Abstract
Turmeric is a known spice indispensable for food preparation and is reported to possess different chemical properties and biological activities. The objective of this study was to determine the effects of variety and type of extracts on 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reducing capacity, antibacterial activity, and chemical composition of essential oil from turmeric varieties cultivated in Ethiopia. The results from the statistical analysis revealed that the highest total curcuminoids content (6.81% m/m), essential oil (6.37% v/m), methanol extract (13.42% v/m) were obtained from Dame variety. On the other hand, the lowest curcuminoids (5.12% m/m), methanol extract (10.58% v/m) and essential oil (3.92% v/m) contents were obtained from HT3/2002 variety. Dame variety also had the highest total polyphenol content (97.55 mg GAE/g) and radical scavenging capacity (46.58 16 μg/mL) with the lowest IC50 value (23.05 μg/mL). Among the tested microorganisms with turmeric extracts, growth inhibition was observed against S. aureus. The results also indicated that three compounds, namely α-Turmerone (32.41 and 35.16%), ar-Turmerone (25.20 and 25.47%), and Curlone (17.98 and 18.19%) dominated 75% of the essential oil component in Dame and Bonga 51/71 varieties, respectively. In summary, the results of this study revealed that extracts from Dame variety have strong biological potential with desired antioxidant and antibacterial activities.
Keywords: Antioxidant, Antibacterial, Curcuma domestica, Ethiopia, Essential oil, Turmeric
Antioxidant; Antibacterial; Curcuma domestica; Ethiopia; Essential oil; Turmeric
1. Introduction
Humankind is often vulnerable to oxidative stress due to reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Tanvir et al., 2017) as well as to diseases caused by bacterial infections (Niamsa and Sittiwet, 2009). Many people take antioxidants and antimicrobials in a form of commercial food additives that are produced synthetically and contain high amounts of preservatives to counter the effects of oxidative stress and growth of food spoiling microbes (Saǧdıç and Özcan, 2003; Akter et al., 2019). However, due to consumer health concerns related to the safety of food containing synthetic chemicals as preservatives, there is a growing interest in using natural antioxidants and antibacterial compounds (Norajit et al., 2007; Vallverdú-Queralt et al., 2015).
Turmeric (Curcuma longa), from a very important genus in the family of Zingiberaceae and Curcuma species is native to tropical south-east Asia, particularly Indonesia and southern India (Ravindran et al., 2007). Essential oils (EOs) and diarylheptanoid curcumin are the major secondary metabolites of turmeric that are used in a wide range of pharmacological applications (Ravindran et al., 2007; Prasad and Aggarwal, 2011). These active principles of turmeric rhizomes show a wide range of biological activities such as antibacterial, antifungal, anticancer, insect repellant and anti-snake venom activity (Negi et al., 1999; Kocaadam and Şanlier, 2017; Dosoky and Setzer, 2018). However, the amount of bioactive extracts, antioxidant and antimicrobial properties depend on the type of chemical composition and cultivated turmeric varieties (Tanvir et al., 2017; Akter et al., 2019).
Although systematic production of C. longa on a commercial scale started in Ethiopia in the 1970s (Peethambaran et al., 2016), only three known turmeric varieties were released by Tepi National Spices Research Center since 2007. This institution introduced ‘Dame’ variety in 2007 and ‘Bonga 51/71’ and ‘HT3/2002’ varieties in 2018. These varieties had been widely adopted in different parts of Ethiopia. They were evaluated for yield (kg/ha) and some quality attributes of the rhizomes (Kifelew et al., 2018; Mekonnen and Garedew, 2019). Other studies focused on the effects of harvesting stage (Hailemichael et al., 2016) and postharvest operations (Mariam et al., 2017) on quality attributes of Dame variety. However, there is no study on the chemical composition and the essential and therapeutic values of turmeric varieties grown in Ethiopia. Therefore, this study aimed to determine the chemical fingerprint of essential oils, abundance of curcumin and other extracts, antibacterial activities against strains of E. coli, S. aureus and Salmonella typhimurium, total phenolic content, and DPPH free radical scavenging assay of turmeric varieties cultivated in Ethiopia.
2. Material and methods
2.1. Plant materials
Nine-month-old rhizomes from three varieties of Curcuma domestica (Bonga 51/71, HT3/2002, and Dame) were harvested after botanically identified and planted at Tepi National Spices Research Center, Ethiopia. The collected plant specimens were identified by a taxonomy specialist, Mr Melaku Wondafrash, at the National Herbarium, College of Natural Sciences, Addis Ababa University, Ethiopia and deposited under a voucher specimen (Voucher Specimen number: T071). The sampling area was located at the Latitude of 7°10′ 54.5″ N; Longitude of 35° 25.04′ 28.2″ E, and altitude of 1200 m.a.s.l. Harvested rhizomes were washed thoroughly to remove soil and dirt particles. After washing, the curing process was conducted by boiling the fresh rhizomes in water, for 45 min. The boiled rhizomes were dried and grounded with a laboratory model grinder (Retsch GmbH mill model 5657, Germany). Ground turmeric was passed through a 0.50 mm sieve to obtain a uniform fine powder, packed with polyethylene bags, and then stored in a refrigerator at 4 °C until further analysis.
2.2. Extraction procedure
2.2.1. Hydrodistillation
Essential oils were obtained from the powdered rhizome by hydrodistillation method using a Clevenger-type apparatus following the procedure described in Singh et al. (2011). Fifty-gram dried turmeric powder was put in a 1000 mL flask, and 500 mL of distilled water along with few glass beads were added. The flask was then filled with an essential oil extraction apparatus fitted with lighter than water type extractor (Clevenger's Apparatus). The distillation was conducted in a thermostatically controlled heater for two hours at 70 °C. The volume of oil in the graduated tube of volatile oil determination tube was recorded, and the percentage of volatile oil in (v/m) air-dried material was calculated.
