Abstract
Backgrounds: Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique for the treatment of several psychiatric disorders, e.g., mood disorders and schizophrenia. Therapeutic effects of tDCS are suggested to be produced by bi-directional changes in cortical activities, i.e., increased/decreased cortical excitability via anodal/cathodal stimulation. Although tDCS provides a promising approach for the treatment of psychiatric disorders, its neurobiological mechanisms remain to be explored.
Objectives: To review recent findings from neurophysiological, chemical, and brain-network studies, and consider how tDCS ameliorates psychiatric conditions.
Findings: Enhancement of excitatory synaptic transmissions through anodal tDCS stimulation is likely to facilitate glutamate transmission and suppress gamma-aminobutyric acid transmission in the cortex. On the other hand, it positively or negatively modulates the activities of dopamine, serotonin, and acetylcholine transmissions in the central nervous system. These neural events by tDCS may change the balance between excitatory and inhibitory inputs. Specifically, multi-session tDCS is thought to promote/regulate information processing efficiency in the cerebral cortical circuit, which induces long-term potentiation (LTP) by synthesizing various proteins.
Conclusions: This review will help understand putative mechanisms underlying the clinical benefits of tDCS from the perspective of neurotransmitters, network dynamics, intracellular events, and related modalities of the brain function.
Keywords: transcranial direct current stimulation, non-invasive brain stimulation, neurotransmitter, LTP, neuromodulation, neural network
Introduction
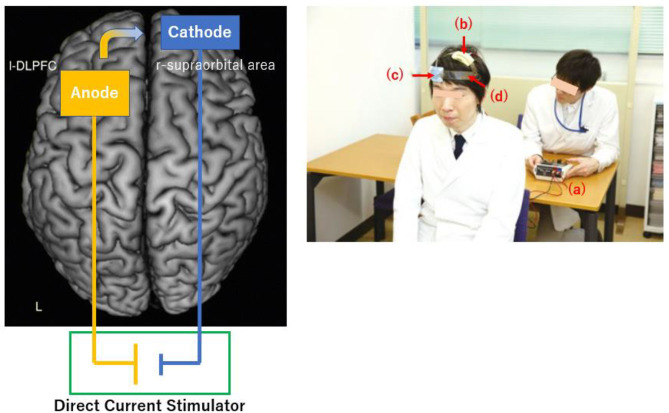
Transcranial direct current stimulation (tDCS) is a non-invasive method that modulates neural activities in the brain by delivering low-amplitude (usually no more than 2 mA) over a short period (no more than 30 min) between electrodes (anode and cathode). At least, one of the electrodes is placed on the scalp, through which electronic currents penetrate the skull to enter the brain and facilitate or inhibit spontaneous neural activities in the vicinity of electrodes (Yokoi et al., 2018; Figure 1).
Figure 1.
Schematic diagram (Left) and experimental setup (Right) for tDCS. (Left) The anode and cathode electrodes are positioned over the left dorsolateral prefrontal cortex and over right supraorbital region, respectively. The direction of current flow is from the anode to cathode. (Right) An administrator controls the stimulator (a). Anodal (b) and cathodal (c) electrodes of 35-cm2 in size are placed on F3 and right supraorbital region, respectively. A head strap (d) is used as needed to increase reproducibility.
Effectiveness of tDCS in the treatment of major depressive disorder (MDD) has been reported (Yokoi et al., 2018). Thus, a meta-analysis has shown a moderate effect of tDCS on depressive symptoms in patients with acute depression (Hedges'g = 0.37) (Shiozawa et al., 2014). Also, there has been a series of reports showing the ability of tDCS to ameliorate positive/negative symptoms of schizophrenia (Kim et al., 2019). For example, hallucinations (positive symptoms) (Hedges'g = 0.86) and negative symptoms (0.41) have been found to be improved by multi-session tDCS on the frontal or frontotemporal lobe (see montages in Table 1) twice daily for 5 days (Kim et al., 2019). Moreover, meta-analysis for cognitive function in patients with schizophrenia indicates the ability of multi-session tDCS on the prefrontal cortex (see montage in Table 1) to improve working memory (Hedges'g = 0.49), an important cognitive domain (Narita et al., 2020; Table 1).
Table 1.
Meta-analyses of the effects of tDCS.
| Study | Target disease | No. of RCT (n) | Montage (anode/cathode) | Intensity (mA) | Duration (min) | No. of sessions | Outcomes | Effect size (Hedges'g) |
|---|---|---|---|---|---|---|---|---|
| Shiozawa et al. (2014) | MDD | 7 RCTs (259) | F3/RSO, F3/F4, F3/F8 | 1–2 | 20–30 | 5–15 | HAMD MADRS |
0.37 |
| Kim et al. (2019) | Schizophrenia (positive symptoms) | 5 RCTs (186) | Between F3 and FP1/Between T3 and P3 | 2 | 20 | 10–15 | AHRS PANSS PSYRATS |
0.86 |
| Kim et al. (2019) | Schizophrenia (negative symptoms) | 7 RCTs (257) | Between F3 and FP1/Between T3 and P3, F3/F4 | 2 | 20–30 | 10–15 | PANSS SANS |
0.41 |
| Narita et al. (2020) | Schizophrenia (cognitive functions) | 9 RCTs (270) | Between F3 and FP1/Between T3 and P3, F3/F4, F3/FP2 | 1–2 | 20–30 | 2–40 | Digit span MCCB, SOPT N-back task 2-back task |
0.49 (working memory) |
MDD, major depressive disorder; RCT, randomized controlled trial; HAMD, Hamilton Rating Scale for Depression; MADRS, Montgomery-Åsberg Depression Rating Scale; AHRS, Auditory Hallucination Rating Scale; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scales; SANS, Scale for the Assessment of Negative Symptoms; MCCB, MATRICS Consensus Cognitive Battery; SOPT, Self-Ordered Pointing Task.
