Abstract
BACKGROUND:
There is a need for valid and reliable biomarkers in hidradenitis suppurativa (HS) for diagnosis and disease activity monitoring. Imaging-based biomarkers have the potential to fulfill this unmet need but no evaluation of analytical or clinical validity has yet been undertaken.
OBJECTIVES:
To evaluate the analytical and clinical validity of sonographic epidermal thickness, Doppler ultrasound and dermal tunnel diameter in patients with Hidradenitis Suppurativa.
METHODS:
22 participants with Hidradenitis Suppurativa were recruited and underwent a total of 65 matched ultrasound and skin biopsies of lesional, perilesional and unaffected tissue. Ultrasound measurements were performed in triplicate with mean values used. Skin biopsies underwent immunohistochemistry as per previously published methods. Analytical validity was assessed in individual ultrasound-biopsy pairs (n=65) by comparisons of sonographic variables with histological correlates. Clinical validity was assessed in individual patients (n=22) by comparing measures of overall disease activity with sonographic outcomes.
RESULTS:
(Epidermal thickness, dermal tunnel diameter and power Doppler intensity were assessed. Sonographic epidermal thickness and dermal tunnel diameter have high analytical validity with corresponding histological measurements. Power Doppler intensity demonstrated high correlation with dermal CD3+ and CD11c+ cell counts but not Neutrophil Elastase positive cells. Power Doppler ultrasound has significant correlation with pain scores, abscess and nodule (AN) count, IHS4 score and number of draining tunnels.
CONCLUSIONS:
Sonographic epidermal thickness and dermal tunnel diameter have acceptable levels of analytical validity in the assessment of HS lesions. Power Doppler intensity demonstrates acceptable clinical and analytical validity suggesting it is a valid imaging-based biomarker in HS.
BACKGROUND
Hidradenitis suppurativa (HS) is a chronic, recurrent inflammatory skin that is characterized by painful or suppurativa nodules, abscesses, and fistulous tracts.1, 2 Clinical scoring systems, such as Hurley staging, Sartorius score, and HiSCR currently serve as entry criteria or end points for assessing therapeutic efficacy in clinical trials.3 Scoring is based upon the counting of lesions, which has poor interobserver reliability,4 thus limiting accuracy and reproducibility. The use of clinical biomarkers (either serum, tissue or imaging based) presents an opportunity to utilize a reproducible and objective measurement outcome. Concerns regarding serum and tissue-based biomarkers include their lack of specificity5,6 and the known compartmentalization of inflammatory cytokines between tissue and serum inpatients with HS7.
To date there have been no studies examining the analytical or clinical validity of image-based biomarkers. Non-invasive imaging modalities (eg ultrasound) can visualize skin lesions not visible to the naked eye and can provide additional qualitative and quantitative variables in the assessment of disease. It is a safe, relatively affordable non- modality which has been utilized in a number of inflammatory and fibrotic skin diseases8,9. Ultrasound has established clinical validity in psoriasis and scleroderma8,9 and sonographic findings have been well characterized in HS.10–13, What is currently absent from the current literature is an examination of how sonographic findings in HS correlate with clinical, histological and immunological markers of disease. Such correlates have been hypothesized by our group based upon the results of a published scoping review of the existing literature13. Establishing analytical and clinical validity are the first stages in the identification and validation of potential biomarkers of disease15.
This study aims to assess the analytical and clinical validity of sonographic outcome measurements in HS. Specific pre-defined14 target measurements have included: epidermal thickness; dermal tunnel diameter and power Doppler intensity.
Methods:
All participants were recruited under an IRB-approved protocol at the Rockefeller University Hospital. Participants were recruited as part of a study into the natural history of Hidradenitis Suppurativa with the participants being a sample of convenience. Given the lack of previous validation studies no power calculations were undertaken. All participants provided written informed consent to take part in the study and were not on active therapy (including oral antibiotics, systemic retinoids or biologic therapy).
Participants underwent full cutaneous examination and assessment of HS disease severity using previously validated outcome clinical outcome measures including HiSCR/AN counts16, IHS4 score17 patient and physician-measured Visual Analogue Scales (VAS) of pain, itch and global disease severity. Quality of life was assessed using the DLQI18.
Lesional, perilesional and unaffected tissue biopsies were taken according to previously published recommendations19. Cutaneous sonography of all biopsy sites was performed with a multifrequency linear GE LogicQ probe (10–22MHz) by one HS clinician experienced with sonography in HS (JF). Healthy controls also underwent site-matched cutaneous biopsies and ultrasound measurements. All ultrasound measurements were performed in triplicate with average values used. Power Doppler intensity was assessed using a semi-quantitative scoring method (1–3 with 1 being minimal focal or no intensity, 2 indicating focal high intensity or diffuse low intensity and 3 indicating diffuse high intensity). Tissue sections were prepared with H&E and IHC staining as per previously published methods20. Measurement of epidermal thickness was undertaken in triplicate with average values used. Measurement of tunnels was performed using triplicate values of the maximum transverse dimension (thickness) in cm with average values used.