2.2.2. Solvent extraction
The methanolic extract was prepared as described by Green et al. (2008), but with slight modifications. Fifteen-gram grounded turmeric powder was put in a 1 L conical flask and aliquot of 250 mL of methanol (99%) was added, and the conical flask was covered with aluminum foil to minimize solvent loss through evaporation. The mixture was then placed on a magnetic stirrer and extracted for six hours. The extract was filtered by gravity when the extraction was completed. The residue obtained from the filtration was re-extracted with 100 mL of ethanol for two hours. Both filtrates were pooled together and concentrated in a rotary evaporator (Colen-Parmer, SB-1200) in a water bath set at 50 °C. The obtained methanol extract was quantified, and the yield was calculated as a percentage (w/w).
2.3. Estimation of total polyphenol content
The total polyphenol content (TPC) of the turmeric extracts was determined following the Singleton et al. (1999) procedure adopted by Tanvir et al. (2017) using Folin-Ciocalteu reagent. Briefly, 5% methanolic extract was prepared by adding turmeric powder (5 g) in 70% methanol solution to make a 100 mL solution. Then the solution was filtered through Whatman No. 44 filter paper. After filtration, 0.1 mL of the extract (0.5 mg/mL) was mixed with 2 mL of 7.5% sodium carbonate solution. Then, 2.5 mL of 10-fold diluted Folin-Ciocalteu reagent was added, and then distilled water was added to make the solution 10 mL. The resulting final reaction mixture was incubated for one hour in the dark. The intensity of the blue-colored complex was measured at 765 nm using a JASCO V-630 spectrophotometer (Shimadzu Corporation, Japan). The TPC present was expressed as milligram of Gallic acid equivalent (GAE)/gram of extracts on a dry weight basis (DW) of plant material after plotting calibration curve for different concentrations (5–30 μg/mL) of Gallic acid.
2.4. Quantitative determination of total curcuminoids
Total curcuminoids content in each sample of C. longa was performed as described in Pothitirat and Gritsanapan (2006) with some modifications. For the preparation of the standard solution, standard curcumin (100 mg) (cat # C-75300, purity >95%) was accurately weighed and transferred to a 100 mL volumetric flask. The stock solution was prepared by adding methanol and adjusting to a final volume of 100 mL. Then 2 mL stock solution was added to 25 mL volumetric flask, and the volume was made with methanol. From this solution, concentrations of 1.6, 3.2, 4.8, 6.4 and 8.0 μg/mL were prepared and used for the preparation of the calibration curve.
For the preparation of the sample solution from turmeric powder, the powder (100 mg) of each sample was separately transferred to a 10 mL volumetric flask. Methanol was added to volume and mixed. Then the mixture was set aside at room temperature for 24 h with frequent shaking. One mL of the clear supernatant liquid was transferred and diluted with methanol to 25 mL volume. One mL of this solution was then transferred to a 50 mL volumetric flask and diluted to volume with methanol. The absorbance of these solutions and serial standard dilutions were taken at 425 nm using UV-Visible spectrophotometry (Model: JASCO V-630, Shimadzu Corporation, Tokyo, Japan) having two matched quartz cells with 1 cm path length.
2.5. DPPH radical scavenging activity
To determine radical scavenging activity by DPPH method, 5% methanolic extract was prepared by adding turmeric powder (5 g) in methanol to make a 100 mL solution. Briefly, between 0.05 and 0.4 mL of the extract was mixed with 2 mL of 0.1 mM 1, 1-diphenyl-2-picrylhydrazyl (DPPH) in methanol. The final solution was adjusted to 3 mL using methanol to produce different concentrations (16.00–130.00 μg/mL). The %DPPH radical scavenging activity was calculated using the following equation:
where ADPPH is the absorbance of DPPH in the absence of a sample. As is the absorbance of DPPH in the presence of a sample.
The percentage of radical scavenging activity was plotted against the corresponding extract concentration to obtain the IC50 (half-maximal inhibitory concentration) value. Vitamin C (ascorbic acid) was used as a positive control.
2.6. Antibacterial activity
The agar disc diffusion method was used to check turmeric extracts' antibacterial activity as described in Gupta et al. (2015) with minor modifications. In this study, antibacterial patterns were studied for different extracts of C. domestica (rhizome) viz methanol extract (oleoresin) and aqueous extract (essential oil) for comparing three turmeric varieties. The standard strains Staphylococcus aureus ATCC 25923, Salmonella typhimurium ATCC 25953, and Escherichia coli ATCC 25922 used as test organism were obtained from collections of Ethiopian Biodiversity Institute, Addis Ababa. Turmeric extracts were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich USA) to get 100 mg/mL concentration. Sterile filter paper discs that have 8 mm diameter were impregnated with 50 μl of diluted extract solution and then incubated for 15 min to ensure proper diffusion of the extract. The serial dilution was then surface spread onto nutrient agar plates with 0.1 ml of bacterial culture standardized to 0.5 McFarland standards (1.5 × 108 CFU ml−1) and incubated at 37 °C for 24 h. The inhibition zones' diameter was measured, and the mean value of three independent experiments (n = 3) was used in the statistical analysis. The control discs contained DMSO only, and the standard antibiotic gentamycin (10 μg/disc) was included in the assay as a positive control.