In spite of accumulated evidence for the efficacy of tDCS in treating psychiatric disorders, particularly schizophrenia and mood disorders, its mechanism of action has not been fully elucidated (Stagg and Nitsche, 2011). Therefore, the current review aimed to provide an overview of the actions of tDCS, especially anodal stimulation, on neurotransmission and neural networks in the brain, to help understand the mechanisms underlying its therapeutic effects.
The effect of tDCS on psychiatric symptoms has been mainly reported in studies using anodal stimulation over the frontal cortex. On the other hand, where the cathodal electrode is placed has not been uniform, indicating anodal stimulation has attracted interests to consider the mechanism of tDCS (Fregni et al., 2020). As the clinical benefits of tDCS have been found when multi-sessions are applied (Shiozawa et al., 2014; Kim et al., 2019; Narita et al., 2020), emphasis was placed on long-term changes of neural events produced by tDCS.
Neurophysiological Understanding of tDCS
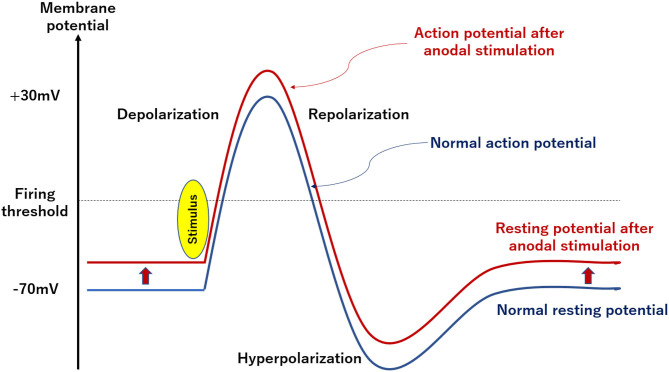
Anodal stimulation with tDCS (1–2 mA) by itself is not strong enough to depolarize the membrane potential of neurons to the firing threshold, and only increases the rate of spontaneous combustion and their excitability (Nitsche and Paulus, 2000; Philip et al., 2017; Figure 2). Conversely, cathodal stimulation is thought to deepen the resting membrane potential, making it difficult for neurons to depolarize, which reduces spontaneous combustion rates and excitability of neurons (Nitsche and Paulus, 2000; Philip et al., 2017; Figure 2). Importantly, these effects of tDCS depend on the intensity and duration of stimulation (Nitsche and Paulus, 2000), and radial electric field (Seo and Jun, 2019).
Figure 2.
Schematic diagram of changes in neuron potential by tDCS. Anodal stimulation with tDCS (1–2 mA) is not strong enough to depolarize the membrane potential of neurons to the firing threshold. Conversely, cathodal stimulation is thought to deepen the resting membrane potential and make it difficult for neurons to depolarize.
Electrophysiological understanding of tDCS may be facilitated by the stimulation-dependent model (Fertonani et al., 2011). In this model, anodal stimulation is considered to promote depolarization of neurons, and cathodal one causes hyperpolarization to suppress it. Moreover, electrical stimulation affects multiple neurons and increases their membrane potentials to induce depolarization. These events in the vicinity of neural membranes has been proposed to explain the ability of tDCS to improve brain functions (Silvanto et al., 2008).
Increased excitability of local neurons by anodal stimulation is thought to increase blood flow around the stimulation site, and induce subsequent metabolic changes. Specifically, blood-flow changes through tDCS on the prefrontal cortex have been measured by functional near-infrared spectroscopy (fNIRS) (Merzagora et al., 2010). In this study, the increase of oxygenated hemoglobin concentrations under the anodal electrode was significantly larger than those for the cathode. This is thought to reflect the ability of anodal stimulation to induce metabolic changes among neurons (Merzagora et al., 2010).
Biochemical Effects of tDCS
Changes in neurotransmissions by anodal stimulation have been reported in relation to metabolic changes in the brain. Here, we review the accumulated evidence for the effect of stimulation of the motor cortex in patients with chronic pain and those receiving post-stroke motor rehabilitation (Medeiros et al., 2012; Tables 2, 3). For example, the effect of anodal stimulation is suppressed by carbamazepine (sodium channel inhibitor) (Liebetanz et al., 2002), indicating that inhibition of intracellular influx of extracellular sodium ion suppresses anode-induced depolarization of neurons, and subsequent excitements.
Table 2.