Clinical validity was assessed through comparison of each sonographic outcome measurement with clinical outcome measures including AN count, IHS4 score, patient visual analogue scale (VAS) pain and itch; tunnel drainage and global disease severity. Analytical validity was undertaken through comparison of sonographic outcome measurements against objective pre-defined measurements of the outcome of interest14. Sonographic epidermal thickness and tunnel diameter was compared to histologic epidermal thickness and tunnel diameter of the corresponding biopsy specimen. Sonographic measures of epidermal thickness, presence of tunnels and dermal power Doppler intensity was compared to quantitative measurements of histological inflammatory infiltrate density (CD3+, CD11c+ and Neutrophil elastase (NE)).
Statistical Methods:
All statistical calculations were performed in R v.5.3.321 Comparison between groups was undertaken using the Wilcoxon sign rank test for related groups and the Mann-Whitney U Test for independent groups. Chi squared and fisher exact tests were used for categorical data based upon sample size. Scatterplots and Spearman rank correlation (R2) were calculated for comparison of continuous data. Bland-Altman plots were calculated for comparisons of continuous data. P<0.05 was considered statistically significant and adjustment for multiple comparisons was made using the Bonferroni correction in each group of analyses.
RESULTS:
DEMOGRAPHIC RESULTS:
A total of 22 individuals with untreated moderate to severe Hidradenitis Suppurativa and ten healthy controls were recruited for this study. All participants were not on active treatment and had a washout period of at least 5 half-lives prior to study enrollment. A total of 65 cutaneous sites underwent matched clinical assessment, ultrasound and skin biopsy. Detailed demographic and clinical data are presented in Supplementary Table 1.
ANALYTICAL VALIDITY
Analytical validity results are presented in Table 1 and discussed by specific sonographic measurement variable below.
Table 1:
Analytical and Clinical Validity Potential Image -Based Biomarkers in Hidradenitis Suppurativa. Correlation coefficient (R2) with 95% confidence intervals in parentheses. Non-parametric tests are presented with comparison of medians with interquartile range (IQR) in parentheses. P-values are also presented for each comparison. R2 interpretation: >0.64: strong correlation; 0.25–0.64: moderate correlation; <0.25: minimal-to-no correlation. Additional Scatterplots relating to this table are available in Supplementary Figure 3.
| IMAGE-BASED BIOMARKER | ANALYTICAL VALIDITY | IMAGE-BASED BIOMARKER | CLINICAL VALIDITY | ||||
|---|---|---|---|---|---|---|---|
| VARIABLE | STATISTIC (SIGNIFICANCE) | INTERPRETATION | VALIDITY VARIABLE | STATISTIC (SIGNIFICANCE) | INTERPRETATION | ||
| Sonographic Epidermal Thickness | Histological Thickness (Figure 1d) |
R2 (95%CI) = 0.8247 (0.749–0.899) (p<0.0001) |
Strong correlation | Sonographic Epidermal Thickness | Pain (Fig 5g) | R2(95%CI) = 0.09360 (−0.036–0.223) (p=0.02) |
Minimal-to-no correlation |
| Lesional vs Control Skin (Fig 1c) | Medians (IQR): 0.03 (0.02–0.05) vs 0.01 (0.01–0.02) (p<0.001) |
Significant difference between groups | Patient Global Assessment (Fig 5i) |
R2(95%CI) = 0.01340 (−0.040–0.067) (p=0.391) |
Minimal-to-no correlation | ||
| CD3+ Dermal Cell Counts (Fig 1e) | R2 (95%CI) = 0.3946 (0.215–0.573) (p=0.001) |
Moderate correlation | |||||
| CD11c+ Dermal Cell Counts (Fig 1f) | R2(95%CI) = 0.2010 (0.032–0.369) (p=0.003) |
Minimal-to-no correlation | Itch (Fig 5h) | R2 (95%CI) =0.0003 (−0.0078–0.0084) (p=0.891) |
Minimal-to-no correlation | ||
| NE+ Dermal Cell Counts (Fig 1g) | R2(95%CI) = 0.1435 (−0.009–0.296) (p=0.02) |