2.7. Analysis of essential oil from two varieties of Curcuma
Essential oil components were determined for two of the varieties (Dame and Bonga 51/71) chosen based on their good previously analyzed quality parameters. The analysis was conducted with Gas chromatograph (GC) of 890B series instrument (Agilent Technologies, Palo Alto, CA, USA). Then it was fitted with quadrupole mass spectrometer (MS) (Agilent 5977A MSD), and DB-5MS fused silica capillary column (30 m × 0.25 mm [internal diameter film thickness 0.25 mm]). Oven temperature was increased to 60–75 °C at 4 °C/min; 75–140 °C at 7 °C/min; 140–145 °C at 2 °C/min; and 145–270 °C at 20 °C/min. Both auto-injector and detector temperatures were kept at 280 °C; sample injection volume, 1 μL; split ratio was 10:1. The carrier gas was helium at a flow rate of 1 ml/min. GC/MS (70eV) data were measured on the same gas chromatography coupled with MSD 5977. The settings used were MS source temperature at 280 °C, ion source temperature at 280 °C, quadrupole temperature at 150 °C, solvent delay of 3 min, and acquisition mode scan of 50–550 amu. The retention index was calculated using a homologous series of n-alkanes (C8–C18).
2.8. Identification of chemical constituents of essential oils
Characterization of chemical components from EO of Curcuma domestica was done based on their retention time; retention indices (RI) determined using a homologous series of n-alkanes (C8–C18) under the same temperature-programmed conditions, co-injection with standards (Aldrich and Fluka). Compounds were identified by comparing their retention indices and mass spectra with National Institute of Standards and Technology (NIST) and Wiley, and then by comparing the given mass spectral data with literature data (Adams, 2007). The relative amount of each component of the essential oil and its fractions were expressed as a percentage of the peak area relative to the total peak area.
2.9. Statistical analysis
The effect of Variety (3 levels: Bonga 51/71, HT3/2002, and Dame) on % Curcuminoids, % Methanol extract, % EO yield and TPC, and the effect of Treatment (4 levels: Bonga 51/71, HT3/2002, Dame, and Control) on % DPPH scavenging capacity and IC50 were determined using one-way analysis of variance (ANOVA). The effects of Variety (3 levels: Bonga 51/71, HT3/2002, and Dame) and type of Extract (2 levels: essential oil and methanolic extract) on inhibition zone against S. aureus were determined using two-way ANOVA. Normal distribution and constant variance assumptions on the error terms were verified by examining the residuals as described in Montgomery (2020). Since the main effects of Variety and Treatment were significant on all response variables, multiple means comparison was completed using Duncan's method (Montgomery, 2020) and letter groupings were generated at the 5% level of significance. The analysis was completed using SAS version 9.4 software (SAS, 2014). Excel (in Microsoft Office, Microsoft, WA, USA) was used to produce the figures.
3. Results and discussion
This study is the first to report the chemical composition of essential oil, total polyphenol, antioxidant and antimicrobial properties of turmeric varieties grown in Ethiopia.
3.1. Total curcuminoids, methanol extracts and essential oil yields
The mean curcuminoids contents of the three turmeric varieties were significantly different from each other and the highest (6.81%) and the lowest (5.12%) curcuminoids contents were obtained from Dame and HT3/2002 varieties, respectively (Table 1). The results shown in Table 1 indicate that the mean methanol extracts and essential oil yields obtained from Bonga 51/71 and Dame varieties were not significantly different. The highest (13.62%) methanol extract was obtained from Bonga 51/71, while the lowest (10.58%) was obtained from HT3/2002 variety. Similarly, the mean essential oil yields from Dame and Bonga 51/71 varieties were not statistically different but were significantly higher than the one from HT3/2002 variety (Table 1).
Table 1.
Mean total curcuminoids, methanol extracts and essential oil yield obtained from three turmeric varieties.
| Variety | % Curcuminoids (m/m) | % Methanol extracts (m/m) | % Essential oil (v/m) |
|---|---|---|---|
| Bonga 51/71 | 6.49b | 13.62a | 6.27a |
| HT3/2002 | 5.12c | 10.58b | 3.92b |
| Dame | 6.81a | 13.42a | 6.37a |
| 0.07 | 0.44 | 0.09 |
Within each column, means sharing the same letter are not significantly different at the 5% level. = the square root of the Mean Square Error (MSE), which estimates the common standard deviation (σ).
The content of curcumin in turmeric determines its color, quality, and therapeutic utility. Extraction of turmeric powder with methanol resulted in a yellow-colored pigment commercially known as oleoresin, the mother liquor of curcuminoids that becomes curcumin (Negi et al., 1999; Teow et al., 2016). The extracted essential oils from turmeric powders through hydrodistillation were slightly viscous liquid with orange-colored, spicy odor, and insoluble in water characteristics. However, the essential oils are soluble in acetone and ethanol. Hence, a combination of both curcuminoids and essential oils had a wide range of biological activities including antioxidant, anti-inflammatory, anticancer, antimicrobial, neuroprotective, cardioprotective, radio-protective effects and anti-Alzheimer activities in both preclinical and clinical studies (Amalraj et al., 2017; Meng et al., 2018).
The use of turmeric is spreading internationally, and its production must be increased to meet national and international demands. In this study, Dame is the most promising variety containing the highest amount of curcuminoids, methanol extract and essential oil yield among the turmeric varieties grown in Ethiopia. Kifelew et al. (2018) reported that Bonga 51/71, HT3/2002 and Dame were the best performing genotypes in terms of growth parameters and yield attributes before they were released as turmeric varieties. Previous research (Singh et al., 2014; Aarthi et al., 2020) indicated that growth and yield parameters impact curcuminoids, oleoresin and essential oil yield of turmeric rhizomes positively. To introduce turmeric cultivation into non-traditional areas, varieties that are good in productivity, and that give high bioactive extract need to be identified. Therefore, the results of this study show a promising opportunity for selecting a turmeric variety with good amount of chemical composition that can bring socio-economic transformations and improve the livelihoods of turmeric farmers in Ethiopia.