Effects of concomitant medication on anodal tDCS on the motor cortex of healthy subjects (adapted from Medeiros et al., 2012).
| Study | N | Stimulation intensity (mA) | Stimulation duration (min) | Stimulation site | Pharmacological intervention | Effects |
|---|---|---|---|---|---|---|
| Liebetanz et al. (2002) | 11 | 1 | 5 | Left M1 | Carbamazepine (CBZ) Dextromethorphan (DMO) |
Both drugs suppressed the effect of tDCS. |
| Nitsche et al. (2003) | 11–14 | 1 | 11~13 | Left M1 | CBZ, DMO Flunarizine (FLU) |
All suppressed the effect of tDCS. |
| Nitsche et al. (2004a) | 6–12 | 1 | 11 | Left M1 | Lorazepam (LOR) | Delayed the effect of tDCS. |
| Nitsche et al. (2004b) | 12 | 1 | 13 | Left M1 | d-cycloserine (d-CYC) | Prolonged the effect of tDCS. |
| Nitsche et al. (2004c) | 5–12 | 1 | 13 | – | Amfetaminil (AMP) Propranolol (PRO) |
AMP enhanced and prolonged the effect of tDCS while PRO shortened it. |
| Nitsche et al. (2006) | 4–12 | 1 | 13 | Left M1 | Sulpiride | Suppressed and delayed the effect of tDCS. |
| Kuo et al. (2007) | 10–12 | 1 | 13 | Left M1 | Rivastigmine (RIVA) | Suppressed the effect of tDCS. |
| Kuo et al. (2008) | 7–11 | 1 | 13 | Left M1 | Levodopa (L-dopa) | Suppressed the non-specific effects of tDCS while enhanced local effects on synapses of specific neurons. |
| Rango et al. (2008) | 10 | 1.5 | 15 | Right M1 | None | Increased the myo-inositol content under the anode electrode. |
| Nitsche et al. (2009) | 12 | 1 | 13 | – | Citalopram (CIT) | Enhanced and prolonged the effect of tDCS. |
| Stagg et al. (2009) | 7–11 | 1 | 10 | – | None | Locally decreased GABA in the cortex. Glutamic acid decreased in correlation with the decrease in GABA due to cathodal stimulation. |
| Monte-Silva et al. (2010) | 12 | 1 | 13 | Left M1 | L-dopa | Suppressed the effect of tDCS. |
| Stagg et al. (2011) | 12 | 1 | 10 | Left M1 | None | Decreased GABA. Positive correlation was found between motor learning and changes in the fMRI signal on the left M1. |
| Thirugnanasambandam et al. (2011) | 48 | 1 | 13 | Left M1 | Nicotine | Suppressed the effect of tDCS. |
| Chaieb et al. (2012) | 8 | 1 | 5 | Left M1 | d-CYC | Suppressed the effect of tDCS. |
tDCS, transcranial direct current stimulation; M1, primary motor cortex.
Table 3.
Pharmacological actions.
| Drug | Pharmacological action |
|---|---|
| Carbamazepine (CBZ) | Sodium channel inhibitor |
| Dextromethorphan (DMO) | NMDA receptor inhibitor |
| Flunarizine (FLU) | Calcium channel inhibitor |
| Lorazepam (LOR) | GABA receptor agonist |
| d-cycloserine (d -CYC) | NMDA receptor partial agonist |
| Amfetaminil (AMP) | Adrenergic receptor agonist |
| Propranolol (PRO) | Adrenergic receptor inhibitor |
| Sulpiride (SUL) | Dopamine receptor inhibitor |
| Pergolide (PGL) | Dopamine receptor agonist |
| Rivastigmine (RIVA) | Cholinesterase inhibitor |
| Levodopa (L-dopa) | Dopamine precursor |
| Citalopram (CIT) | Serotonin reuptake inhibitor |
| Nicotine | Nicotinic acetylcholine receptor agonist |
Glutamate receptor subtypes governing excitatory synaptic transmissions include AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-D-aspartate) receptors, both of which are coupled with ion-channels. The AMPA receptor is involved in the intracellular influx of sodium ion during neuronal depolarization, causes transient action potentials, and accounts for most of the excitatory synaptic transmissions. On the other hand, the NMDA receptor is involved in the intracellular influx of calcium ion during depolarization, produces prolonged action potentials, and mediates neural circuits governing memory and learning. Therefore, actions on NMDA receptors, inducing plasticity of neurons, play a dominant role in improving symptoms of psychiatric disorders. Accordingly, dextromethorphan (NMDA receptor inhibitor) suppresses the effect of anodal stimulation (Liebetanz et al., 2002; Nitsche et al., 2003, 2004a), while d-cyclo-serine (partial NMDA receptor agonist) prolongs it (Nitsche et al., 2004b). This is in line with the observations that NMDA receptor agonists enhance excitatory synaptic transmissions, while NMDA receptor inhibitors suppress it (Liebetanz et al., 2002; Nitsche et al., 2003, 2004a,b). Also, GABA (gamma-aminobutyric acid: γ-aminobutyric acid), a neurotransmitter that inhibits synaptic transmissions, may play a role. Thus, lorazepam, a GABA receptor agonist, delays the effect of anodal stimulation (Nitsche et al., 2004c). On the other hand, the anodal stimulus itself causes a local decrease in GABA concentrations in the cortex (Stagg et al., 2009, 2011).
Monoamine neurotransmitters, such as dopamine, serotonin, and acetylcholine have been reported to mediate the effect of tDCS (Nitsche et al., 2006, 2009; Kuo et al., 2007, 2008; Monte-Silva et al., 2010; Thirugnanasambandam et al., 2011). For example, sulpiride, a dopamine receptor blocker (Nitsche et al., 2006), suppresses the effects of anodal stimuli, while levodopa, a dopamine precursor (Kuo et al., 2008; Monte-Silva et al., 2010), locally enhances excitement of certain synaptic transmissions (Kuo et al., 2008). These findings suggest that the action of tDCS may include regulation of dopamine transmissions. Also, citalopram, a serotonin reuptake inhibitor, enhances anodal stimulation (Nitsche et al., 2009). Regarding acetylcholine transmissions, rivastigmine, a cholinesterase inhibitor, suppresses the effect of tDCS (Kuo et al., 2007). In sum, the direction of influence on actions of anodal stimulation varies depending on monoamine neurotransmitters.
