Abstract
Purpose
Laboratory stressors have been shown to impact the activity of the intrinsic laryngeal muscles (ILMs), which may be part of the final causal pathway in some stress-induced voice disorders. Previous research suggests that personality traits such as stress reaction might increase one's susceptibility to these problems. Also, the autonomic nervous system response is implicated in the pathogenesis of voice disorders putatively involving ILM hyperfunction. The purpose of this study was to investigate personality and autonomic nervous system predictors of ILM responses to stressor exposure.
Method
Thirty-seven physically and vocally healthy female adults completed a personality questionnaire and were subjected to a speech preparation task intended to induce stress. Fine wire electromyography of the ILMs was performed so that the activity of these muscles could be measured prior to and during the stressor. Participants' trait stress reaction was measured as a personality-based predictive variable, as was respiratory-corrected respiratory sinus arrhythmia, a putative measure of vagal outflow to the heart.
Results
The personality measure trait stress reaction uniquely predicted thyroarytenoid, trapezius, and tibialis activity, whereas respiratory sinus arrhythmia uniquely predicted the activity of all muscles studied. Differences were observed in the autonomic predictor variable as a function of whether or not effects of respiration were accounted for in the variable's calculation.
Conclusions
This study explores the potential mediating roles of personality and autonomic function in ILM activity during a stressor. Both variables have value in predicting ILM activity during stressor exposure.
We previously examined the claim that the intrinsic laryngeal muscles (ILMs) respond to physical and psychosocial stressors with increased activation and characterized the nature of the ILM stress response (Helou et al., 2013, 2018). In cohorts of vocally and physically healthy women, we showed that rapid activation of the ILMs is a common response to experimentally induced stress. However, underlying mechanisms of this laryngeal stress response remain unknown. In this study, which was conducted in the same cohort described by Helou et al. (2018), we sought to probe potential mechanisms underlying the ILM stress response. A number of psychological variables (e.g., trait anxiety) and physiological variables (e.g., glottal closure as viewed endoscopically, electroglottography) were considered, and we ultimately selected cardiac vagal control as a physiological variable and trait stress reaction as a psychological variable that may predict ILM response to stress.
Respiratory Sinus Arrhythmia as an Autonomic Predictor
Autonomic function has been proposed as one possible mechanism relevant to ILM response, by this group and others (e.g., Dietrich & Verdolini Abbott, 2012; Scherer, 1986). Cardiac vagal control is conceptualized as the strength of the inhibitory effect or “brake” of the parasympathetic vagus nerve over the excitatory effects of the sympathetic nervous system on the heart. In healthy individuals, parasympathetic dominance over sympathetic influences is maintained in the heart during rest conditions; that is, the heart is under tonic inhibitory control by the parasympathetic nervous system (Thayer & Sternberg, 2006). Individuals with purportedly low parasympathetic/vagal tone are more likely to suffer from recent stress, depression, anxiety, low self-esteem, or other such disorders (e.g., Davis et al., 2002; Delaney & Brodie, 2000; Jönsson, 2007; Licht et al., 2009; Martens et al., 2008).
To capitalize on the accessibility of the electrocardiographic (ECG) signal for providing a general snapshot of autonomic function, we measured respiratory sinus arrhythmia (RSA). RSA, a measure extracted from the ECG signal, is thought to index cardiac vagal control. It reflects rhythmic increases (during inspiration) and decreases (during expiration) of efferent cardiac vagal effects on the sinoatrial node (Berntson et al., 1997; Grossman & Taylor, 2007). Also known as high-frequency heart rate variability, RSA represents the high-frequency variation in the beat-to-beat cardiac rhythm and is measured by calculating the time between R spikes (i.e., the R–R interval, also referred to as interbeat interval) on an electrocardiograph trace and submitting this time series to a spectral or autoregressive analysis.
Of relevance to this study, states of acute anxiety, worry, and stress are often accompanied by a cardiac autonomic imbalance in the direction of depressed cardiac vagal (i.e., parasympathetic) control (Friedman, 2007; Sheps & Sheffield, 2001). The preponderance of findings suggests that a broad array of stressful states (e.g., panic, recollection of stressful events, exposure to traumatic stimuli) trigger cardiac vagal withdrawal, as do laboratory stressors such as mental arithmetic and shock avoidance. These findings corroborate analogous outcomes in several animal models (Goldstein, 2001). Moreover, anxiety disorders (e.g., panic disorder, posttraumatic stress disorder, generalized anxiety disorder, specific phobias, childhood anxiety disorders) are generally linked to low RSA (Friedman, 2007).
Of course, the parasympathetic nervous system tends to exhibit a high degree of specificity across autonomic effectors, and activity observed in the heart may be of little or no relevance to activity observed in the laryngeal muscles. However, for a number of reasons, we elected to press RSA into service for this study. First, RSA is thought to provide some general insight about an individual's autonomic state and health and could also serve to validate whether a stressor impacted the participants' autonomic status. While an anatomical substrate for parasympathetic outflow to the laryngeal region is likely negligible and certainly debatable (Hisa et al., 1999; Ibanez et al., 2010; Maranillo et al., 2008; Ramaswamy et al., 1994), higher order regions of the central nervous system do have the capacity to impact the outflow to laryngeal musculature in the face of a stressor. Coordinated autonomic effects of a functional nature are likely at play in the ILMs, on the basis of the fact that they (a) comediate airway resistance with the smooth musculature of the airways and (b) valve for the lungs. Since the respiratory system falls under profound autonomic control, we reason that the larynx likely plays a functional role as autonomic status varies. Thus, while we do not endorse a causal mechanism per se for Autonomic Nervous System (ANS) impacting the laryngeal muscles, we do seek to interrogate the functional response of the ILMs during a stress-induced shift in participants' autonomic status.
Trait Stress Reaction as a Personality Predictor
Roy and Bless proposed a seminal theory regarding how personality might be involved in the pathogenesis of muscle tension dysphonia and vocal fold lesions (Roy & Bless, 2000a, 2000b; Roy et al., 2000). This theory, today referred to as the “trait theory of voice disorders,” draws from theories relating to the intertwinement of mind (i.e., personality) and body (i.e., biology). The trait theory of voice disorders holds that personality is a predisposing factor that influences how individuals respond—emotionally, cognitively, and vocally—to environmental cues. These trait-specific responses are thought to be conditioned and therefore predictable within adult individuals. Findings of other investigators generally support the trait theory of voice disorders. Relatively elevated levels of neuroticism or negative emotionality (and subfactors of these constructs) are commonly observed in and thought to hold a causal role in certain voice disorders, such as primary muscle tension dysphonia and vocal fold nodules (Freidl et al., 1990; Gerritsma, 1991; House & Andrews, 1987; Kinzl et al., 1988; McGrory et al., 1997; Pfau, 1975).
Both neuroticism and negative emotionality are broad constructs composed of several facets such as anxiety and stress reaction. Of particular interest in the proposed study, stress reaction is a core affective facet of negative emotionality that is closely linked to questionnaire measures of anxiety (Patrick et al., 2002). Thus, in general, individuals with high scores on a stress reaction measure are relatively more likely to feel easily upset, anxious, worried, tense, vulnerable, and so forth, than others. Conversely, those with low stress reaction scores are generally more likely to recover quickly from upsetting experiences, can put worries and fears aside, and do not tend to feel especially vulnerable (Patrick et al., 2002).