Minimal-to-no correlation | |||||
| Power Doppler Intensity | CD3+ Dermal Cell Counts (Fig 3m) | R2(95%CI) =0.4025 (0.224–0.581) (p=0.001) |
Moderate correlation | Power Doppler Intensity | Pain (Fig 4d) |
R2(95%CI) = 0.673 (0.547–0.799) (p<0.0001) |
Strong correlation |
| CD11c+ Dermal Cell Counts (Fig 3n) | R2(95%CI) =0.3515 (0.171–0.532) (p<0.0001) |
Moderate correlation | Patient Global Assessment (Fig 4f) | R2(95%CI) = 0.056 (−0.048–0.162) (p=0.062) |
Minimal-to-no correlation | ||
| NE+ Dermal Cell Counts (Fig 3o) | R2(95%CI)= 0.2871 (0.108–0.4 67) (p=0.0004) |
Moderate correlation | Itch (Fig 4e) |
R2(95%CI) = 0.019 (−0.044–0.083) (p=0.269) |
Minimal-to-no correlation | ||
| Presence of Tunnels (Fig 4g) | Medians (IQR): 2.00 (1.00–3.00) vs 1.00 (0.00–1.00) P=0.0096 |
Significant difference between groups | |||||
| Dermal Tunnel Diameter | Histological Diameter (Fig 2d) |
R2(95%CI) = 0.8401 (0.771–0.908) (p<0.0001) |
Strong correlation | Dermal Tunnel Diameter | Pain (Supp Fig 3) |
R2 (95%CI) = 0.211 (−0.153–0.537) (p<0.05) |
Minimal-to-no correlation |
| Histological Diameter of Draining vs Non-Draining Tunnel (Fig 2k) |
Medians (IQR): 0.40 (0.20–0.50) vs 0.30 (0.10–0.40) p=0.046 |
Significant difference between groups | |||||
| Pain (Draining Tunnels Only) (Supp Fig 3) |
R2 (95%CI) =0.048 (−0.049–0.146) (p=0.245) |
Minimal-to-no correlation | |||||
| Histological Diameter of Draining Tunnels Only (Fig 2e) | R2(95%CI) =0.8909 (0.8425–0.939) (p<0.0001) |
Strong correlation | Patient Global Assessment (Supp Fig 3) |
R2 (95%CI) =0.746 (0.643–0.849) (p<0.0001) |
Strong correlation | ||
| Patient Global Assessment (Draining Tunnels Only) (Supp Fig 3) |
R2 (95%CI) =0.741 (0.636–0.845) (p<0.0001) |
Strong correlation | |||||
| Histological Diameter of Non-Draining Tunnels Only (Fig 2f) | R2(95%CI) =0.6413 (0.506–0.776) (p<0.0001) |
Strong correlation | |||||
| Itch (Supp Fig 3) |
R2 (95%CI) =0.1759 (0.013–0.338) (p=0.02) |
Minimal-to-no correlation | |||||
| Sonographic Draining vs Non-Draining Tunnels (Fig 2l) |
Medians (IQR): 0.359 (0.187–0.508) vs 0.294 (0.108–0.410) (p<0.05) |
Significant difference between groups | Itch (Draining Tunnels Only) (Supp Fig 3) |
R2 (95%CI) =0.08 (−0.042–0.202) (p=0.02) |
Minimal-to-no correlation | ||
EPIDERMAL THICKNESS:
Ultrasound of HS lesional skin identifies a prominent hyperechoic linear band with adjacent hypoechogenicity - the entry-signal/ entrance echo and surface reflection. This corresponds histologically with areas of psoriasiform epidermal hyperplasia (Figure 1Ai). This degree of hyperechogenicity and adjacent hypoechogenicity was not seen in healthy controls (Figure 1Aii). As previously described in psoriasis and HS, the thickness of the entry-signal correlates with the maximum thickness of the rete ridges of the epidermis22. We therefore refer to this entry-signal as “epidermal thickness” for the remainder of the study. Statistically significant differences were seen between HS lesional skin and skin of site-matched healthy controls were seen in both histological and sonographic measurements of epidermal thickness (Figure 1B–1C) (p<0.001). Very strong correlation was noted between histological and sonographic measurements of epidermal thickness (R2=0.8247) (Fig 1D) indicating a high level of analytical validity. Bland-Altman plots identified a high level of agreement between measures with evidence of proportional bias (Supplementary Figure 1A). Sonographic epidermal thickness was consistently greater than histological epidermal thickness. Sonographic epidermal thickness displayed moderate correlation to quantitative CD3+ cell counts (R2= 0.3946) and with only fair-to-poor correlation with CD11c+ (R2= 0.2010) and NE+ (R2= 0.1435) quantitative cell counts.