The above considerations overall lead to the concept that anodal stimulation enhances excitatory synaptic transmissions by stimulating glutamate transmissions and suppressing GABA transmissions in the cortex. On the other hand, it modulates the dopamine system, enhances and suppresses serotonin and acetylcholine transmissions, respectively. These effects of tDCS on monoamine transmissions are considered to be associated with change of the balance between excitatory and inhibitory inputs in the brain (Okun and Lampl, 2008).
Neuroanatomical Understanding of the Effect of tDCS
Impaired functional connectivity between brain regions has been reported in patients with psychiatric disorders, such as schizophrenia and bipolar disorder (Yamada et al., 2020). In schizophrenia patients, a study using resting functional magnetic resonance imaging (fMRI) found a separation between the medial prefrontal cortex and the dorsolateral prefrontal cortex (Chai et al., 2011). Another study found changes of dynamic functional connectivity mainly in the thalamus and cerebellum, as well as frontal, temporal, occipital, fusiform, post-central, cuneus, supramarginal, and calcarine cortices in patients with schizophrenia or bipolar disorder. Specifically, functional connectivities involving the post-central, frontal, and cerebellar cortices are weakened across schizophrenia and bipolar disorder, while those involving the insular, temporal, frontal, fusiform, lingual, occipital, supramarginal cortices, as well as thalamus and cerebellum, are strengthened (Du et al., 2017). Kunze et al. systematically applied tDCS to a large-scale network model consisting of 74 brain regions to investigate the functional connectivity of dynamic states. They found alterations of the competitive interrelationship of functional networks by tDCS (Kunze et al., 2016).
Based on these findings, the mechanism of action of tDCS on neural circuits are summarized in Table 4. Anodal tDCS may enhance excitatory synaptic transmissions by changing the balance between glutamate and GABA activities (Clark et al., 2011; Stagg et al., 2014; Bachtiar et al., 2015; Hunter et al., 2015), leading to modification of functional connectivity between brain regions, including the stimulation site (Polanía et al., 2011; Stagg et al., 2014; Bachtiar et al., 2015; Hunter et al., 2015). Furthermore, the effects of tDCS may be extended in the brain, through an increased/decreased release of monoamine transmitters, such as dopamine, on neural circuits that do not necessarily involve the anodal stimulation site (Polanía et al., 2011; Hunter et al., 2015; Fonteneau et al., 2018). These neural events are thought to improve psychiatric symptoms and cognitive function (Fukai et al., 2019). In summary, anodal stimulation is likely to modify activity levels of both specific brain regions and multiple network systems (Luft et al., 2014).
Table 4.
Changes in the brain networks by anodal tDCS.
| Study | Subject (n) | Stimulation intensity (mA) | Stimulation duration (min) | Stimulation site | Pharmacological intervention | Results |
|---|---|---|---|---|---|---|
| Clark et al. (2011) | Healthy subjects (7) | 2 | 30 | Right parietal lobe | None | Increased glutamic acid concentrations under anode electrodes. |
| Polanía et al. (2011) | Healthy subjects (13) | 1 | 10 | Left primary motor cortex | None | Reduced direct functional connectivity to gray matter away from the left somatomotor cortex (SM1). Enhanced functional connectivity between the premotor area and the parietal lobe via the left SM1. Enhanced functional connectivity between the left posterior cingulate cortex and the right DLPFC. |
| Stagg et al. (2014) | Healthy subjects (10) | 1 | 10 | Left primary motor cortex | None | Negative correlation between GABA concentrations and functional connectivity of the resting motor network. Decreased GABA concentrations. Enhanced the functional connectivity of the resting motor network. |
| Hunter et al. (2015) | Healthy subjects (9) | 2 | 30 | Right parietal lobe | None | Increased glutamic acid concentrations under anode electrodes. Enhanced the functional connectivity of the superior parietal-inferior parietal-left frontal parietal-cerebellum. Suppressed the functional connectivity of the anterior cingulate-basal ganglia. |
| Bachtiar et al. (2015) | Healthy subjects (12) | 1 | 20 | Left primary motor cortex | None | Decreased GABA concentrations. Enhanced the functional connectivity of the resting motor network. Decreased GABA concentrations and enhanced functional connectivity of motor networks by different mechanisms. |
| Fonteneau et al. (2018) | Healthy subjects (32) | 2 | 20 | Both dorsolateral prefrontal cortex (DLPFC) | None | Increased dopamine release in the striatum. |
| Wörsching et al. (2018) | Healthy subjects (28) | 2 | 20 | Right DLPFC | None | Decreased resting-state fMRI connectivity in a medial part of the left prefrontal cortex. Decreased regional brain activity during a delayed-response working-memory (DWM) retrieval. Faster responses to the DWM task. |
| Fukai et al. (2019) | Healthy subjects (20) | 2 | 26 | Left DLPFC | None | Increased dopamine release in the right ventral striatum. |
| Bulubas et al. (2019) | Patients with major depression (52) | 2 | 30 (22 sessions) | Left DLPFC | Escitalopram 20 mg/day |
Association between larger gray matter volumes and depression improvement assessed over a treatment period of 10 weeks. |
Mechanism of Action of tDCS via Long-Term Potentiation and Glial Cells
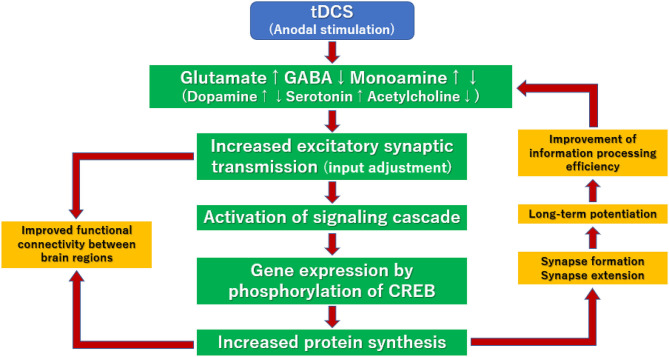
Long-term potentiation (LTP), continuous enhancement of signal transduction between neurons, is thought to mediate the effect of tDCS (Figure 3). First, action potentials in presynaptic neurons are converted into chemical signals at the presynaptic membrane. Subsequently, neurotransmitters (glutamate, GABA, dopamine, serotonin, acetylcholine, etc.) are released into the synaptic gap. The process by which this neurotransmitter is transmitted to post-synaptic neurons is called the signal transduction cascade. In this cascade, various neurotransmitters activate/inhibit transduction cascades bound to G-proteins or ion-channels, leading to phosphorylation of cAMP-responsive element binding protein (CREB) and activation of genes in the nucleus of neurons. Also, the neurotrophic factor-bound transduction cascade may play a role by activating various kinase enzymes (Stephen, 2013). These signal transduction cascades enhance the synthesis of various proteins, such as neurotransmitter synthases, receptors, ion channels, and intracellular signal proteins. Facilitative actions of these proteins that regulate efficiency of neurotransmissions in the cerebral cortex circuit may explain the ability of tDCS to induce LTP (Figure 3).