3.2. Total polyphenols content (TPC)
The mean values of TPC in turmeric rhizomes of different varieties are shown in Table 2. These multiple means comparison results revealed that TPC of Bonga 51/71 and Dame varieties are not significantly different from each other at the 5% level of significance. The two highest TPCs were found in Dame (97.55 mg GAE/g) and Bonga 51/71 (96.52 mg GAE/g), whereas the lowest was found in HT3/2002 (83.21 mg GAE/g). This difference among varieties is in line with a previous study by Tanvir et al. (2017) who reported that different turmeric varieties cultivated in Bangladesh gave different TPC. A similar pattern was obtained in Japan (Akter et al., 2019) where the minimum (37.9) and the maximum (157.4) mg GAE/g TPC were obtained from a methanolic extract of C. aromatica and Ryudai gold turmeric varieties, respectively.
Table 2.
Mean concentrations of total phenolic content (TPC), % DPPH scavenging capacity, and IC50 obtained from four treatments.
| Treatment | Total phenolic content (mg GAE/g DW) | % DPPH scavenging capacity of 16 μg/mL | IC50 (μg/mL) |
|---|---|---|---|
| Bonga 51/71 | 96.52a | 40.80c | 44.32a |
| HT3/2002 | 83.21b | 43.68b | 32.25b |
| Dame | 97.55a | 46.58a | 23.05c |
| Control | - | 48.83a | 17.81d |
| 1.20 | 1.29 | 1.07 |
Within each column, means sharing the same letter are not significantly different at the 5% level. DW = dry weight basis, IC50 = Half maximal (antioxidant activity) effective concentration. = the square root of the Mean Square Error (MSE) that estimates the common standard deviation (σ).
Total phenolic contents are essential constituents of plant materials that contribute to the functional quality (color, and flavor) and minimize molecular damage through quenching singlet oxygen and scavenging free radicals (Tanvir et al., 2017). Sukati and Khobjai (2019) observed a significant positive correlation between TPC and 1/IC50 values for DPPH, indicating the considerable contribution of phenolic compounds to these antioxidant assays. Moreover, the TPC of turmeric extracts can decrease when the extraction is performed with an aqueous methanol mixture. For example, Nisar et al. (2015) reported that turmeric samples from Faisalabad, Pakistan extracted with 60 and 80% methanol in water showed TPC of 5.24 and 6.82 mg GAE/g, respectively. Similarly, Sukati and Khobjai (2019) reported that turmeric rhizomes from Southern Thailand exhibited TPC of 19.79 mg GAE/g for aqueous extract and 23.69 mg GAE/g for 80% methanol extract. From these results, we postulate that the difference in TPC might be due to the solvent used in extraction, the variety, and the geographic area where the turmeric is cultivated.
3.3. DPPH radical scavenging activity
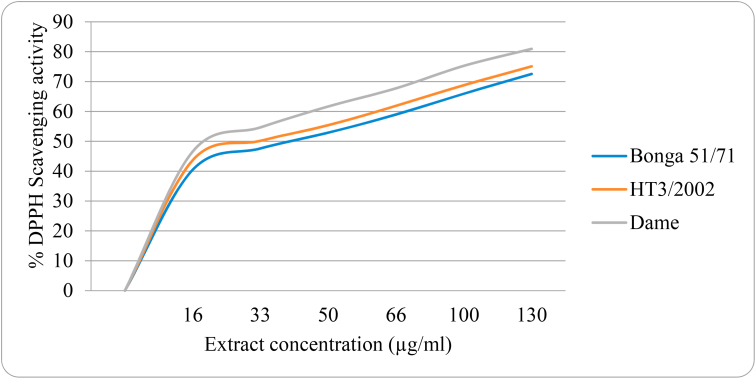
Figure 1 shows a relationship between the concentration (μg/mL) of methanolic extracts and DPPH radical scavenging capacity. The results indicate that as the extracts' concentration increases from 16 to 130 μg/mL, the percentage of scavenging capacity rises by 77.9, 71.9, and 73.8 % for Bonga 51/71, HT3/2002, and Dame varieties, respectively. The scavenging capacity of DPPH versus concentration of extracts (μg/mL) was plotted for the determination of half-maximal inhibitory concentration (IC50) of the DPPH-radical scavenging activity.
Figure 1.
DPPH radical scavenging activity of three turmeric varieties (Bonga 51/71, HT3/2002 and Dame) at different extract concentrations (μg/mL) in methanol.
Table 2 shows the calculated percentage of inhibition at 16 μ g/mL and IC50 values of the standard and the extracts. Amongst the three turmeric varieties, Dame revealed the highest scavenging activity (46.58%) with an IC50 value of 23.05 μg/mL, and Bonga 51/71 variety exhibited the lowest scavenging activity (40.80%) and higher IC50 value (44.32 μg/mL). Here, IC50 explains the antioxidant concentration required for 50% scavenging of DPPH radicals in the specified time. The smaller IC50 values correspond to the plant extracts' higher antioxidant activity (Khatoon et al., 2013).
Free radicals play a substantial role in a wide variety of pathological manifestations. Antioxidants fight against free radicals and protect us from various diseases (Umamaheswari and Chatterjee, 2008). They exert their action either by scavenging free radicals or by safeguarding the antioxidant defense mechanisms (Nimse and Pal, 2015). Additionally, antioxidants' polarity and hydrophobicity play an essential role in the antioxidant activity, especially in the biomembrane systems (Kumar et al., 2016). The DPPH assay principle is based on the antioxidant extracts that could reduce the stable DPPH to yellow. The antioxidants scavenge DPPH radical by donating hydrogen atoms leading to a non-radical state (DPPH-H) with yellow color (Afroz et al., 2014).