Figure 1:
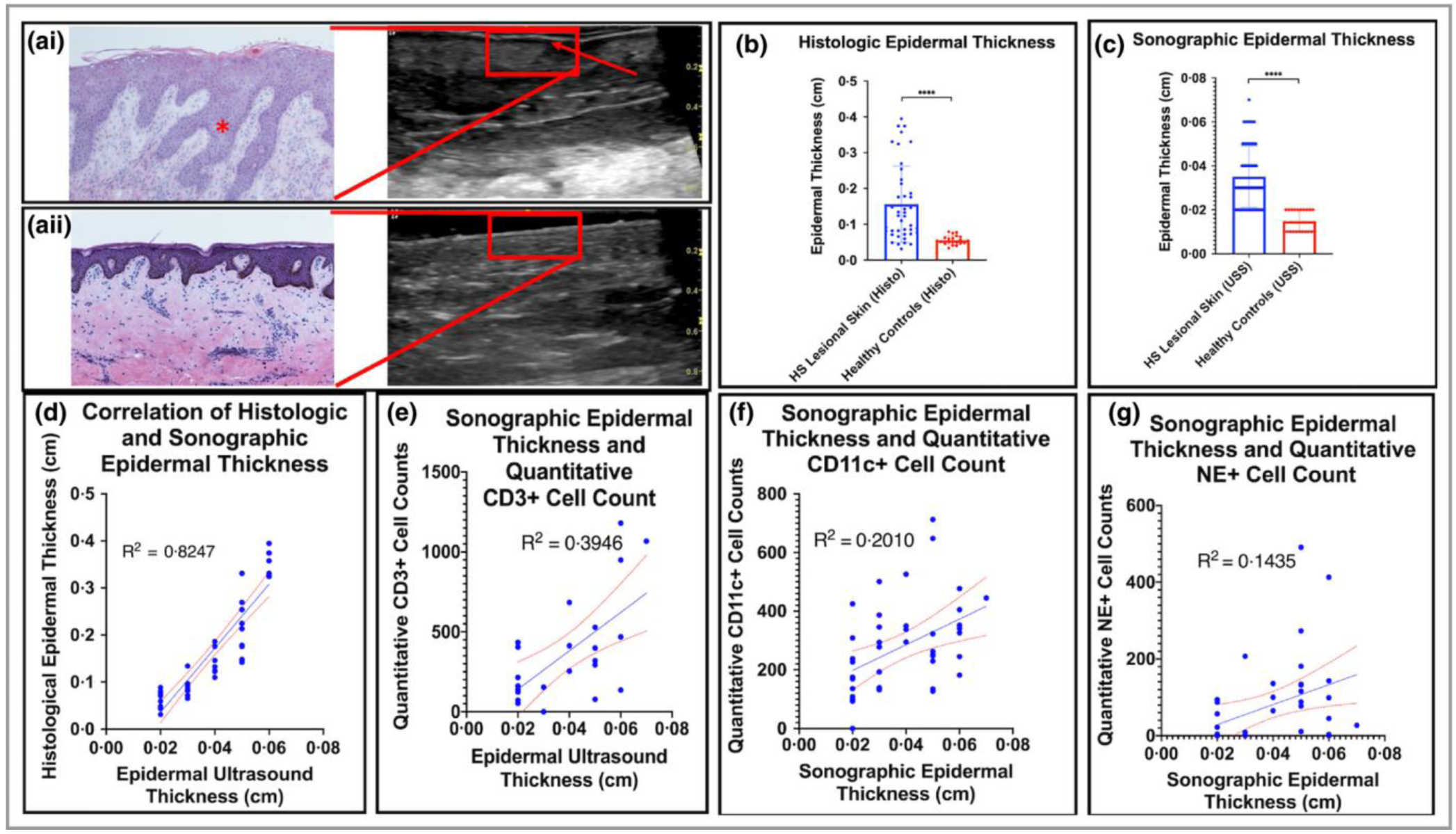
Assessment of the Clinical and Analytical Validity of Ultrasound measurement of epidermal thickness in Hidradenitis Suppurativa (HS). (Ai): Psoriasiform epidermal hyperplasia (*) correlated with prominent hyperechoic thickening on Ultrasound (red arrow) compared with normal skin (Aii). (B): Histological epidermal thickness was significantly different between HS lesional skin and healthy controls. (C) Sonographic epidermal thickness was also significantly different between HS lesional skin and controls. (D) A high degree of correlation is seen in measures of epidermal thickness by ultrasound and epidermal thickness by validated methods in histopathology (R2=0.82) (E): Sonographic epidermal thickness correlated moderately with CD34+ cell counts (E), CD11c+ cell counts (F) but not with NE+ cell counts (G).
