma is composed of different types of trophoblasts, which are a component of the placenta11. In humans, cytotrophoblasts and syncytiotrophoblasts must be present for a definitive diagnosis of choriocarcinoma1. In mice, choriocarcinoma is generally composed of the following three types of trophoblastic tumor cells: cytotrophoblasts, trophoblastic giant cells, and syncytiotrophoblasts. However, Alison et al. reported eight cases of ovarian choriocarcinoma in B6C3F1 mice; five cases showed only trophoblastic giant cells, while the other three cases contained all three types of trophoblastic cells5. Additionally, in another report of three cases of choriocarcinoma in B6C3F1 mice, only trophoblastic giant cells were detected6, 7. Similarly, only trophoblastic giant cells were detected in the tumor in the present study. Furthermore, no mitotic figures were observed in this case, which could be correlated with the characteristics of trophoblastic giant cells that exhibit polyploid DNA without intervening mitoses12. Additionally, binucleated tumor cells were rarely observed in the mass, which is similar to previous cases5, 6, 7. Moreover, these were considered as syncytiotrophoblasts based on the nuclei diameter of 10–20 μm in previous studies5, 7. However, nuclei of binucleated cells in the present study were 40–50 μm in diameter, and the histopathological features these cells not consistent with the previous case.
In the present study, the large pleomorphic cells could not be assessed immunohistologically due to sample restrictions. However, histological features of large pleomorphic cells suggested that they were trophoblastic giant cells. Previous reports in 8-week-old B6C3F1 mice have shown that trophoblastic giant cells in choriocarcinoma are positive for human placental lactogen (HPL), and weakly positive for human chorionic gonadotropin (hCG) or pregnancy-specific beta-1-glycoprotein (SP-1)6. In particular, syncytrophoblastic cells were positive for hCG5.
In conclusion, we described a case of ovarian choriocarcinoma occurring in a young ICR mouse. Choriocarcinoma is rarely observed in young mice, and, to the best of our knowledge, this is the first report of choriocarcinoma in young ICR mice.
Disclosure of Potential Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to Dr. Satoshi Furukawa for his kind advice.
References
- 1.Axe SR, Klein VR, and Woodruff JD. Choriocarcinoma of the ovary. Obstet Gynecol. 66: 111–114. 1985. [PubMed] [Google Scholar]
- 2.Talerman A. Germ cell tumors of the ovary. In: Pathology of the Female Genital Tract. Springer New York. 602–664. 1982. [Google Scholar]
- 3.Sobis H. Choriocarcinoma, uterus, rat. In: Monographs on pathology of laboratory animals (genital system). TC Jones, U Mohr, and RD Hunt (eds). Springer-Verlag Berlin Heidelber. 138–139. 1987. [Google Scholar]
- 4.Alison RH, and Morgan KT. Ovarian neoplasms in F344 rats and B6C3F1 mice. Environ Health Perspect. 73: 91–106. 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alison RH, Lewis DJ, and Montgomery CA. Ovarian choriocarcinoma in the mouse. Vet Pathol. 24: 226–230. 1987. [DOI] [PubMed] [Google Scholar]
- 6.Koujitani T, Kikawa E, Toyosawa K, Okimoto K, and Yasuda M. Spontaneous ovarian choriocarcinoma in two B6C3F1 mice. J Toxicol Pathol. 8: 435–439. 1995. [Google Scholar]
- 7.Frith CH, and Evans MG. Spontaneous ovarian choriocarcinoma, yolk sac carcinoma, and teratoma in B6C3F1 mice: a case report. Toxicol Pathol. 21: 91–98. 1993. [DOI] [PubMed] [Google Scholar]
- 8.Toyosawa K, Okimoto K, Koujitani T, and Kikawa E. Choriocarcinoma and teratoma in the ovary of a cynomolgus monkey. Vet Pathol. 37: 186–188. 2000. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann-Bart M, and Fischer I. Choriocarcinoma with metastasis in a rabbit (Oryctolagus cuniculi). Vet Pathol. 45: 77–79. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Kurman RJ, and Norris HJ. Malignant germ cell tumors of the ovary. Hum Pathol. 8: 551–564. 1977. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa S, Kuroda Y, and Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 27: 11–18. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zybina EV, and Zybina TG. Polytene chromosomes in mammalian cells. Int Rev Cytol. 165: 53–119. 1996. [DOI] [PubMed] [Google Scholar]