Perceptions of and responses to stressors are idiosyncratic and variable across individuals (i.e., a person-by-situation interaction exists). When individual traits are poorly matched to a situation (e.g., a shy person being called upon to lead a group), the stress response is greater than when traits are well matched to a situation (e.g., an outgoing person being called upon to lead a group; Cohen & Hamrick, 2003). While responses across individuals may vary greatly, individual responses within tasks are quite reliable over time (Cohen & Hamrick, 2003). This person-by-situation interaction is situated at the topmost level of the psychobiological framework advanced by Dietrich and Verdolini Abbott (2012).
Overall, the trait theory of voice disorders seems to be supported empirically. However, studies testing this theory have primarily examined higher order aspects of personality (e.g., neuroticism), setting aside finer parsing of broader constructs such as negative emotionality. Negative emotionality (comparable in certain ways to both introversion and neuroticism) seems to be causal in certain types of voice disorders such as primary muscle tension dysphonia, but multiple subfactors of negative affect (for instance, social closeness, alienation, aggression) also exist. It is unlikely that all of these subfactors are equally relevant to the voice and disorders of the voice, and other investigators have proposed a special role of trait stress reaction in so-called “functional” voice disorders (Dietrich et al., 2012, 2019). The relationship between subfactors of negative emotionality and ILM response to stress has not yet been explored.
This study seeks to address this gap by investigating trait stress reaction as measured by the Multidimensional Personality Questionnaire–Brief Form (MPQ-BF). Trait stress reaction is included in the proposed study because it represents a finer element of a broad personality construct—negative emotionality, broadly vis-à-vis introversion—that has been empirically shown to be germane to the development of primary muscle tension dysphonia (Roy & Bless, 2000a, 2000b).
Research Questions
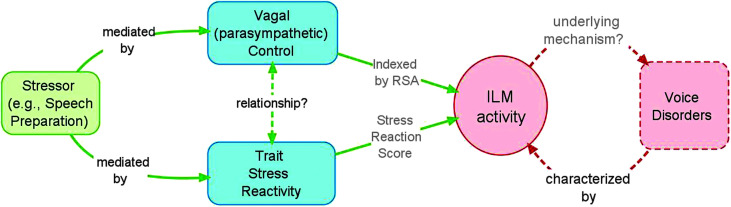
We sought to determine whether trait stress reaction scores and RSA predict magnitude of at-rest 1 ILM response to an experimental stressor. We hypothesized that higher values of trait stress reaction, which is strongly related to neuroticism and anxiety, would predict greater magnitude of electromyographic (EMG) activity for each of the ILMs and the upper trapezius muscle (a positive control muscle), but not for the anterior tibialis muscle (a negative control muscle). Also, if strong and fast-resolving physiological lability is a sign of autonomic health as proposed by numerous investigators (e.g., see McEwen, 2007), then in a healthy cohort of participants, higher values of RSA (i.e., stronger parasympathetic outflow) should predict greater activity of the ILMs. While stress tends to be more strongly associated with sympathetic rather than parasympathetic nervous system activity, we chose RSA as a key variable because of its known relationship to anxiety and sensitivity to stress. These questions are schematized in Figure 1.
Figure 1.
Schematic of research questions. RSA = respiratory sinus arrhythmia; ILM = intrinsic laryngeal muscle.
Method
The study was approved by the institutional review boards at the University of Pittsburgh and University of Pittsburgh Medical Center (PRO12110063), and written informed consent was obtained from the study participants prior to initiation of the study. All research questions were investigated using a single-subject experimental design with multiple subjects. Some methods for this study are described in detail in the companion article by Helou et al. (2018), which was based on findings in the same cohort during the same experimental session. A brief recapitulation of the study follows, with additional detail provided as needed. Physically and vocally healthy women aged 18–30 years were invited to participate in a study aimed at measuring muscle responses during “different speech and nonspeech tasks,” when in fact the intention was to experimentally induce stress. Thus, this element of the study was the first of several moments of intentional deception in the paradigm, all aimed at minimizing anticipatory stress responses in advance of the stressor or maximizing stress responses during the stressor. Women in a narrow age range were included in this preliminary study in an attempt to minimize the heterogeneity of physiological responses to stress.
At the time of screening, which was web-based, participants completed the paper-and-pencil version of the MPQ-BF in its entirety according to test instructions (Patrick et al., 2002). The stress reaction subscore (SRscore) of the MPQ-BF (Tellegen, 1995) was calculated for each participant according to test instructions and using a custom SPSS script provided by the authors (research permissions obtained from the University of Minnesota Press). The SRscore, one of the predictive independent variables used in this study, has a high internal consistency (Cronbach's α = .84) in a cross-validation sample (Patrick et al., 2002).
Following the web-based screening (Stage I) and a live screening session (Stage II) on a separate day, eligible participants attended an experimental session. At the beginning of this session, participants were fitted with (a) a left arm cuff for measuring average heart rate and blood pressure; 20-mm bipolar Ag/AgCl surface electrodes (Grass Technologies, Astro-Med, Inc.) positioned over (b) the left upper trapezius muscle (selected as a positive control site) to measure EMG activity, (c) the left anterior tibialis muscle (selected as a negative control site) to measure EMG activity, and (d) the thorax to measure ECG activity in a three-lead configuration; (e) Piezo Crystal respiratory effort transducers situated on the chest wall between Ribs 5 and 8, at the point where maximal expansion was observed during inhalation; and (f) ground and reference electrodes on the right olecranon and the right earlobe, respectively.
Next, participants engaged in Stage III, a paced breathing task, during which recordings were made from all of the devices listed above. This paced breathing task was conducted according to Egizio et al. (2011), for the purpose of later controlling for the confounding effects of respiratory rate and tidal volume on RSA values. The respiratory band measured pressure changes due to expansion and contraction of the thoracic cavity with breathing. After a brief (approximately 1 min) practice trial, participants were paced across four breathing conditions (8, 10.5, 13, and 18 breaths/min) for 2 min per condition, using audiovisual cues provided by a computer software program (EZ Air Plus 1.0, 2009, Biofeedback Foundation of Europe). Breaks of approximately 2 min were given between each set of breathing conditions. ECG and respiratory band–derived signals were simultaneously recorded for later analysis.
Next, participants engaged in Stage IV, a “true baseline” condition during which the participant rested quietly in a seated position for 180 s while exposed to emotionally neutral video stimuli (participants watched a video of waves crashing on the beach, with no humans in the scene). Next, participants underwent Stage V, which involved inserting hook wire electrodes (see Helou et al., 2013, for electrode construction details) into the right posterior cricoarytenoid (PCA), bilateral thyroarytenoid (TA)/lateral cricoarytenoid (LCA) muscle complex, and bilateral Cricothyroid (CT) according to previously published methods (Helou et al., 2018; Hillel, 2001; Munin et al., 2003). ILM EMG recordings were made using g.USBamp Biosignal Amplifiers and BCI 2000 acquisition software (Albany). Signals were digitized using a sampling rate of 9,600 samples/s/channel, and no online filtering was applied during data acquisition.
Next, heart rate and blood pressure were monitored until they returned to true baseline levels (Stage VI), at which point participants engaged in two (counterbalanced) baseline conditions aimed at obtaining at-rest data from all equipment during a second restful video-watching condition (baseline rest, Stage VIIa) and a nonstressful, nonverbal activity designed to be as parallel as possible to the upcoming stressor without actually being stressful (baseline subvocalization condition, hereafter referred to as “Baseline Subvoc,” Stage VIIb).