DERMAL TUNNEL DIAMETER:
Dermal tunnels were detected in 36/44 (81.8%) lesional and perilesional sites. They were represented sonographically by hypoechoic/anechoic bands bordered by hyperechoic strips corresponding to epithelialized tunnels seen on histology. (Fig 2a–c). The hyperechoic strips of keratinised tunnels had greater echogenicity and less branching than surrounding areas of fibrosis which can also appear as linear hyperechoic bands on ultrasound. Tunnel diameter as measured by ultrasound (median diameter = 500μm) had a very high level of correlation with histological tunnel diameter (R2=0.8401). Stratification by draining and non-draining tunnels (Fig 2e–f) suggested a higher correlation with draining as opposed to non-draining tunnels (Table 1). Bland-Altman plots demonstrated a high level of agreement between measures although ultrasound measures were consistently higher than histological measures and a systematic error was suggested when comparing tunnel diameters (Supp Fig 1). Visual differences in echogenicity of linear bands and power Doppler intensities were observed between draining and non-draining tunnels (Fig 2g–j) Statistically significant differences in both histologic and sonographic tunnel diameter were observed (Fig 2k–l) (p<0.05) between draining and non-draining tunnels.
Figure 2:

(a) The clinical presentation of dermal tunnels correlates with Ultrasound Findings highlighting hyperechoic keratinized dermal tunnels (Black arrow and red brackets) (b), and correlated histology specimen (c). Correlation between diameter of tunnels based on Ultrasound and Histological measurements (d). with variation in correlation in the presence of draining (e) and non-draining (f) tunnels. Comparison of Doppler ultrasound findings between draining (g)(i), and non-draining (h)(j) tunnels. Significant differences in the diameter of tunnels are seen on histology (k) and sonographically (i) when stratified by the drainage of tunnels.
DERMAL POWER DOPPLER INTENSITY:
Measurement of inflammation through the use of semi-quantitative power Doppler intensity was able to measure and localise inflammation in a variety of clinical presentations of HS (Fig 4a–d). The degree and location of power Doppler intensity signaling was more superficial in lesions such as nodules (Fig 3a), and deeper in deeper lesions such as abscesses (Fig 3b). The intensity of power Doppler signal also correlated with degree of neutrophil infiltration as indicated by NE+ IHC staining (Fig 3i–l). Quantitative Dermal CD3+ cell counts had the highest correlation to semiquantitative power Doppler intensity (Fig 3m) followed by CD11c+ dermal cell counts (Fig 3n) with quantitative NE+ IHC staining demonstrating the lowest correlation.
Figure 4:

Semiquantitiative power Doppler signaling significantly correlates with AN count (A), IHS4 count (B) number of draining tunnels (C) and Clinical Pain Scores (D). Itch (E) and global assessment scores (F) demonstrated little to no correlation. Power Doppler signaling differentiates between the presence and absence of dermal tunnels (G) and is associated with differences in CD3+ but not CD11c+ and NE+ dermal cell counts (H).
Figure 3:

Clinical and Analytical Validity of Power Doppler Ultrasound across different clinical manifestations of Hidradenitis Suppurativa (HS). Clinical and Ultrasound correlation of (a) Nodular, (b), Deep Abscesses, (c) draining tunnels and (d) non-draining tunnels demonstrate localization and intensity of power doppler flow consistent with clinical manifestations (e)- (h). Histological correlation (i)- (l) demonstrates psoriasiform acanthotic epidermis more prominent in (i) and (k) compared to (j) and (l), with superficial neutrophil elastase staining showing more superficial localization of neutrophils in nodular lesions and actively draining tunnels. Semiquantitative scoring of power Doppler intensity has moderate correlation with CD3 Dermal cell counts (m), CD11c dermal cell counts (n) and NE+ dermal cell counts (o).
CLINICAL VALIDITY:
Clinical Validity results are presented in Table 1 and discussed under each sonographic outcome measure listed below.
EPIDERMAL THICKNESS:
Epidermal thickness demonstrated poor correlation with clinical measures of overall disease severity (AN count, IHS4 score and number of draining tunnels) (Supp Figure 2a–c). Patient reported outcomes of pain, itch and global disease severity also demonstrated poor correlation with histologic epidermal thickness (Supp Figure 2d–f) and sonographic epidermal thickness (Supp Figure 2g–i). Similarly, tunnel diameter demonstrated no significant correlation with these clinical measures of disease activity and severity (Supplementary Figure 3).