However, compared with control (ascorbic acid), Dame variety had similar potency in the percentage of DPPH scavenging capacity at 16 μ g/mL with slightly higher IC50 value. Ascorbic acid is a potent antioxidant that directly interacts with a broad spectrum of harmful ROS, terminates the chain reaction initiated by free radicals via electron transfer, and is involved in the regeneration of other antioxidants, such as tocopherol, to their functional state (Chan, 1993). The high content of TPC and the considerable DPPH free radical-scavenging activity of Dame variety suggest that it is a promising source of natural antioxidants.
3.4. Antibacterial activities
The preliminary screening of antibacterial activity of turmeric extracts against different bacterial strains is shown in Table 3. There was no observed growth inhibition of Curcuma domestica extracts against E.coli and S. typhimurium (both Gram-negative) in the present study. On the other hand, a remarkable bacterial growth inhibition pattern of extracts was observed against S. aureus (Gram-positive) among the tested microorganisms.
Table 3.
Inhibition effect of turmeric extracts, having antibacterial activity against test bacteria.
| Test Pathogen | Treatment |
|||
|---|---|---|---|---|
| Essential oils | Methanol extract | Gentamicin | DMSO | |
| S.aureus | +ve | +ve | +ve | -ve |
| E.coli | -ve | -ve | +ve | -ve |
| S. typhimurium | -ve | -ve | +ve | -ve |
+ve = inhibits bacterial growth; -ve = does not inhibit bacterial growth.
The varying sensitivity degree of turmeric extracts against bacterial strains might be due to the difference in the cell wall structure of Gram-positive and Gram-negative bacteria. It was described by Chouhan et al. (2017) that, Gram-negative bacteria such as E. coli and S. typhimurium, exhibit rigid lipopolysaccharide on an outer membrane, thereby limiting the diffusion of hydrophobic compounds through it. On the other hand, a thick peptidoglycan wall is not dense enough to resist small antimicrobial molecules, facilitating access to the Gram-positive bacteria's cell membrane. Besides, as Kwiatkowski et al. (2020) mentioned, Gram-positive bacteria may ease the infiltration of hydrophobic compounds of EOs due to the lipophilic ends of lipoteichoic acid present in the cell membrane. These compounds impart crucial processes in the cell due to the increased membrane permeability, consequently inducing leakage of ions and important cell contents, that eventually leads to cell death.
Medicinal herbs possess curative properties due to the various complex chemical substances of different composition, which are found as secondary plant metabolites in one or more parts of these plants (Kuruppu et al., 2019). Several researchers have explored the antimicrobial properties of extracts obtained from multiple Curcuma species. For example, in a study conducted by Jose and Thomas (2014), extracts obtained from C. caesia and C. aeruginosa rhizome with different solvent showed inhibitory action against three Gram-positive (S. aureus, S. haemolyticus, B. cereus) and five Gram-negative (S. typhi, T. enterobacter, V. cholerae, P. aeruginosa and S. marcescense) bacteria. Stanojević et al. (2015) indicated that, essential oils obtained from Curcuma longa exhibit a more potent antimicrobial activity on Gram-positive bacteria (compared to Gram-negative bacteria). Similarly, Gupta et al. (2015) reported extracts obtained from Curcuma longa rhizome exhibited antimicrobial activity against S. aureus.
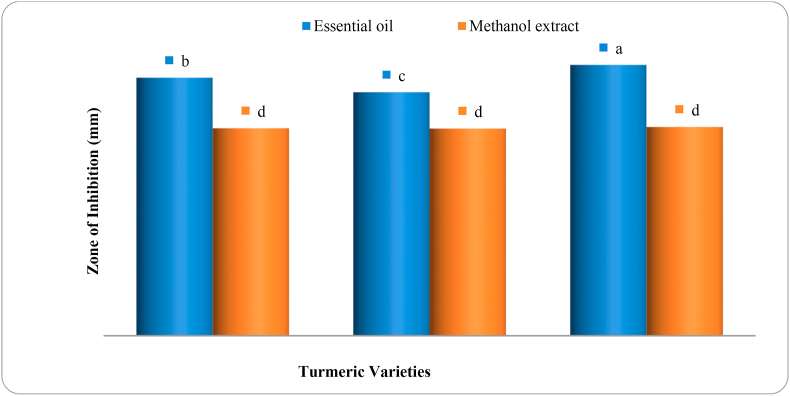
Figure 2 shows the quantitative antibacterial activity of essential oils and methanol extracts from three varieties of Curcuma domestica against the S. aureus. It is visible in Figure 2 that, unlike EOs, there is no significant difference among the mean inhibition zones of the three varieties when the type of extract is methanol. In this study, EO was more effective than methanol extracts within each turmeric variety. A more potent inhibition was achieved from EO of Dame (16.85 mm) followed by HT3/2002 (15.15 mm). Methanol extract from HT3/2002 showed a minimal inhibitory zone (12.90 mm). Our results agree with those obtained by Singh et al. (2002) regarding the minimal inhibition zone exhibited by methanol extracts than EOs. A critical review by Shahidi and Hossain (2018) revealed that the reduced potential toxicity of methanol extract against S. aureus was due to poor solubility and low bioavailability of curcumin in the extract. In this case, Teow et al. (2016) suggested that the addition of antibiotics or combination with several phytochemicals such as cinnamaldehyde, ellagic acid, and eugenol with curcuminoids/curcumin could create a synergistic antibacterial effect on S. aureus.
Figure 2.