Figure 3.
Putative mechanisms for the enhancement of long-term potentiation by tDCS. Various neurotransmitters activate/inhibit transduction cascades bound to G-proteins or ion-channels, leading to phosphorylation of cAMP-responsive element binding protein (CREB) and activation of genes in the nucleus of neurons. These signal transduction cascades enhance the synthesis of various proteins, such as neurotransmitter synthases, receptors, ion channels, and intracellular signal proteins. Facilitative actions of these proteins that regulate efficiency of neurotransmissions in the cerebral cortex circuit may explain the ability of tDCS to induce LTP.
Brain-derived neurotrophic factor (BDNF) may also mediate the development of LTP (Cocco et al., 2018). So far, multi-session anodal simulations on the left dorsolateral prefrontal cortex (DLPFC) has been shown to improve mood symptoms without significant change in BDNF concentrations in the blood of patients with major depressive disorder (Brunoni et al., 2015). Further study is warranted to see if tDCS affects BDNF levels in other psychiatric disorders.
Glial cells, including astrocytes, have been reported to be activated by tDCS (Ruohonen and Karhu, 2012). As these cells regulate the concentrations of chemical substances and neurotransmitters in the outer space of neurons, the mechanisms by which tDCS ameliorate psychiatric symptoms may involve some modalities other than direct actions on neuronal cells. For example, findings from animal studies suggest involvement of LTP and glial cells (see Table 5). In rats, anodal tDCS stimulation on hippocampal CA3-CA1 synapses has been reported to induce LTP (Ranieri et al., 2012). Also, tDCS increases cAMP accumulation in the polarized cortex (Hattori et al., 1990), and changes mRNA expressions, leading to the increase in the density of dendritic spines in subjects with strokes (Jiang et al., 2012).
Table 5.
Animal studies on the mechanism of long-term potentiation (LTP) by anodal tDCS.
| Study | Age or weight, species | Stimulation intensity, duration | Stimulation site | Results |
|---|---|---|---|---|
| Hattori et al. (1990) | 190–240 g Wistar rats |
0.3, 3, or 30 μA 30–240 min, 3–5 times per day for several days |
Sensory-motor cortex | Increased cAMP accumulation in the polarized cortex in 3 μA. Decreased cAMP accumulation in the polarized cortex in 0.3 μA. |
| Fritsch et al. (2010) | Male 6–8 weeks old mice | 10 μA | Motor cortex (M1) slices | Induced synaptic plasticity in vitro by DCS, which was dependent on enhanced BDNF-secretion and TrkB-activation. |
| Jiang et al. (2012) | Adult, Sprague-Dawley rats, model of middle cerebral artery occlusion | mA, 30 min 3, 7, or 14 sessions |
Primary motor cortex | Enhanced density of dendritic spines after stroke. No change in the up-regulated PX1 mRNA expression after stroke. Improved post-stroke motor function on days 7 and 14. |
| Ranieri et al. (2012) | 150–200 g Wistar rats |
200–250 μA, 20 min | Hippocampal slices | Modulated LTP at rat hippocampal CA3-CA1 synapses. |
| Podda et al. (2016) | Male 30–45 days old mice | 350 μA, 20 min | Left hippocampus | Exhibited 1-week lasting enhancement in hippocampal LTP, learning, and memory, which were associated with enhanced acetylation of BDNF promoter I, expression of BDNF exons I and IX, and BDNF protein levels. Enhanced CREB phosphorylation, pCREB binding to BDNF promoter I, and recruitment of CREB-binding protein. |
| Monai et al. (2016) | 8–12 weeks old mice | 0.1 mA, 10 min | Primary visual cortex | Induced surges of Ca2+ influx into astrocytes across the entire cortex by using a transgenic mouse expressing G-CaMP7 in astrocytes. Changed the meta-plasticity of the cortex through astrocytic Ca2+/IP3 signaling. |
| Yu et al. (2019) | 8 weeks old male Sprague-Dawley rats | 250 μA, 30 min | Hippocampal CA1 slices | Enhanced LTP in hippocampal CA1 slices from rats. Exhibited high levels of BDNF in the hippocampal CA1 region. |
Monai et al. reported that tDCS augments noradrenaline levels by increasing intracellular calcium ion concentrations through stimulation of α1 adrenergic receptors on astrocytes in genetically modified mice (Monai et al., 2016). Also, an increase in intracellular concentrations of calcium ions by tDCS has been found in human cells (Dubé et al., 2012), suggesting the involvement of astrocytes in the ability of tDCS to induce LTP.