POWER DOPPLER SIGNALING:
Abscess and Nodule counts (AN Count), IHS4 scores and number of draining tunnels were significantly different between patients when stratified by semiquantitative power Doppler intensity (Fig 4a–c). No significant difference was seen between power Doppler intensity ratings of 1 and 2 (Fig 4a–c) but ratings of 3 showed significantly higher AN counts, IHS4 scores and number of draining tunnels than ratings of 1 or 2. Clinical pain scores correlated well with semiquantitative power Doppler intensity (R2= 0.673) (Fig 4d) with no significant correlation seen between clinical itch and global disease severity scores (Fig 4e,f). The median level of power Doppler intensity was significantly different (p<0.01) between patients with and without tunnels (Fig 4G); and this was replicated when analyzed by quantitative IHC for CD3+ cells. (Fig 4H).
Discussion and Limitations:
We present quantitative data to explore the analytical and clinical validity of ultrasound-based biomarkers in HS. The overall level of analytical validity was high regarding epidermal thickness, tunnel diameter and power Doppler intensity (Table 1). This suggests that ultrasound-based measures can be a potential surrogate measurement for histology-based outcomes in HS. The ability for ultrasound to identify tunnels in HS is of vital importance, particularly as the extent of tunnels is difficult to appreciate with clinical examination alone11. Epidemiological evidence indicates that the presence of tunnels is associated with decreased response to therapy with Adalimumab, highlighting the potential utility of tunnel identification in patients with HS23.
Our results go one step further in demonstrating that the activity of these tunnels can be ascertained using the dermal tunnel diameter, with median values of tunnel diameter significantly elevated in draining tunnels compared with non-draining tunnels (Fig 3). Power Doppler intensity also correlates significantly with AN counts, IHS4 scores, draining tunnels counts and clinical pain scores, demonstrating both acceptable analytical and clinical validity of semiquantitative power Doppler intensity measurements.
The analytical validity of epidermal thickness and dermal tunnel diameter was acceptable, however the clinical validity demonstrated little to no correlation. (Supp Figure 2). Previous studies have correlated pain and erythema to the sonographic diameter of nodules, but this is the first examination of clinical correlation with epidermal thickness and dermal tunnel diameter specifically24. The lack of clinical correlation we have observed does not necessarily imply that epidermal thickness measurements and tunnel diameter measurements are not useful in the assessment of HS. This lack of correlation may be due to the inherent variability and questionable reliability23 of clinical outcomes dependent on counts (AN counts, IHS4); or it may only be highly clinically relevant in a subset of patients (such as those with draining tunnels). It may also indicate a temporal disconnect between clinical symptoms (eg pain) and the delayed changes (over days or weeks) to the thickness of epithelium and tunnels from acute changes in levels of inflammation. This would also be supported by the lack of correlation between epidermal thickness and inflammatory cell infiltrates (Table 1). The high analytical validity of tunnel diameter and epidermal thickness suggests that it is a potential biomarker for longitudinal change in disease activity over time and in the setting of pharmacologic interventions. The systematic error identified in tunnel diameter comparisons may limit the responsiveness of this measure, but this requires quantification in longitudinal treatment studies. Additionally, overall measures of disease activity may be influenced by external psychosocial factors which are not captured via sonographic measurements25,26. Therefore, measurement of epidermal thickness and tunnel diameter at only one body site may be of greater utility in the assessment of localized disease activity, although measurement across all disease sites have been reported to produce accurate assessment of general disease activity27. This also emphasizes the utility of ultrasound in the selection of biopsy sites, given the high inflammatory activity surrounding tunnels. Inadvertent biopsy of a perilesional or non-lesional site with an underlying tunnel may introduce errors in measurement of inflammatory mediators19. Therefore, pre-biopsy assessment of sites with ultrasound has high utility in assessment for occult tunnels, and the high degree of analytical validity presented by our data supports this notion.
The use of imaging -based biomarkers is well established in oncology with well-characterized developmental pathways for validation and clinical implementation15. In order for an imaging-based biomarker to be useful in the clinical setting, it must cross two translational gaps. The novel biomarker must first establish of accurate and reproducible measures of given parameter (analytical validity) that correlate with established clinical markers of disease severity (clinical validity). This is termed the first translational gap. Along with our presented data, power Doppler intensity has been independently shown to correlate with patient pain scores and severity assessment25,26, which begins the process of establishing the multicenter reproducibility of these image-based biomarkers25,26. The second translational gap pertains to establishing the use of the image-based biomarker as positively influencing patient care15. This would involve cost benefit analyses which hopefully (given the relatively low cost of ultrasound) would return a positive result. Multiple studies have addressed how sonographic imaging can improve diagnostic accuracy and severity staging11,28. Examining image-based biomarkers as a predictive biomarker for treatment response would be a component of crossing the second translational gap. Stratifying patients by the sonographic presence and absence of tunnels may be one method which could evaluate the utility of sonographic biomarkers in directing patient care. In the absence of one predictive biomarker, novel sonographic scoring systems employing measures of power Doppler intensity, epidermal thickness and tunnel diameter may be developed and evaluated for utility in directing therapeutic decisions. Although we have established the validity of baseline sonographic characteristics as potential image-based biomarkers in HS, one downside of our study is the lack of longitudinal evaluation pre and post intervention. Assessing the responsiveness of sonographic biomarkers to therapeutic interventions would enable direct comparison to change in measurements of clinical response. Additionally, given that our study is based at a single site, measurement of inter-operator repeatability of sonographic measurements is required to ensure reliability across multi-site studies.