Mean zone of inhibition (mm) obtained from the combinations of the two extract types (essential oil and methanol extract) and the three turmeric varieties against S. aureus (Gram-positive bacteria). Means sharing the same letter are not significantly different at the 5% significant level. The value of = 0.21.
3.5. Analysis of essential oil composition
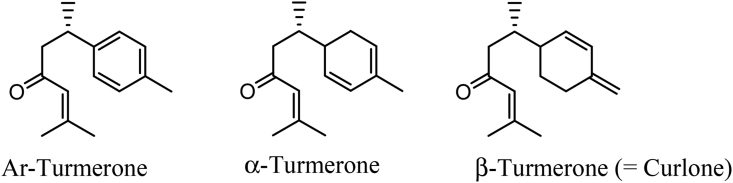
The analytical procedures revealed the identification of 24 compounds in the rhizome of C. domestica essential oil (Table 4). The most abundant components were oxygenated sesquiterpenes with α-Turmerone (32.41 and 35.16%), ar-turmerone (25.20 and 25.47%), and curlone (β-turmerone) (17.98 and 18.19%) (Figure 3) followed by sesquiterpenes with α-Curcumene (4.20 and 3.74%), α-Zingiberene (4.96 and 4.53%), and β-Sesquiphellandrene (5.51 and 4.85%) for Dame and Bonga 51/71 varieties, respectively (Table 4). These findings agree with those of Gounder and Lingamallu (2012) that sesquiterpenoid (i.e., ar-turmerone [28.3%], α-turmerone [24.8%] and curlone [21.1%]) were the major constituents, whereas α-phellandrene (1.2%), α-zingiberene (2.4%), β-sesquiphellandrene (2.1%), ar-curcumene (1.8%) and eucalyptol (0.4%) were the minor constituents of turmeric rhizomes.
Table 4.
Constituents of turmeric rhizome essential oil from Dame and Bonga 51/71 varieties.
| S/No | Constituent identifieda | Molecular formula | RIb | Composition (%) |
|
|---|---|---|---|---|---|
| Dame | Bonga 51/71 | ||||
| 1 | α-Phellandrene | C10H16 | 9.4 | 0.57 | 0.41 |
| 2 | o-Cymene | C10H14 | 10.4 | 0.36 | 0.24 |
| 3 | Eucalyptol | C10H18O | 10.8 | 0.40 | 0.21 |
| 4 | Trans- α-Bergamotene | C15H24 | 25.06 | 0.12 | 0.13 |
| 5 | Caryophyllene | C15H24 | 25.61 | 0.36 | 0.39 |
| 6 | (E)- β-Famesene | C15H24 | 26.54 | 0.22 | 0.17 |
| 7 | β-Curcumene | C15H24 | 27.38 | 0.13 | 0.15 |
| 8 | α-Curcumene | C15H22 | 27.50 | 4.20 | 3.74 |
| 9 | α-Zingiberene | C15H24 | 28.0 | 4.96 | 4.53 |
| 10 | β-Sesquiphellandrene | C15H24 | 29.1 | 5.51 | 4.85 |
| 11 | Caryophyllene oxide | C15H24O | 30.5 | 0.44 | 0.41 |
| 12 | Humulene epoxide I | C15H24O | 30.7 | 0.17 | 0.19 |
| 13 | (E)-Nuciferol | C15H22O | 32.8 | 0.90 | 0.64 |
| 14 | (Z)-α-trans-Bergamotene | C15H24 | 33.0 | 0.99 | 0.62 |
| 15 | β-Bisabolene | C15H24 | 28.5 | 1.24 | 1.02 |
| 16 | ar-Turmerol | C15H22O | 31.6 | 1.21 | 0.95 |
| 17 | Bergamotol, Z- α -trans- | C15H24O | 32.98 | 0.99 | 0.62 |
| 18 | 7-epi-cis-sesquisabinene hydrate | C15H26O | 33.6 | 0.56 | 0.45 |
| 19 | ar-Turmerone | C15H20O | 36.9 | 25.20 | 25.47 |
| 20 | α-Turmerone | C15H22O | 37.3 | 32.41 | 35.16 |
| 21 | Curlone | C15H22O | 39.8 | 17.98 | 18.19 |
| 22 | 6R, 7R-Bisabolone | C15H24O | 43.7 | 0.27 | 0.25 |
| 23 | (Z)-α-Atlantone | C15H22O | 44.7 | 0.59 | 0.58 |
| 24 | (E)-Atlantone | C15H22O | 47.1 | 0.67 | 0.76 |
The compounds are listed in the order of elution from a DB-5MS column.
Retention indices (RI) relative to C8–C18 n-alkanes on the DB-5MS column.
Figure 3.
Chemical structures of key volatile components in the essential oil from Curcuma spp. Rhizomes (Dosoky and Setzer, 2018).
This study also reveals a new insight into the chemical composition of essential oil from turmeric varieties cultivated in Ethiopia. When our results are compared with those of Oyemitan et al. (2017), there is a high level of similarity with the most abundant components of the volatile oil obtained from C. longa rhizome in Ondo (Nigeria) (i.e., oxygenated sesquiterpenes with α-Turmerone [35.9%], curlone [12.9%], and ar-turmerone [10.0%]). However, their finding on α-phellandrene (15.5%) was very different from ours (0.57–0.41%).