BDNF binds to TrkB receptors that regulate the growth and synaptic activity of neurons, and are thought to be involved in the formation of LTP (Stephen, 2013). For example, anodal tDCS induces synaptic plasticity in vitro, which is dependent on enhanced BDNF-secretion and TrkB-activations (Fritsch et al., 2010). Moreover, Podda et al. (2016) reported that mice subjected to anodal tDCS exhibited hippocampal LTP and improvement of learning and memory. These effects have been reported to be associated with enhancement of acetylation of BDNF promoter I, expression of BDNF exons I and IX, and BDNF protein levels (Podda et al., 2016). The hippocampi of mice receiving tDCS also exhibit enhanced CREB phosphorylation, and phosphorylated CREB at Ser133 (pCREB133) binds to BDNF promoter I, and recruits of CREB-binding proteins. These findings suggest that anodal tDCS increases hippocampal LTP and memory via mechanisms related to BDNF genes (Podda et al., 2016; Yu et al., 2019).
Conclusions
In this review, we discussed the electrophysiological understanding of tDCS on the basis of the stimulation-dependent model. Biochemically, enhancement of excitatory synaptic transmissions through anodal stimulation is likely to facilitate glutamate transmission and suppress gamma-aminobutyric acid transmission in the cortex. Accordingly, tDCS may positively or negatively regulate dopamine, serotonin, and acetylcholine transmissions. These neural events may change the balance between excitatory and inhibitory inputs. In this way, anodal stimulation may modulate activity levels of multiple network systems.
LTP may also provide putative mechanisms underlying the ability of tDCS to treat psychiatric disorders. Future studies should consider other domains of symptoms of psychiatric conditions of schizophrenia and mood disorders, e.g., social cognition and meta-cognition (Nishida et al., 2018; Yamada et al., 2019), as a target of treatment with tDCS. Also, identifications of predictors for its therapeutic benefits in clinical settings deserve further endeavors (Bulubas et al., 2019).
Limitations
The current review is narrative and the articles were not systematically searched. Moreover, many of the papers presented in this review targeted healthy individuals rather than those with psychiatric disorders. It should be noted that patients with mental illnesses might respond differently to tDCS from healthy people. Furthermore, some articles included in this review were on the effect of tDCS over motor cortex. Further study is warranted to examine evidence for stimulation on prefrontal cortex that has been the main target of psychiatric disorders, such as depression and schizophrenia (Mezger et al., 2020).
Author Contributions
YY and TS planned and designed the review, made substantial contributions, and approved the final manuscript. YY collected the data and drafted the first manuscript. TS critically reviewed the draft and revised it. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Drs. Kazuyuki Nakagome, Shinsuke Kito, and Naotsugu Hirabayashi at National Center of Neurology and Psychiatry for supporting our research activities.
Footnotes
Funding. This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI No. 20K16635, Intramural Research Grant (29-1, 30-1, and 30-8) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP) and JH 2020-B-08, and AMED under Grant Numbers 18dk0307069 and 18dk0307081.
References
- Bachtiar V., Near J., Johansen-Berg H., Stagg C. J. (2015). Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 4:e08789. 10.7554/eLife.08789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A. R., Baeken C., Machado-Vieira R., Gattaz W. F., Vanderhasselt M. A. (2015). BDNF blood levels after non-invasive brain stimulation interventions in major depressive disorder: a systematic review and meta-analysis. World J. Biol. Psychiatry 16, 114–122. 10.3109/15622975.2014.958101 [DOI] [PubMed] [Google Scholar]
- Bulubas L., Padberg F., Bueno P. V., Duran F., Busatto G., Amaro E., Jr., et al. (2019). Antidepressant effects of tDCS are associated with prefrontal gray matter volumes at baseline: evidence from the ELECT-TDCS trial. Brain Stimul. 12, 1197–1204. 10.1016/j.brs.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Chai X. J., Whitfield-Gabrieli S., Shinn A. K., Gabrieli J. D., Nieto Castañón A., McCarthy J. M., et al. (2011). Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36, 2009–2017. 10.1038/npp.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb L., Antal A., Terney D., Paulus W. (2012). Pharmacological modulation of the short-lasting effects of antagonistic direct current-stimulation over the human motor cortex. Front. Psychiatry 3:67. 10.3389/fpsyt.2012.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. P., Coffman B. A., Trumbo M. C., Gasparovic C. (2011). Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a 1H magnetic resonance spectroscopy study. Neurosci. Lett. 500, 67–71. 10.1016/j.neulet.2011.05.244 [DOI] [PubMed] [Google Scholar]
- Cocco S., Podda M. V., Grassi C. (2018). Role of BDNF signaling in memory enhancement induced by transcranial direct current stimulation. Front. Neurosci. 12:427. 10.3389/fnins.2018.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pearlson G. D., Lin D., Sui J., Chen J., Salman M., et al. (2017). Identifying dynamic functional connectivity biomarkers using GIG-ICA: application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum. Brain Mapp. 38, 2683–2708. 10.1002/hbm.23553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé J., Rochette-Drouin O., Lévesque P., Gauvin R., Roberge C. J., Auger F. A., et al. (2012). Human keratinocytes respond to direct current stimulation by increasing intracellular calcium: preferential response of poorly differentiated cells. J. Cell. Physiol. 227, 2660–2667. 10.1002/jcp.23008 [DOI] [PubMed] [Google Scholar]