At this point, corresponding to Stage VIII of the experiment, participants were told they had 3 min to prepare an opening speech on the theme of a job interview, which must include the following: (a) present three of your best and worst characteristics; (b) use math to make a case for how much this job is worth to you (i.e., how much you want to be paid)—factor in how much you have spent to date on education, travel and living expenses, and any other relevant financial details; and (c) describe your goals for the future in the form of a “5-year plan.” Participants were told that they must present to an audience for 5 min. Four confederates were shown on a computer screen who had “skyped in to observe the speech preparation and delivery and make ratings of participants' nonverbal communication behaviors.” Participants were also told that one confederate would be videotaping them in order to have groups of students observe and rate their nonverbal communication at a later date. This stage involves multiple elements of intentional deception: Participants were not actually being observed by a real live audience (i.e., the four confederates were prerecorded), the participants would silently prepare but not verbally deliver a speech in this experiment, and they were not being videotaped during the speech preparation task (SPT). Following the stressful SPT, participants were debriefed and engaged in a repeat baseline task (Stage IX) during which they sat quietly while attending to the emotionally neutral video.
During all tasks involving measurement of ILM activity, overt contaminants to the signal were marked, such as coughing, sneezing, throat clearing, and so forth. As needed and able, the investigator slightly extended that epoch's recording time to account for the post hoc removal of the contaminant to ensure a sufficient amount of uncontaminated data for analysis. Such events were rare in this study. Other intermittent events such as swallow and breath holding were also marked in the recording file when patently obvious, which was not always the case, but were not marked as contaminants or removed prior to data analysis. The logic for nonremoval was that these behaviors should be roughly equal over time within participants, and if they increased during a certain epoch (e.g., the stressor), we have no reason to rule that behavior out as a legitimate manifestation of a stress response.
Following the experimental session, values for the second Independent variable (IV) were calculated. Respiratory-corrected values of RSA (RSACORR_DIFF), the two-channel files calculated for the true baseline and SPT epochs were loaded into MindWare (MindWare 3.0.21, MindWare Technologies LTD), which automatically marked each QRS peak in the ECG waveform and calculated output variables. ECG data were visually inspected, and any errors were corrected manually. Respiratory band data were inspected to ensure that the program's automatic calculation matched the number of breaths reflected in the waveform. Corrections were then applied for the confounding influences of respiratory rate 2 according to previously utilized methods (Egizio et al., 2011; Jain et al., 2011). In short, the spectral power of the 0.15- to 0.40-Hz frequency band (hereafter referred to just as RSA) using fast Fourier transform on interbeat intervals was obtained from the MindWare output. To correct for the effects of respiration, (a) average respiratory cycle length in seconds was calculated for each task by dividing the total time of the task (approximately 3 min) by the respiration rate during that period; (b) separate within-subject regressions were calculated regressing RSA on average respiratory rate (Egizio et al., 2011); (c) regression data were inspected to ensure R 2 values were above .70 and/or direct inspection of scatter plots showing the relationship between RSA and respiratory rate confirmed that each parameter's activity increased with that of the other parameter; and (d) the regression line of RSA on respiratory variables was then utilized to estimate the task-related changes in RSA that were systematically lower or higher than anticipated values (i.e., respectively reflecting cardiac vagal decline or augmentation; Grossman & Taylor, 2007). To correct for the effects of respiration on RSA, these predicted RSA values for true baseline and SPT were subtracted from the measured RSA values for each respective task. It is this value, RSACORR_DIFF, which served as one independent variable in subsequent regression analyses and will be described in greater detail below.
Data Reduction and Analysis
Data reduction and analysis were performed using MATLAB 7.8.0 (R009a). A 10-Hz high-pass filter was applied to all EMG channels in order to remove drift and offset. Notch filters of 60, 120, and 180 Hz were applied to all data channels, and all data channels were full-wave rectified for analysis. For RSACORR_DIFF processing, true baseline and SPT data files were (a) duplicated, (b) filtered as above and down-sampled to 1000 Hz, (c) modified so that only the ECG and respiratory band channels remained, and (d) converted to two-channel (ECG and respiratory band) comma delimited text files.
Obtaining Dependent Variables
To obtain values for each dependent variable (DV), the following data reduction procedures were performed for signals from each ILM and the two control sites. First, the magnitude of ILM/trapezius/tibialis activity change (from baseline) was calculated. Recall that two baseline epochs were obtained in this study—Baseline Rest and Baseline Subvoc—and that for the primary research question(s) relating to the predictive roles of trait stress reaction and RSA, the Baseline Subvoc epoch served as the baseline to which the experimental condition was compared. The Baseline Subvoc value was represented by calculating the mean of the entire Baseline Subvoc 120-s task epoch. The EMG value during SPT was calculated in the same fashion. Values representing magnitude of ILM/trapezius/tibialis activity change were recorded as the absolute difference from Baseline Subvoc to SPT for EMG values. These values were used as the DV for regression models.
Because the TA/LCA and CT muscles were sampled bilaterally to protect against loss of data in the event of electrode displacement, the muscle displaying the clearest change from baseline in absolute value was included for analysis in the regression model. Hence, data from three ILMs were included in separate statistical analyses for each subject: PCA, one TA/LCA complex (left or right), and CT (left or right). In addition, only data from these three muscles were included in the resolution latency analysis, described next.
Statistical Approach
Five separate multiple regressions were run to predict the magnitude of change in the three ILMs and two control muscles of interest, by the independent variables RSACORR and SRscore. In addition, the same multiple regressions were rerun using the “raw,” uncorrected RSA values calculated as a difference score from true baseline to the SPT (RSARAW_DIFF). Participants with outlier values were removed from the analyses (n = 2 for all muscles, n = 1 for all ILMs, n = 1 for the CT, n = 1 for the upper trapezius muscle, and n = 1 for the anterior tibialis muscle). Regressions were then rerun, and results are given below. Data from no more than two participants were removed from any analysis based on outlier status.
Finally, this article represents the second main part of a larger study, the first part of which was described previously. In the partner article, we showed that the ILMs commonly increase in activity during a silent SPT that healthy female participants considered to be stressful (Helou et al., 2018). Data in this study are derived from the same cohort. Subject-level data for change in heart rate, blood pressure, and respiration can be referenced in our previous publication.
Results
Seventy-eight potential participants completed the web-based screening survey, and 50 potential participants attended the face-to-face screening. Forty participants satisfied all of the inclusion criteria and participated in the study. Of those 40 participants, one could not tolerate the placement of the fine wire electrodes and was dismissed from the study. Data from two participants were corrupted for unknown reasons and could not be analyzed. Thus, complete data sets for 37 individuals are presented herein.
Of the 37 participants who engaged in all tasks from which RSA values were to be derived, RSA data from n = 32 were usable. Two participants' data became corrupted toward the end of the experimental session, including during the SPT task (one lost respiratory band trace, the other lost ECG trace), and three participants' ECG data were corrupt for all tasks. With respect to within-subject regressions to correct for the effects of respiration on RSA, most participants (n = 24) had R 2 values ranging from .701 to .974 (M = .847, SD = .088). A remaining n = 8 participants had values just below .70, but visual inspection of their data confirmed the positive relationship between RSA and rate, and thus, their data were included in analyses.