Similarly, Chowdhury et al. (2008) have identified 54 compounds from the yellow type of C. longa rhizomes in Bangladesh. According to their findings, ar-turmerone (27.78%), α-Turmerone (17.16%), culone (13.82%), 2-carene (4.78%), zingiberene (4.37%) and β-sesquiphellandrene (5.57%) comprise the major constituents of turmeric rhizomes. Stanojević et al. (2015) reported that C. longa that originated from Turkey consists of 65.4% oxygenated sesquiterpenes represented by α-Turmerone, ar-turmerone and curlone as the primary compound, followed by 8% benzene derivatives and 9.5% sesquiterpene hydrocarbons of total oil composition. Singh et al. (2011) reported the essential oil composition of C. longa rhizome collected from Orissa (India) bear turmerone (49.1%), α-phellandrene (5.3%), ar-curcumene (3.5%), eucalyptol (2.6%), and β-sesquiphellandrene (1.8%).
On the other hand, Li et al. (2011) and Morsy (2017) reported that turmeric essential oil's chemical composition depends on geographic origin and part of the plant used. Li et al. (2011) stated that volatile oils from leaves and flowers of C. longa are dominated by monoterpenes, particularly p-cymene, β-phellandrene (β-felandrene), terpinolene (terpenoline), p-cymen-8-ol, cineole, and myrcene.
4. Conclusions
The findings of this study can be recognized as the first detection of the bioactive components, antioxidant and antibacterial activities of turmeric grown in Ethiopia. The study on the turmeric varieties revealed that Dame variety exhibits prominent antioxidant and antibacterial activities followed by Bonga 51/71 variety. The other finding of this study is that curlone, ar-turmerone and α-Turmerone are the major components of the EOs. In vitro test on antibacterial activities of turmeric EOs and methanol extract confirmed that S. aureus is the susceptible bacterium to the extracts among the three bacterial strains. In conclusion, this research provided useful information on the functional and therapeutic potential of turmeric varieties grown in Ethiopia.
Declarations
Author contribution statement
Belay Haile: Conceived and designed the experiments; Wrote the paper.
Sirawdink Fikreyesus Forsido; Yetenayet B. Tola: Contributed reagents, materials, analysis tools or data.
Tessema Astatkie: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the College of Agriculture and Veterinary Medicine, Jimma University, Ethiopia.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aarthi S., Suresh J., Leela N., Prasath D. Multi environment testing reveals genotype-environment interaction for curcuminoids in turmeric (Curcuma longa L.) Ind. Crops Prod. 2020;145:112090. [Google Scholar]
- Adams R.P. Allured Publishing Co. Carol Stream; Illinois: 2007. Identification of Essential Oil Components by Gas Chromatography/mass- Spectrometry. [Google Scholar]
- Afroz R., Tanvir E., Islam M.A., Alam F., Gan S.H., Khalil M.I. Potential antioxidant and antibacterial properties of a popular Jujube fruit: apple Kul (Zizyphus mauritiana) J. Food Biochem. 2014;38:592–601. [Google Scholar]
- Akter J., Hossain M.A., Takara K., Islam M.Z., Hou D.-X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): isolation of active compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;215:9–17. doi: 10.1016/j.cbpc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017;7:205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.C. Partners in defense, vitamin E and vitamin C. Can. J. Physiol. Pharmacol. 1993;71:725–731. doi: 10.1139/y93-109. [DOI] [PubMed] [Google Scholar]
- Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J.U., Nandi N.C., Bhuiyan M.N.I., Mobarok M.H. Essential oil constituents of the rhizomes of two types of Curcuma longa of Bangladesh. Bangladesh J. Sci. Ind. Res. 2008;43:259–266. [Google Scholar]
- Dosoky N.S., Setzer W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients. 2018;10:1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder D.K., Lingamallu J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012;38:124–131. [Google Scholar]
- Green C.E., Hibbert S.L., Bailey-Shaw Y.A., Williams L.A., Mitchell S., Garraway E. Extraction, processing, and storage effects on curcuminoids and oleoresin yields from Curcuma longa L. grown in Jamaica. J. Agric. Food Chem. 2008;56:3664–3670. doi: 10.1021/jf073105v. [DOI] [PubMed] [Google Scholar]
- Gupta A., Mahajan S., Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol Rep. 2015;6:51–55. doi: 10.1016/j.btre.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemichael G., Nebiyu A., Mohammed A., Belew D., Tilahun D. Effect of stage of maturity at harvest on the quality of different accessions of turmeric (Curcuma domestica Val) in Southwestern Ethiopia. S. J. Agri. Res. 2016;5:87–90. [Google Scholar]
- Jose S., Thomas T.D. Comparative phytochemical and antibacterial studies of two indigenous medicinal plants Curcuma caesia Roxb. and Curcuma aeruginosa Roxb. Int. J. Green Pharm. 2014;8:65–71. [Google Scholar]
- Khatoon M., Islam E., Islam R., Rahman A.A., Alam A.K., Khondkar P., Rashid M., Parvin S. Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Res. Note. 2013;6:121. doi: 10.1186/1756-0500-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifelew H., Bekele D., Yadesa L., Getu A., Getachew W., Hailemichael G., Mitiku H. Result of turmeric variety trial in Ethiopia. Int. J. Res. Stud. Agric. Sci. 2018;4(9):34–38. [Google Scholar]
- Kocaadam B., Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh M., Singh P., Singh S., Raj P., Pandey K. Antioxidant efficacy and curcumin content of turmeric (Curcuma-longa L.) flower. Int. J. Curr. Pharm. Res. 2016;8:112–114. [Google Scholar]