- Fertonani A., Pirulli C., Miniussi C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 31, 15416–15423. 10.1523/JNEUROSCI.2002-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau C., Redoute J., Haesebaert F., Le Bars D., Costes N., Suaud-Chagny M. F., et al. (2018). Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb. Cortex. 28, 2636–2646. 10.1093/cercor/bhy093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F., El-Hagrassy M. M., Pacheco-Barrios K., Carvalho S., Leite J., Simis M., et al. (2020). Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. Int. J. Neuropsychopharmacol. pyaa051. 10.1093/ijnp/pyaa051. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B., Reis J., Martinowich K., Schambra H. M., Ji Y., Cohen L. G., et al. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. 10.1016/j.neuron.2010.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai M., Bunai T., Hirosawa T., Kikuchi M., Ito S., Minabe Y., et al. (2019). Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: a study with positron emission tomography. Transl. Psychiatry 9:115. 10.1038/s41398-019-0443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Moriwaki A., Hori Y. (1990). Biphasic effects of polarizing current on adenosine sensitive generation of cyclic AMP in rat cerebral cortex. Neurosci. Lett. 116, 320–324. 10.1016/0304-3940(90)90094-P [DOI] [PubMed] [Google Scholar]
- Hunter M. A., Coffman B. A., Gasparovic C., Calhoun V. D., Trumbo M. C., Clark V. P. (2015). Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Res. 1594, 92–107. 10.1016/j.brainres.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Xu R. X., Zhang A. W., Di W., Xiao Z. J., Miao J. Y., et al. (2012). Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 226, 421–426. 10.1016/j.neuroscience.2012.09.035 [DOI] [PubMed] [Google Scholar]
- Kim J., Iwata Y., Plitman E., Caravaggio F., Chung J. K., Shah P., et al. (2019). A meta-analysis of transcranial direct current stimulation for schizophrenia: “Is more better?” J. Psychiatr. Res. 110, 117–126. 10.1016/j.jpsychires.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Kunze T., Hunold A., Haueisen J., Jirsa V., Spiegler A. (2016). Transcranial direct current stimulation changes resting state functional connectivity: a large-scale brain network modeling study. Neuroimage 140, 174–187. 10.1016/j.neuroimage.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Kuo M. F., Grosch J., Fregni F., Paulus W., Nitsche M. A. (2007). Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J. Neurosci. 27, 14442–14447. 10.1523/JNEUROSCI.4104-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. F., Paulus W., Nitsche M. A. (2008). Boosting focally-induced brain plasticity by dopamine. Cereb. Cortex. 18, 648–651. 10.1093/cercor/bhm098 [DOI] [PubMed] [Google Scholar]
- Liebetanz D., Nitsche M. A., Tergau F., Paulus W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 125(Pt 10), 2238–2247. 10.1093/brain/awf238 [DOI] [PubMed] [Google Scholar]
- Luft C. D., Pereda E., Banissy M. J., Bhattacharya J. (2014). Best of both worlds: promise of combining brain stimulation and brain connectome. Front. Syst. Neurosci. 8:132. 10.3389/fnsys.2014.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros L. F., de Souza I. C., Vidor L. P., de Souza A., Deitos A., Volz M. S., et al. (2012). Neurobiological effects of transcranial direct current stimulation: a review. Front. Psychiatry 3:110. 10.3389/fpsyt.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzagora A. C., Foffani G., Panyavin I., Mordillo-Mateos L., Aguilar J., Onaral B., et al. (2010). Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage 49, 2304–2310. 10.1016/j.neuroimage.2009.10.044 [DOI] [PubMed] [Google Scholar]
- Mezger E., Rauchmann B. S., Brunoni A. R., Bulubas L., Thielscher A., Werle J., et al. (2020). Effects of bifrontal transcranial direct current stimulation on brain glutamate levels and resting state connectivity: multimodal MRI data for the cathodal stimulation site. Eur. Arch. Psychiatry Clin. Neurosci. 10.1007/s00406-020-01177-0. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monai H., Ohkura M., Tanaka M., Oe Y., Konno A., Hirai H., et al. (2016). Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 7:11100. 10.1038/ncomms11100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K., Liebetanz D., Grundey J., Paulus W., Nitsche M. A. (2010). Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J. Physiol. 588(Pt 18), 3415–3424. 10.1113/jphysiol.2010.190181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Z., Stickley A., DeVylder J., Yokoi Y., Inagawa T., Yamada Y., et al. (2020). Effect of multi-session prefrontal transcranial direct current stimulation on cognition in schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 216, 367–373. 10.1016/j.schres.2019.11.011 [DOI] [PubMed] [Google Scholar]