Visual inspection of respiratory band signals indicated that respiratory depth measures were contaminated by oversaturation of the signal during the 8 and 10.5 breaths/min conditions of the paced breathing tasks. This occurred in approximately one third of participants and was likely a function of the respiratory band being slightly tighter than necessary to accommodate the more exaggerated thoracoabdominal expansion observed during slow, deep breathing (though participants were asked about their physical comfort level and none reported discomfort with the respiratory band).
SRscore values for this cohort (n = 37) were well within the range of normative data (T-score mean = 44.01, SD = 9.40). Table 1 provides high-frequency heart rate variability (i.e., RSA) change values without correction for the effects of respiration on the heart rate signal (RSARAW_DIFF). Values suggested to relate to sympathetic activity are also provided, though it should be noted that some scientists have compellingly challenged whether the low-frequency band indeed reflects sympathetic contributions to heart rate variability (Reyes del Paso et al., 2013). Finally, the natural log values (ln) for both low- and high-frequency power and the ratio of low- and high-frequency power are also provided.
Table 1.
Heart rate variability descriptive data.
| Variable | M | SD | Min | Max |
|---|---|---|---|---|
| True baseline | ||||
| Interbeat interval (ms) | 959.76 | 142.93 | 683.22 | 1251.64 |
| High-frequency power (RSARAW, ms2) | 1974.45 | 2010.45 | 34.51 | 9235.80 |
| Low-frequency power (ms2) | 1095.78 | 1502.02 | 52.36 | 6675.41 |
| Low frequency–high frequency ratio | 0.83 | 1.31 | 0.06 | 7.72 |
| ln high-frequency power (ln ms2) | 3.05 | 0.54 | 1.54 | 3.97 |
| ln low-frequency power (ln ms2) | 2.74 | 0.53 | 1.72 | 3.82 |
| Speech preparation task | ||||
| Interbeat interval (ms) | 725.28 | 145.40 | 485.15 | 975.38 |
| High-frequency power (RSARAW, ms2) | 567.10 | 613.42 | 7.95 | 2976.17 |
| Low-frequency power (ms2) | 462.89 | 386.36 | 25.93 | 1541.86 |
| Low frequency–high frequency ratio | 1.20 | 0.90 | 0.35 | 3.79 |
| ln high-frequency power (ln ms2) | 2.51 | 0.54 | 0.90 | 3.47 |
| ln low-frequency power (ln ms2) | 2.49 | 0.45 | 1.41 | 3.19 |
Note. RSA = respiratory sinus arrhythmia.
For analyses where muscle activation was predicted by RSACORR_DIFF and SRscore, values for some participants were found to include outliers and were thus removed from the analyses. Regressions were then rerun, and results are given below. For analyses where muscle activation was predicted by RSARAW_DIFF and SRscore, values for some participants were found to include outliers that led to violation of statistical assumptions and were thus removed from the analyses. Data from no more than two participants were removed from any analysis based on outlier status. Regressions were then rerun, and results are given below. Negative values of the DV reflect a decrease in muscle activity from baseline to stressor, whereas positive DV values reflect an increase.
Individual multiple regressions were performed to predict magnitude of change in the muscle of interest using RSACORR and SRscore, and all assumptions were met. The relationship between RSACORR_DIFF and SRscore was not found to be statistically significant, p = .408. The relationship between RSARAW_DIFF and SRscore was also assessed and found to be nonsignificant, p = .210. Regression coefficients and errors for each model can be found in Table 2.
Table 2.
Summary of multiple regression analyses.
| Variable | B | SE B | β | Variable | B | SE B | β |
|---|---|---|---|---|---|---|---|
| Posterior cricoarytenoid | |||||||
| Intercept | 100.54 | 143.49 | Intercept | 262.02 | 221.49 | ||
| RSACORR | 0.083 | 0.031 | .529* | RSARAW | 0.030 | 0.137 | .050 |
| SRscore | 40.27 | 28.02 | .282 | SRscore | 66.12 | 39.99 | .380 |
| Thyroarytenoid/lateral cricoarytenoid complex | |||||||
| Intercept | 864.01 | 243.75 | Intercept | 856.01 | 333.54 | ||
| RSACORR | 0.116 | 0.055 | .355* | RSARAW | 0.044 | 0.185 | .046 |
| SRscore | −125.83 | 52.443 | −.402* | SRscore | −28.51 | 61.46 | −.091 |
| Cricothyroid | |||||||
| Intercept | 192.44 | 151.03 | Intercept | 400.28 | 169.81 | ||
| RSACORR | .100 | 0.034 | .530* | RSARAW | .125 | 0.097 | .265 |
| SRscore | 15.81 | 30.18 | .094 | SRscore | 33.19 | 34.46 | .198 |
| Upper trapezius (positive control) | |||||||
| Intercept | 2.63 | 2.22 | Intercept | 5.37 | 2.46 | ||
| RSACORR | .001 | 0.000 | .377* | RSARAW | 0.001 | 0.001 | .252 |
| SRscore | 1.23 | 0.431 | .446* | SRscore | 1.48 | 0.514 | .477* |
| Anterior tibialis (negative control) | |||||||
| Intercept | 12.62 | 5.29 | Intercept | 18.85 | 5.13 | ||
| RSACORR | .002 | 0.001 | .347* | RSARAW | 0.003 | 0.002 | .313 |
| SRscore | 2.39 | 0.958 | .407* | SRscore | 3.40 | 0.99 | .536* |
Note. B = unstandardized regression coefficient; SE B = standard error of the coefficient; β = standardized coefficient; RSACORR = respiratory-corrected value of RSA; SRscore = stress reaction subscore.
p < .05 for the full model and for the variable.
PCA
A multiple regression was run to predict magnitude of change in the PCA by RSACORR_DIFF and SRscore. The overall model statistically significantly predicted PCA activity, F(2, 16) = 5.081, p = .020, R 2 = .388. RSACORR_DIFF added statistically significantly to the prediction, p = .016, but trait stress reaction did not, p = .170. An additional multiple regression was run for the same variables, but with RSARAW_DIFF as the first IV. All assumptions were met. The model did not statistically significantly predict PCA activity, F(2, 17) = 1.373, p = .280, R 2 = .139.
TA/LCA Complex
A multiple regression was run to predict magnitude of change in the TA/LCA by RSACORR_DIFF and SRscore. The overall model statistically significantly predicted TA/LCA activity, F(2, 26) = 4.838, p = .016, R 2 = .271. RSACORR_DIFF added statistically significantly to the prediction, p = .044, as did trait stress reaction, p = .024. An additional multiple regression was run for the same variables, but with RSARAW_DIFF as the first IV. The model did not statistically significantly predict TA/LCA activity, F(2, 26) = .142, p = .868, R 2 = −.011.
Cricothyroid
A multiple regression was run to predict magnitude of change in the CT by RSACORR_DIFF and SRscore. The overall model statistically significantly predicted CT activity, F(2, 22) = 4.614, p = .021, R 2 = .296. RSACORR_DIFF added statistically significantly to the prediction, p = .007, but trait stress reaction did not, p = .606. An additional multiple regression was run for the same variables, but with RSARAW_DIFF as the first IV. This model did not statistically significantly predict CT activity, F(2, 22) = 1.123, p = .343, R 2 = .093.