- Kuruppu A.I., Paranagama P., Goonasekara C. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi. Pharm. J. 2019;27:565–573. doi: 10.1016/j.jsps.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski P., Łopusiewicz Ł., Kostek M., Drozłowska E., Pruss A., Wojciuk B., Sienkiewicz M., Zielińska-Bliźniewska H., Dołęgowska B. The antibacterial activity of lavender essential oil alone and in combination with Octenidine Dihydrochloride against MRSA strains. Molecules. 2020;25:95. doi: 10.3390/molecules25010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yuan W., Deng G., Wang P., Yang P., Aggarwal B. Chemical composition and product quality control of turmeric (Curcuma longa L.) Pharm. Crops. 2011;2:28–54. [Google Scholar]
- Mariam L.W., Mohamed A., Tilahun D. Effect of rhizome types, drying thickness and drying materials on the quality of turmeric (Curcuma longa L.) in Tepi, south Western Ethiopia. Agric. Res. Technol. 2017;11(2):555809. [Google Scholar]
- Mekonnen B., Garedew W. Nitrogen fertilizer rate and time of application affected yield and quality of turmeric (Curcuma longa L.) at Tepi, Southwestern Ethiopia. Am. J. Agric. For. 2019;7(4):126–132. [Google Scholar]
- Meng F.-C., Zhou Y.-Q., Ren D., Wang R., Wang C., Lin L.-G., Zhang X.Q., Ye W.-C., Zhang Q.-W. Chapter 10 - turmeric: A review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In: Grumezescu A.M., Holban A.M., editors. Handbook of Food Bioengineering, Natural and Artificial Flavoring Agents and Food Dyes. Academic Press; 2018. pp. 299–350. [Google Scholar]
- Montgomery D.C. tenth ed. Wiley; New York: 2020. Design and Analysis of Experiments. [Google Scholar]
- Morsy N.F.S. Active Ingredients from Aromatic and Medicinal Plants. InTech; London, UK: 2017. Chemical structure, quality indices and bioactivity of essential oil constituents; pp. 175–206. [Google Scholar]
- Negi P., Jayaprakasha G., Jagan Mohan Rao L., Sakariah K. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J. Agric. Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- Niamsa N., Sittiwet C. Antimicrobial activity of Curcuma longa aqueous extract. J. Pharmacol. Toxicol. 2009;4:173–177. [Google Scholar]
- Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc. Adv. 2015;5:27986–28006. [Google Scholar]
- Nisar T., Iqbal M., Raza A., Safdar M., Iftikhar F., Waheed M. Estimation of total phenolics and free radical scavenging of turmeric (Curcuma longa) Am. Eurasian J. Agric. Environ. Sci. 2015;15:1272–1277. [Google Scholar]
- Norajit K., Laohakunjit N., Kerdchoechuen O. Antibacterial effect of five Zingiberaceae essential oils. Molecules. 2007;12(8):2047–2060. doi: 10.3390/12082047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oyemitan I.A., Elusiyan C.A., Onifade A.O., Akanmu M.A., Oyedeji A.O., Mcdonald A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in Southwest Nigeria. Tox. Rep. 2017;4:391–398. doi: 10.1016/j.toxrep.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peethambaran C.K., Hailemichael G., Mitiku H., Kifelew H. 2016. Ethiopia Adores Turmeric in Hearth and Fields.http://www.indianspicesociety.in/iss/pdf/15.%20Ethiopia%20adores%20turmeric%20in%20hearth%20and%20fields%20-%20Spice%20India.pdf [Online]. India: Indian Society for Spices. Available: [Google Scholar]
- Pothitirat W., Gritsanapan W. Variation of bioactive components in Curcuma longa in Thailand. Curr. Sci. 2006;91:1397–1400. [Google Scholar]
- Prasad S., Aggarwal B.B. Turmeric, the golden spice. From traditional medicine to modern medicine. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal Medicine: Biomolecular and Clinical Aspects. second ed. CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- Ravindran P., Babu K.N., Sivaraman K. CRC Press; 2007. Turmeric: the Genus Curcuma. [Google Scholar]
- Saǧdıç O., Özcan M. Antibacterial activity of Turkish spice hydrosols. Food Contr. 2003;14:141–143. [Google Scholar]
- SAS . SAS Institute Inc.; Cary, NC: 2014. SAS/STAT® 9.4 User’s Guide. [Google Scholar]
- Shahidi F., Hossain A. Bioactives in spices, and spice oleoresins: phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018;3:8–75. [Google Scholar]
- Singh R., Chandra R., Bose M., Luthra P.M. Antibacterial activity of Curcuma longa rhizome extract on pathogenic bacteria. Curr. Sci. 2002:737–740. [Google Scholar]
- Singh S., Sankar B., Rajesh S., Sahoo K., Subudhi E., Nayak S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Ess. Oil Res. 2011;23:11–18. [Google Scholar]
- Singh S., Sahoo S., Dash S., Nayak S. Association of growth and yield parameters with bioactive phytoconstituents in selection of promising turmeric genotypes. Ind. Crops Prod. 2014;62:373–379. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999;299:152–178. [Google Scholar]
- Stanojević J.S., Stanojević L.P., Cvetković D.J., Danilović B.R. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.) Adv. Met. Technol. 2015;4:19–25. [Google Scholar]
- Sukati S., Khobjai W. Total phenolic content and DPPH free radical scavenging activity of young turmeric grown in southern Thailand. Appl. Mech. Mater. 2019;886:61–69. [Google Scholar]
- Tanvir E.M., Hossen M., Hossain M., Afroz R., Gan S.H., Khalil M., Karim N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017 [Google Scholar]
- Teow S.Y., Liew K., Ali S.A., Khoo A.S.B., Peh S.C. Antibacterial action of curcumin against Staphylococcus aureus: a brief review. J. Trop. Med. 2016 doi: 10.1155/2016/2853045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umamaheswari M., Chatterjee T. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr. J. Tradit., Complementary Altern. Med. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- Vallverdú-Queralt A., Regueiro J., Alvarenga J.F.R., Martinez-Huelamo M., Leal L.N., Lamuela-Raventos R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015;35:189–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.