Conclusions:
Sonographic epidermal thickness and dermal tunnel diameter have acceptable levels of analytical validity in the assessment of HS lesions compared with histological correlates. Power Doppler signaling demonstrates both acceptable levels of clinical and analytical validity suggesting it is a valid imaging-based biomarker in HS. Future work should focus upon assessing the response of these biomarkers to established therapeutic interventions and assessment of the utility of image-based biomarkers as diagnostic and predictive biomarkers for directing and optimizing patient care and outcomes.
Supplementary Material
Supplementary Figure 3: Additional Scatterplots of Correlations presented in Table 1: Sonographic Tunnel Diameter and Draining Tunnel Sonographic Diameter graphed against Pain (A, D), Global Disease Severity (B, E) and Itch (C, F).
Supplementary Table 1: Demographic and Disease Activity features of patients included in this study including location of paired biopsy/ultrasound sites. M=Male, F=Female, BMI= Body Mass Index.
Supplementary Figure 1: Bland Altman Plots of significant correlations in Figures 1 and 2, demonstrating proportional bias in the correlation between histological and sonographic epidermal thickness, and no evidence of bias in other comparisons.
Supplementary Figure 2: AN Count (A), IHS4 Count (B) and number of tunnels (C) does not demonstrate correlation with sonographic epidermal thickness. Pain, itch and global assessment scores also do not correlate with histological (D)–(F) or sonographic (G)–(I) measures of epidermal thickness.
What’s already known about this topic?
Image-based biomarkers have potential for HS disease monitoring but no evaluation of analytical or clinical validity of image-based biomarkers currently exists.
What does this study add?
Power Doppler intensity has greater analytical and clinical validity than epidermal thickness and dermal tunnel thickness. It is a valid imaging biomarker for disease activity in HS.
Acknowledgement:
We acknowledge GE for their assistance in the loan of the GE LogiQ Doppler Ultrasound Unit for this study.
Funding and Disclosures: J.W.F. was supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. K.N. was supported by a MSTP grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program.
Footnotes
Conflicts of Interest: J. G. Krueger has received research support (grants paid to institution) from AbbVie, Amgen, BMS, Boehringer, EMD Serono, Innovaderm, Kineta, LEO Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, and Vitae and personal fees from AbbVie, Acros, Allergan, Aurigne, BiogenIdec, Boehringer, Escalier, Janssen, Lilly, Novartis, Pfizer, Roche, and Valeant. The other authors declare they have no relevant conflicts of interest.
References:
- 1.Jemec GB, Clinical practice. Hidradenitis suppurativa. N Engl J Med, 2012. 366(2): p. 158–64. [DOI] [PubMed] [Google Scholar]
- 2.Saunte DML and Jemec GBE, Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA, 2017. 318(20): p. 2019–2032. [DOI] [PubMed] [Google Scholar]
- 3.Kimball AB, et al. , Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med, 2016. 375(5): p. 422–34. [DOI] [PubMed] [Google Scholar]
- 4.van Straalen KR, et al. , Poor interrater reliability of hidradenitis suppurativa phenotypes. Journal of the American Academy of Dermatology, 2018. 79(3): p. 577–578. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, et al. , Increased serum interleukin-6 levels in patients with hidradenitis suppurativa. Postepy Dermatol Alergol, 2017. 34(1): p. 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter CA and Jones SA, IL-6 as a keystone cytokine in health and disease. Nat Immunol, 2015. 16(5): p. 448–57. [DOI] [PubMed] [Google Scholar]
- 7.Kanni T, et al. , Compartmentalized Cytokine Responses in Hidradenitis Suppurativa. PLoS One, 2015. 10(6): p. e0130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta AK, et al. , The use of high-frequency ultrasound as a method of assessing the severity of a plaque of psoriasis. Arch Dermatol, 1996. 132(6): p. 658–62. [PubMed] [Google Scholar]
- 9.Lis-Swiety A, et al. , A systematic review of tools for determining activity of localized scleroderma in paediatric and adult patients. J Eur Acad Dermatol Venereol, 2017. 31(1): p. 30–37. [DOI] [PubMed] [Google Scholar]
- 10.Wortsman X and Jemec GB, Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg, 2007. 33(11): p. 1340–2. [DOI] [PubMed] [Google Scholar]