Upper Trapezius
A multiple regression was run to predict magnitude of change in the upper trapezius muscle by RSACORR_DIFF and SRscore. The model statistically significantly predicted upper trapezius muscle activity, F(2, 28) = 6.542, p = .005, R 2 = .318. Both RSACORR_DIFF and SRscore contributed significantly to the overall prediction, p < .023 and p < .008, respectively. An additional multiple regression was run for the same variables, but with RSARAW_DIFF as the first IV. The model statistically significantly predicted upper trapezius muscle activity, F(2, 26) = 5.217, p = .012, R 2 = .286. RSARAW_DIFF did not contribute significantly to the overall prediction, p = .140, but SRscore did, p = .008.
Anterior Tibialis
A multiple regression was run to predict magnitude of change in the anterior tibialis muscle by RSACORR_DIFF and SRscore. The overall model statistically significantly predicted tibialis activity, F(2, 28) = 4.945, p = .014, R 2 = .261. Both RSACORR_DIFF and SRscore contributed significantly to the overall prediction, p < .042 and p < .019, respectively. An additional multiple regression was run for the same variables, but with RSARAW_DIFF as the first IV. The model statistically significantly predicted anterior tibialis muscle activity, F(2, 26) = 7.730, p = .002, R 2 = .373. RSARAW_DIFF did not contribute significantly to the overall prediction, p = .054, but SRscore did, p = .002.
Additional regressions were run to determine if the results for the derived indices might be due to differences in their fundamental components. We examined models using baseline heart rate, heart rate change, baseline RSA, baseline blood pressure, and blood pressure change (baseline minus SPT values) as variables in place of RSA. None were equivalent predictors to the RSA indices described above.
Discussion
This study explored autonomic and personality-based predictors of ILM responses to a psychosocial stressor. We previously described robust physiological and self-reported stress responses that were observed in this same cohort of physically and vocally healthy young women and showed that increased activation of the ILM was commonly observed in the face of stress. Here, we describe the value of RSA (a physiological variable) and trait stress reaction (a psychological variable) in predicting the ILM response. We hypothesized that for every unit increase in RSA and trait stress reaction, we would observe an increase in ILM activity. We examined RSA values that were both corrected (RSACORR_DIFF) and uncorrected (RSARAW) for respiratory effects. Consistent with RSA literature (e.g., Berntson et al., 1997; Grossman & Taylor, 2007; Ritz, 2009), the regression models were substantially impacted by whether the RSA value was corrected or not.
RSACORR_DIFF as a Predictor of ILM Activity
In the model where SRscore and RSACORR_DIFF predicted the magnitude of change in muscle activity from baseline to stressor, the independent variable RSACORR_DIFF significantly predicted the activity of all the muscles examined. Specifically, higher values of RSACORR_DIFF were associated with increases in muscle activity from Baseline Subvoc to SPT. This relationship is consistent with the proposal that the characteristic laryngeal response in healthy, non–voice-disordered women is one of dramatic yet fast-resolving muscular activity increases (Helou et al., 2018). This liability might be related to parasympathetic/vagal influences in the body, which should not dismiss the possibility of concurrent sympathetic mechanisms at play. Indeed, evidence of sympathetic activation also coexisted by way of increases in heart rate, blood pressure, and respiratory rate. It should be emphasized that a causal autonomic mechanism at the level of the ILMs is not suggested here, and indeed, a causal model was not employed in this study. Rather, we interpret these findings as reflecting some functional response of the ILMs during a stressor-induced shift in autonomic status.
Findings herein are consistent with the concept of healthy allostatic resilience proposed by McEwen (2002). That is, participants in this study generally exhibited physiological flexibility, as is expected in healthy normal individuals. They demonstrated robust cardiovascular, state, and somatic muscle responses to a potent stressor and then rapidly returned to baseline values once the perceived threat was removed. One implication for these findings relates to the visual observation of laryngeal hyperfunction during clinical diagnosis of disorders such as muscle tension dysphonia. Laryngeal hyperfunction (excessive laryngeal tension) is thought to underlie vocal hyperfunction (Hillman et al., 1989), which is a broad term used to describe conditions in which the vocal mechanism is misused such that the muscular forces are excessive and/or imbalanced (e.g., Aronson, 1980; Koufman & Blalock, 1991; Morrison et al., 1983). For many patients, flexible laryngoscopy and perhaps even the clinical environment might trigger stress; thus, the laryngeal hyperfunction that can be appreciated on physical exam might partially be due to acute stress. Alternatively or additionally, it may be highly valuable for patients with suspected muscle tension dysphonia to undergo some stress provocation protocol under flexible laryngoscopy to observe the laryngeal muscle response to induced stress. Analogous practices are commonplace in asthma and allergy clinics, and the (cardiovascular) reactivity hypothesis described by Obrist (1981) also relates to classifying and predicting disease via reactivity measures.
Experts in the measurement and interpretation of RSA caution investigators against assuming that what is true for cardiac parasympathetic control is also true for other systems in the body. Fractioning of responses occurs commonly across organ systems (Grossman & Taylor, 2007; Ritz, 2009). Regarding the airway, solid evidence exists that vagal excitation is associated with smooth muscle constriction and subsequent narrowing of the airway (see a review by Ritz, 2004). It appears that this study is the first to examine RSA changes associated with the ILMs, which play a critical airway valving role. Unfortunately, it remains unknown to what extent the larynx is yoked to the needs of the lungs versus to what extent it “acts on its own behalf.” Combining that particular gap in knowledge with the fact that this study did not examine the glottic response to the stressor exposure, the airway status (i.e., dilated vs. constricted) of these participants cannot be described empirically. Germane to the present discussion, laryngeal muscle activity, specifically as a function of increased vagal outflow to the airway, remains wholly unknown.
Also in this model, stress reaction score (SRscore) significantly predicted the activity of the upper trapezius and anterior tibialis muscles, such that for every unit increase in SRscore, a greater magnitude of change in muscle activity was observed from Baseline Subvoc to SPT. This directionality was consistent with our hypothesis that the higher the SRscore, the greater the activity of the muscles would be. High scorers on this subscale of the MPQ-BF tend to be tense and nervous, often feel worried, anxious, and emotionally volatile, and feel vulnerable (Patrick et al., 2002). On the other hand, low scorers are able to quickly recover from upsetting situations, rarely feel emotional turmoil, and can easily set fears and worries aside.
SRscore also significantly predicted the activity of the TA/LCA muscle, but the direction of the relationship unexpectedly differed from that of SRscore and the control muscles. For every unit increase in SRscore, an increase in TA/LCA muscle activity was expected from baseline to stressor. However, the opposite was observed in that SRscore increases predicted a decrease in TA/LCA muscle activity. Assuming this finding is not spurious, it could be that the high stress reaction–low muscle activation relationship is aligned with others' empirical observations of behavioral inhibition as it pertains to voice disorders (Dietrich & Verdolini Abbott, 2012; van Mersbergen et al., 2008). Those studies found that individuals with muscle tension dysphonia scored higher on stress reaction as measured by the MPQ-BF and also exhibited blunted facial and extralaryngeal muscle responses to emotion induction. Although this study involved participants with unimpaired voices and did not utilize an emotion induction paradigm, its observations are consistent with the notion that behavioral inhibition might be related to high levels of stress reaction.