- Nishida K., Toyomaki A., Koshikawa Y., Niimura H., Morimoto T., Tani M., et al. (2018). Social cognition and metacognition contribute to accuracy for self-evaluation of real-world functioning in patients with schizophrenia. Schizophr. Res. 202, 426–428. 10.1016/j.schres.2018.06.071 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Fricke K., Henschke U., Schlitterlau A., Liebetanz D., Lang N., et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 553(Pt 1), 293–301. 10.1113/jphysiol.2003.049916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M. A., Grundey J., Liebetanz D., Lang N., Tergau F., Paulus W. (2004a). Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb. Cortex. 14, 1240–1245. 10.1093/cercor/bhh085 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Jaussi W., Liebetanz D., Lang N., Tergau F., Paulus W. (2004b). Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology 29, 1573–1578. 10.1038/sj.npp.1300517 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Kuo M. F., Karrasch R., Wächter B., Liebetanz D., Paulus W. (2009). Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol. Psychiatry 66, 503–508. 10.1016/j.biopsych.2009.03.022 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Lampe C., Antal A., Liebetanz D., Lang N., Tergau F., et al. (2006). Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur. J. Neurosci. 23, 1651–1657. 10.1111/j.1460-9568.2006.04676.x [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Liebetanz D., Schlitterlau A., Henschke U., Fricke K., Frommann K., et al. (2004c). GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur. J. Neurosci. 19, 2720–2726. 10.1111/j.0953-816X.2004.03398.x [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Paulus W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 527(Pt 3), 633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M., Lampl I. (2008). Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 11, 535–537. 10.1038/nn.2105 [DOI] [PubMed] [Google Scholar]
- Philip N. S., Nelson B. G., Frohlich F., Lim K. O., Widge A. S., Carpenter L. L. (2017). Low-intensity transcranial current stimulation in psychiatry. Am. J. Psychiatry 174, 628–639. 10.1176/appi.ajp.2017.16090996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda M. V., Cocco S., Mastrodonato A., Fusco S., Leone L., Barbati S. A., et al. (2016). Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci. Rep. 6:22180. 10.1038/srep22180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R., Paulus W., Antal A., Nitsche M. A. (2011). Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage 54, 2287–2296. 10.1016/j.neuroimage.2010.09.085 [DOI] [PubMed] [Google Scholar]
- Rango M., Cogiamanian F., Marceglia S., Barberis B., Arighi A., Biondetti P., et al. (2008). Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn. Reson. Med. 60, 782–789. 10.1002/mrm.21709 [DOI] [PubMed] [Google Scholar]
- Ranieri F., Podda M. V., Riccardi E., Frisullo G., Dileone M., Profice P., et al. (2012). Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J. Neurophysiol. 107, 1868–1880. 10.1152/jn.00319.2011 [DOI] [PubMed] [Google Scholar]
- Ruohonen J., Karhu J. (2012). tDCS possibly stimulates glial cells. Clin. Neurophysiol. 123, 2006–2009. 10.1016/j.clinph.2012.02.082 [DOI] [PubMed] [Google Scholar]
- Seo H., Jun S. C. (2019). Relation between the electric field and activation of cortical neurons in transcranial electrical stimulation. Brain Stimul. 12, 275–289. 10.1016/j.brs.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Shiozawa P., Fregni F., Benseñor I. M., Lotufo P. A., Berlim M. T., Daskalakis J. Z.. (2014). Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 17, 1443–1452. 10.1017/S1461145714000418 [DOI] [PubMed] [Google Scholar]
- Silvanto J., Muggleton N., Walsh V. (2008). State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 12, 447–454. 10.1016/j.tics.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Stagg C. J., Bachtiar V., Amadi U., Gudberg C. A., Ilie A. S., Sampaio-Baptista C., et al. (2014). Local GABA concentration is related to network-level resting functional connectivity. Elife 3:e01465. 10.7554/eLife.01465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. J., Bachtiar V., Johansen-Berg H. (2011). The role of GABA in human motor learning. Curr. Biol. 21, 480–484. 10.1016/j.cub.2011.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. J., Best J. G., Stephenson M. C., O'Shea J., Wylezinska M., Kincses Z. T., et al. (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 29, 5202–5206. 10.1523/JNEUROSCI.4432-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. J., Nitsche M. A. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53. 10.1177/1073858410386614 [DOI] [PubMed] [Google Scholar]
- Stephen M. S. (2013). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications 4th Edn. New York, NY: Cambridge University Press. [Google Scholar]
- Thirugnanasambandam N., Grundey J., Adam K., Drees A., Skwirba A. C., Lang N., et al. (2011). Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology 36, 879–886. 10.1038/npp.2010.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörsching J., Padberg F., Goerigk S., Heinz I., Bauer C., Plewnia C., et al. (2018). Testing assumptions on prefrontal transcranial direct current stimulation: comparison of electrode montages using multimodal fMRI. Brain Stimul. 11, 998–1007. 10.1016/j.brs.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Inagawa T., Sueyoshi K., Sugawara N., Ueda N., Omachi Y., et al. (2019). Social cognition deficits as a target of early intervention for psychoses: a systematic review. Front. Psychiatry 10:333. 10.3389/fpsyt.2019.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Matsumoto M., Iijima K., Sumiyoshi T. (2020). Specificity and continuity of schizophrenia and bipolar disorder: relation to biomarkers. Curr. Pharm. Des. 26, 191–200. 10.2174/1381612825666191216153508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi Y., Narita Z., Sumiyoshi T. (2018). Transcranial direct current stimulation in depression and psychosis: a systematic review. Clin. EEG Neurosci. 49, 93–102. 10.1177/1550059417732247 [DOI] [PubMed] [Google Scholar]
- Yu T. H., Wu Y. J., Chien M. E., Hsu K. S. (2019). Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor. Neuropharmacology 144, 358–367. 10.1016/j.neuropharm.2018.11.012 [DOI] [PubMed] [Google Scholar]