- 11.Wortsman X, et al. , Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg, 2013. 39(12): p. 1835–42. [DOI] [PubMed] [Google Scholar]
- 12.Wortsman X, et al. , Ultrasound Diagnosis and Staging in Pediatric Hidradenitis Suppurativa. Pediatr Dermatol, 2016. 33(4): p. e260–4. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman X, Castro A, Figueroa A. Color Doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS), J Am Acad Dermatol. 2016;75(4):760–7 [DOI] [PubMed] [Google Scholar]
- 14.Grand D, Navrazhina K, and Frew JW, A Scoping Review of Non-invasive Imaging Modalities in Dermatological Disease: Potential Novel Biomarkers in Hidradenitis Suppurativa. Front Med (Lausanne), 2019. 6: p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor JP, et al. , Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol, 2017. 14(3): p. 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimball AB, et al. , HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol, 2016. 30(6): p. 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zouboulis CC, et al. , Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol, 2017. 177(5): p. 1401–1409. [DOI] [PubMed] [Google Scholar]
- 18.Finlay AY and Khan GK, Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol, 1994. 19(3): p. 210–6. [DOI] [PubMed] [Google Scholar]
- 19.Frew JW, et al. , Defining lesional, perilesional and unaffected skin in hidradenitis suppurativa: proposed recommendations for clinical trials and translational research studies. Br J Dermatol, 2019. 181(6): p. 1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentes-Duculan J, et al. , A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol, 2010. 130(10): p. 2412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Team, R.C., R: A language and environment for statistical computing. 2017, R Foundation for Statistical Computing: Vienna, Austria. [Google Scholar]
- 22.el Gammal S, et al. , [100 MHz ultrasound of psoriasis vulgaris plaque]. Ultraschall Med, 1998. 19(6): p. 270–4. [DOI] [PubMed] [Google Scholar]
- 23.Frew JW, et al. , Clinical response rates, placebo response rates, and significantly associated covariates are dependent on choice of outcome measure in hidradenitis suppurativa: A post hoc analysis of PIONEER 1 and 2 individual patient data. J Am Acad Dermatol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarchi K, Yazdanyar N, Yazdanyar S, Wortsman X, Jemec GB. Pain and inflammation in hidradenitis suppurativa correspond to morphological changes identified by high-frequency ultrasound. J Eur Acad Dermatol Venereol. 2015. March;29(3):527–32. [DOI] [PubMed] [Google Scholar]
- 25.Martorell A, et al. , Ultrasound as a Diagnostic and Management Tool in Hidradenitis Suppurativa Patients: A Multicenter Study. J Eur Acad Dermatol Venereol, 2019. [DOI] [PubMed] [Google Scholar]
- 26.Nazzaro G, et al. , Color Doppler as a tool for correlating vascularization and pain in hidradenitis suppurativa lesions. Skin Res Technol, 2019. [DOI] [PubMed] [Google Scholar]
- 27.Zarchi K, Yazdanyar N, Yazdanyar S, Wortsman X, Jemec GB. Pain and inflammation in hidradenitis suppurativa correspond to morphological changes identified by high-frequency ultrasound. J Eur Acad Dermatol Venereol. 2015. March;29(3):527–32 [DOI] [PubMed] [Google Scholar]
- 28.Caposiena Caro RD, et al. , Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: Clinical and ultrasound observations. J Am Acad Dermatol, 2019. 80(5): p. 1314–1321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 3: Additional Scatterplots of Correlations presented in Table 1: Sonographic Tunnel Diameter and Draining Tunnel Sonographic Diameter graphed against Pain (A, D), Global Disease Severity (B, E) and Itch (C, F).
Supplementary Table 1: Demographic and Disease Activity features of patients included in this study including location of paired biopsy/ultrasound sites. M=Male, F=Female, BMI= Body Mass Index.
Supplementary Figure 1: Bland Altman Plots of significant correlations in Figures 1 and 2, demonstrating proportional bias in the correlation between histological and sonographic epidermal thickness, and no evidence of bias in other comparisons.
Supplementary Figure 2: AN Count (A), IHS4 Count (B) and number of tunnels (C) does not demonstrate correlation with sonographic epidermal thickness. Pain, itch and global assessment scores also do not correlate with histological (D)–(F) or sonographic (G)–(I) measures of epidermal thickness.