The prediction of SRscore on the PCA and CT muscles was not statistically significant. Although it is possible that the SRscore indeed has no predictive value in the context of these muscles, it is also possible that the regression tests for the laryngeal muscles were insufficiently powered or the muscle responses were of insufficient magnitude to detect a predictive effect of SRscore on those particular muscles. To this point, the magnitude of change in the TA/LCA muscle (which was significantly predicted by SRscore) was the greatest of all muscles examined in this study. Moreover, if SRscore is actually associated with behavioral inhibition during stress as postulated, then it stands to reason that the regression models would be insufficiently powered to reveal this relationship, because so few participants in this study actually exhibited diminished muscle activity during the stressor as compared to baseline.
RSARAW_DIFF as a Predictor of ILM Activity
In the second model—SRscore and RSARAW_DIFF predicting the magnitude of change in muscle activity from baseline to stressor—RSARAW_DIFF did not significantly predict the activity of any of the muscles in this model. The effects of respiration may actually obscure the relationship between vagal outflow and muscle activity, even in the muscles with the greatest responses from baseline to stressor. It is important to bear in mind that the signals measured via EMG were miniscule relative to the far more robust heart rate and respiratory signals; for instance, the activity of the ILMs during rest would be measured in tens of microvolts. Respiration can heavily influence RSA within individuals. In fact, even when heart rate remains relatively stable across paced breathing tasks ranging from 8 to 18 breaths/min, up to 55% of RSA variance is due to rate and depth of respiration (rate > depth; Grossman et al., 1991; Ritz, 2009; Ritz et al., 2001). Thus, it should not be surprising that respiratory effects on RSA might obscure other relationships.
Ritz (2009) reports the concordance within studies that examined both RSARAW_DIFF and RSACORR_DIFF. Of the 10 studies reviewed, eight showed different findings as a function of which RSA variable was utilized, and most of these differences were in the direction of RSACORR_DIFF revealing a finding that was otherwise obscured by using RSARAW_DIFF. Although the experimental paradigms differ from that employed in this study—emotion induction, erotic imagery, facial expressions, and forehead cooling versus stress induction—our data support others' claim that RSACORR_DIFF and RSARAW_DIFF are two completely different variables rather than slightly adjusted versions of each other (see review by Ritz, 2009). In this study, the latter measure was an ineffective predictor of muscle activity for any of the sites investigated.
In this model, SRscore significantly predicted the activity of both the upper trapezius and anterior tibialis muscles such that increases in SRscore were associated with greater magnitudes of change in muscle activity from baseline to stressor. SRscore did not predict the activity of the laryngeal muscles. We did hypothesize that the upper trapezius muscle activity would be predicted by SRscore, but the significant prediction of anterior tibialis activity was not originally anticipated. Potential explanations regarding the response of the control muscles were delineated earlier and are relevant here as well.
It is possible that no relationship exists between SRscore and the magnitude of ILM change, although there may be other explanations as well. It could be that the participants in this normal cohort were essentially “too normal” to observe the anticipated relationship. In the present cohort, the distribution of trait stress reaction scores was somewhat skewed toward lower (i.e., less stress reactive) scores, perhaps reflecting a self-selection bias in the present sample, since people high in trait stress reaction may be less likely to sign up for an invasive study involving needles in the neck. Nonetheless, all participants exhibited some physiological effects consistent with a sympathetic stress response (e.g., increased heart rate, blood pressure, and/or respiration rate) in addition to self-reported increases in state stress and anxiety (Helou et al., 2018). Also, despite the seemingly large effect sizes for the ILMs observed during the stressor (Helou et al., 2018), it may be that without the correction for the effects of respiration on RSA, the predictive value of SRscore on those variables could not be realized.
Limitations
It remains unknown how well the RSACORR_DIFF variable would predict the activity of the laryngeal muscles if it had included the element of tidal volume; this limitation is discussed subsequently. This omission might be considered a substantial limitation with regard to determining how autonomic changes impact the muscles of interest. Nevertheless, respiratory rate apparently trumps tidal volume in terms of its power to impact RSA values (Berntson et al., 1997; Ritz, 2009). In addition, the idiosyncratic respiratory behaviors (e.g., sighing, breath holding) of some participants (such as those whose data were highlighted in Helou et al., 2018) should be expected to substantially impact RSA measures and thus would not likely be well represented by average values of respiratory rate or volume (Grossman & Taylor, 2007). Thus, RSA-related findings in this study should be interpreted with prudence and attention to the possibility of outstanding respiratory confounds.
However, this very line of thinking may be flawed. Berntson et al. (1991) emphasize that autonomic control is not a continuum extending from parasympathetic to sympathetic outflow. Rather, in the face of stress, a number of modes of autonomic engagement might be seen. For instance, it is possible for both parasympathetic and sympathetic outflow to increase during times of stress. Adding to this consideration the argument by Ritz (2004, 2009) that parasympathetic outflow to the airway might actually be quite different than to the heart, it is entirely possible that RSA, which indexes cardiac vagal outflow, is a poor proxy for airway vagal control, thus rendering interpretations of RSA within the context of airway stress responses fallacious. For instance, could the classically anticipated withdrawal of parasympathetic outflow to the heart during stress be reciprocally complemented by increased parasympathetic outflow to the airway (including to the upper airway comprised, in part, by the laryngeal muscles)? The characteristics and boundaries of the autonomic–cardiac–airway relationship remain to be seen. However, the main point here is that the importance and relevance of sympathetic outflow in laryngeal muscle response perhaps should not be assumed. It may very well be that laryngeal stress responses stem from parasympathetic influences—whether withdrawal or engagement—above and beyond sympathetic influences.
Conclusions
This study explored the potential mediating (not causal) roles of personality and autonomic function in ILM activity during an experimental psychosocial stressor. Both variables evidenced some value in predicting ILM activity during stress. Specifically, after correcting for the effects of respiration rate, increased vagal outflow to the heart predicted increased activity in all muscles studied. With respect to personality, increased trait stress reaction scores predicted increased activity of the upper trapezius and anterior tibialis muscles, and increased trait stress reaction scores predicted decreased activity in the TA muscles. Future studies could build on the existing knowledge base by seeking to disambiguate sympathetic and parasympathetic nervous system contributions to stress-related ILM activity, by exploring whether additional features of personality might also predict ILM stress responses, by examining ILM responses in the context of vocal and speech tasks, and by studying ILM stress responses in individuals with voice disorders.
Acknowledgments
This study was partially supported by the School of Health and Rehabilitation Science Doctoral Award (awarded to Helou), a research grant from the Voice Foundation (awarded to Helou), the University of Pittsburgh Medical Center Rehabilitation Institute (awarded to Wang), and the National Institutes of Health (Grants 3R01NS050256-05S1 [awarded to Wang], 8KL2TR000146 [awarded to Wang], and R01 DC008567 [awarded to Verdolini Abbott]). The authors wish to acknowledge the efforts of Adrianna Shembel and Catherine Bean for their assistance with data collection and Neil Szuminsky for his assistance with fine wire electrode design and construction. The authors also wish to acknowledge the late Samay Jain for his mentorship and guidance pertaining to cardiovascular measurement and analysis.
Funding Statement
This study was partially supported by the School of Health and Rehabilitation Science Doctoral Award (awarded to Helou), a research grant from the Voice Foundation (awarded to Helou), the University of Pittsburgh Medical Center Rehabilitation Institute (awarded to Wang), and the National Institutes of Health (Grants 3R01NS050256-05S1 [awarded to Wang], 8KL2TR000146 [awarded to Wang], and R01 DC008567 [awarded to Verdolini Abbott]).
Footnotes
At-rest ILM response was used instead of a vocal or speech task in order to avoid known effects of speech breathing on RSA (Reilly & Moore, 2003) and to simplify the paradigm and subsequent analysis so that vocal intensity, frequency, and other parameters need not be experimentally controlled.
The initial intention was to also include tidal volume data in the corrections methods. However, data were contaminated as described in the Results section. To maintain consistency of methods without omitting large amounts of data, rate corrections were made on the RSACORR data, but tidal volume corrections were not made. Others have corrected for effects of respiration on RSA in the same fashion (e.g., Overbeek et al., 2014), and some consider respiratory rate a substantially more important variable to control than tidal volume in RSA research (Berntson et al., 1997). Further consideration of this issue will follow in the Discussion section.
References
- Aronson, A. (1980). Clinical voice disorders: An interdisciplinary approach. Brian C. Decker. [Google Scholar]
- Berntson, G. G. , Bigger, J. T., Jr. , Eckberg, D. L. , Grossman, P. , Kaufmann, P. G. , Malik, M. , Nagaraja, H. N. , Porges, S. W. , Saul, J. P. , Stone, P. H. , & van der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Berntson, G. G. , Cacioppo, J. T. , & Quigley, K. S. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487. https://doi.org/10.1037/0033-295X.98.4.459 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , & Hamrick, N. (2003). Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain, Behavior, and Immunity, 17(6), 407–414. https://doi.org/10.1016/S0889-1591(03)00110-7 [DOI] [PubMed] [Google Scholar]
- Davis, M. , Montgomery, I. , & Wilson, G. (2002). Worry and heart rate variables: Autonomic rigidity under challenge. Journal of Anxiety Disorders, 16(6), 639–659. https://doi.org/10.1016/S0887-6185(02)00132-9 [DOI] [PubMed] [Google Scholar]
- Delaney, J. P. A. , & Brodie, D. A. (2000). Effects of short-term psychological stress on the time and frequency domains of heart-rate variability. Perceptual and Motor Skills, 91(2), 515–524. https://doi.org/10.2466/pms.2000.91.2.515 [DOI] [PubMed] [Google Scholar]
- Dietrich, M. , Andreatta, R. D. , Jiang, Y. , Joshi, A. , & Stemple, J. C. (2012). Preliminary findings on the relation between the personality trait of stress reaction and the central neural control of human vocalization. International Journal of Speech-Language Pathology, 14(4), 377–389. https://doi.org/10.3109/17549507.2012.688865 [DOI] [PubMed] [Google Scholar]
- Dietrich, M. , Andreatta, R. D. , Jiang, Y. , & Stemple, J. C. (2019). Limbic and cortical control of phonation for speech in response to a public speech preparation stressor. Brain Imaging and Behavior. https://doi.org/10.1007/s11682-019-00102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, M. , & Verdolini Abbott, K. (2012). Vocal function in introverts and extraverts during a psychological stress reactivity protocol. Journal of Speech, Language, and Hearing Research, 55(3), 973–987. https://doi.org/10.1044/1092-4388(2011/10-0344) [DOI] [PubMed] [Google Scholar]
- Egizio, V. B. , Eddy, M. , Robinson, M. , & Jennings, J. R. (2011). Efficient and cost-effective estimation of the influence of respiratory variables on respiratory sinus arrhythmia. Psychophysiology, 48(4), 488–494. https://doi.org/10.1111/j.1469-8986.2010.01086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidl, W. , Friedrich, G. , & Egger, J. (1990). Persönlichkeit und Stressbearbeitung bei Patienten mit funktioneller Dysphonie [Personality and coping with stress in patients suffering from functional dysphonia]. Folia Phoniatrica, 42(3), 144–149. https://doi.org/10.1159/000266058 [PubMed] [Google Scholar]
- Friedman, B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. https://doi.org/10.1016/j.biopsycho.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Gerritsma, E. J. (1991). An investigation into some personality characteristics of patients with psychogenic aphonia and dysphonia. Folia Phoniatrica, 43(1), 13–20. https://doi.org/10.1159/000266096 [DOI] [PubMed] [Google Scholar]
- Goldstein, D. (2001). The autonomic nervous system in health and disease. Marcel Dekker. [Google Scholar]
- Grossman, P. , Karemaker, J. , & Wieling, W. (1991). Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology, 28(2), 201–216. https://doi.org/10.1111/j.1469-8986.1991.tb00412.x [DOI] [PubMed] [Google Scholar]
- Grossman, P. , & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Helou, L. B. , Rosen, C. A. , Wang, W. , & Verdolini Abbott, K. (2018). Intrinsic laryngeal muscle response to a public speech preparation stressor. Journal of Speech, Language, and Hearing Research, 61(7), 1525–1543. https://doi.org/10.1044/2018_JSLHR-S-17-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helou, L. B. , Wang, W. , Ashmore, R. C. , Rosen, C. A. , & Verdolini Abbott, K. (2013). Intrinsic laryngeal muscle activity in response to autonomic nervous system activation. The Laryngoscope, 123(11), 2756–2765. https://doi.org/10.1002/lary.24109 [DOI] [PubMed] [Google Scholar]
- Hillel, A. D. (2001). The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. The Laryngoscope, 111(4, Pt. 2 Suppl. 97), 1–47. https://doi.org/10.1097/00005537-200104001-00001 [DOI] [PubMed] [Google Scholar]
- Hillman, R. E. , Holmberg, E. B. , Perkell, J. S. , Walsh, M. , & Vaughan, C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech and Hearing Research, 32(2), 373–392. https://doi.org/10.1044/jshr.3202.373 [DOI] [PubMed] [Google Scholar]
- Hisa, Y. , Koike, S. , Tadaki, N. , Bamba, H. , Shogaki, K. , & Uno, T. (1999). Neurotransmitters and neuromodulators involved in laryngeal innervation. Annals of Otology, Rhinology & Laryngology. Supplement, 178, 3–14. https://doi.org/10.1177/00034894991080S702 [DOI] [PubMed] [Google Scholar]
- House, A. , & Andrews, H. B. (1987). The psychiatric and social characteristics of patients with functional dysphonia. Journal of Psychosomatic Research, 31(4), 483–490. https://doi.org/10.1016/0022-3999(87)90006-7 [DOI] [PubMed] [Google Scholar]
- Ibanez, M. , Valderrama-Canales, F. J. , Maranillo, E. , Vazquez, T. , Pascual-Font, A. , McHanwell, S. , & Sanudo, J. (2010). Human laryngeal ganglia contain both sympathetic and parasympathetic cell types. Clinical Anatomy, 23(6), 673–682. https://doi.org/10.1002/ca.20956 [DOI] [PubMed] [Google Scholar]
- Jain, S. , Siegle, G. J. , Gu, C. , Moore, C. G. , Ivanco, L. S. , Jennings, J. R. , Steinhauer, S. R. , Studenski, S. , & Greenamyre, J. T. (2011). Autonomic insufficiency in pupillary and cardiovascular systems in Parkinson's disease. Parkinsonism & Related Disorders, 17(2), 119–122. https://doi.org/10.1016/j.parkreldis.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson, P. (2007). Respiratory sinus arrhythmia as a function of state anxiety in healthy individuals. International Journal of Psychophysiology, 63(1), 48–54. https://doi.org/10.1016/j.ijpsycho.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Kinzl, J. , Biebl, W. , & Rauchegger, H. (1988). Functional aphonia: Psychosomatic aspects of diagnosis and therapy. Folia Phoniatrica, 40(3), 131–137. https://doi.org/10.1159/000265900 [DOI] [PubMed] [Google Scholar]
- Koufman, J. A. , & Blalock, P. D. (1991). Functional voice disorders. Otolaryngologic Clinics of North America, 24(5), 1059–1073. [PubMed] [Google Scholar]
- Licht, C. M. M. , de Geus, E. J. C. , van Dyck, R. , & Penninx, B. W. J. H. (2009). Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosomatic Medicine, 71(5), 508–518. https://doi.org/10.1097/PSY.0b013e3181a292a6 [DOI] [PubMed] [Google Scholar]
- Maranillo, E. , Vazquez, T. , Ibanez, M. , Hurtado, M. , Pascual-Font, A. , McHanwell, S. , Valderrama-Canales, F. , & Sanudo, J. (2008). Anatomic study of human laryngeal ganglia: Number and distribution. Clinical Anatomy, 21(7), 641–646. https://doi.org/10.1002/ca.20699 [DOI] [PubMed] [Google Scholar]
- Martens, A. , Greenberg, J. , & Allen, J. J. B. (2008). Self-esteem and autonomic physiology: Parallels between self-esteem and cardiac vagal tone as buffers of threat. Personality and Social Psychology Review, 12(4), 370–389. https://doi.org/10.1177/1088868308323224 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2002). Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging, 23(5), 921–939. https://doi.org/10.1016/S0197-4580(02)00027-1 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. https://doi.org/10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McGrory, J. J. , Tasko, S. M. , Bless, D. M. , Heisey, D. , & Ford, C. N. (1997). Psychological correlates of functional dysphonia: An investigation using the Minnesota Multiphasic Personality Inventory. Journal of Voice, 11(4), 443–451. https://doi.org/10.1016/S0892-1997(97)80041-0 [DOI] [PubMed] [Google Scholar]
- Morrison, M. D. , Rammage, L. A. , Belisle, G. M. , Pullan, C. B. , & Nichol, H. (1983). Muscular tension dysphonia. The Journal of Otolaryngology, 12(5), 302–306. [PubMed] [Google Scholar]
- Munin, M. C. , Rosen, C. A. , & Zullo, T. (2003). Utility of laryngeal electromyography in predicting recovery after vocal fold paralysis. Archives of Physical Medicine and Rehabilitation, 84(8), 1150–1153. https://doi.org/10.1016/S0003-9993(03)00146-1 [DOI] [PubMed] [Google Scholar]
- Obrist, P. A. (1981). Cardiovascular psychophysiology: A perspective. Plenum Press; https://doi.org/10.1007/978-1-4684-8491-5 [Google Scholar]
- Overbeek, T. J. M. , van Boxtel, A. , & Westerink, J. H. D. M. (2014). Respiratory sinus arrhythmia responses to cognitive tasks: Effects of task factors and RSA indices. Biological Psychology, 99, 1–14. https://doi.org/10.1016/j.biopsycho.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Patrick, C. J. , Curtin, J. J. , & Tellegen, A. (2002). Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment, 14(2), 150–163. https://doi.org/10.1037/1040-3590.14.2.150 [DOI] [PubMed] [Google Scholar]
- Pfau, E.-M. (1975). An investigation into psychological factors contributing to psychogenic dysphonia. Folia Phoniatrica et Logopaedica, 27, 298–306. https://doi.org/10.1159/000263999 [PubMed] [Google Scholar]
- Ramaswamy, S. , Shankar, S. K. , Manjunath, K. Y. , Devanathan, P. H. , & Nityaseelan, N. (1994). Ultrastructure of the ganglion on human internal laryngeal nerve. Neuroscience Research, 18(4), 283–290. https://doi.org/10.1016/0168-0102(94)90164-3 [DOI] [PubMed] [Google Scholar]
- Reilly, K. J. , & Moore, C. A. (2003). Respiratory sinus arrhythmia during speech production. Journal of Speech, Language, and Hearing Research, 46(1), 164–177. https://doi.org/10.1044/1092-4388(2003/013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes del Paso, G. A. , Langewitz, W. , Mulder, L. J. M. , van Roon, A. , & Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477–487. https://doi.org/10.1111/psyp.12027 [DOI] [PubMed] [Google Scholar]
- Ritz, T. (2004). Probing the psychophysiology of the airways: Physical activity, experienced emotion, and facially expressed emotion. Psychophysiology, 41(6), 809–821. https://doi.org/10.1111/j.1469-8986.2004.00247.x [DOI] [PubMed] [Google Scholar]
- Ritz, T. (2009). Studying noninvasive indices of vagal control: The need for respiratory control and the problem of target specificity. Biological Psychology, 80(2), 158–168. https://doi.org/10.1016/j.biopsycho.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Ritz, T. , Thöns, M. , & Dahme, B. (2001). Modulation of respiratory sinus arrhythmia by respiration rate and volume: Stability across posture and volume variations. Psychophysiology, 38(5), 858–862. https://doi.org/10.1111/1469-8986.3850858 [PubMed] [Google Scholar]
- Roy, N. , & Bless, D. M. (2000a). Toward a theory of the dispositional bases of functional dysphonia and vocal nodules: Exploring the role of personality and emotional adjustment. In Kent R. D. & Ball M. J. (Eds.), Voice quality measurement (pp. 461–480). Singular. [Google Scholar]
- Roy, N. , & Bless, D. M. (2000b). Personality traits and psychological factors in voice pathology: A foundation for future research. Journal of Speech, Language, and Hearing Research, 43(3), 737–748. https://doi.org/10.1044/jslhr.4303.737 [DOI] [PubMed] [Google Scholar]
- Roy, N. , Bless, D. M. , & Heisey, D. (2000). Personality and voice disorders: A multitrait–multidisorder analysis. Journal of Voice, 14(4), 521–548. https://doi.org/10.1016/S0892-1997(00)80009-0 [DOI] [PubMed] [Google Scholar]
- Scherer, K. R. (1986). Vocal affect expression: A review and a model for future research. Psychological Bulletin, 99(2), 143–165. https://doi.org/10.1037/0033-2909.99.2.143 [PubMed] [Google Scholar]
- Sheps, D. S. , & Sheffield, D. (2001). Depression, anxiety, and the cardiovascular system: The cardiologist's perspective. The Journal of Clinical Psychiatry, 62(Suppl. 8), 12–18. [PubMed] [Google Scholar]
- Tellegen, A. (1995). Multidimensional Personality Questionnaire–Brief Form (Unpublished test). Used by permisison of the University of Minnesota.
- Thayer, J. F. , & Sternberg, E. (2006). Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–372. https://doi.org/10.1196/annals.1366.014 [DOI] [PubMed] [Google Scholar]
- van Mersbergen, M. , Patrick, C. , & Glaze, L. (2008). Functional dysphonia during mental imagery: Testing the trait theory of voice disorders. Journal of Speech, Language, and Hearing Research, 51(6), 1405–1423. https://doi.org/10.1044/1092-4388(2008/06-0216) [DOI] [PubMed] [Google Scholar]