Abstract
Objective
Due to the global spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), guidance for the use of psychotropic drugs in this context is necessary. We aimed to review clinical evidence regarding the potential toxicity of psychiatric medications in the context of SARS-CoV-2 infection.
Methods
A systematic search for all types of empirical studies and reviews in a broad set of electronic databases and trial registries was conducted up to the 15th of August 2020.
Results
We identified 3 case series and 4 single-case reports on the occurrence of toxicity induced by various psychotropic drugs (lithium, n = 2; clozapine, n = 5; risperidone n = 2; haloperidol n = 1; duloxetine, n = 1). In addition, we provide a new case report on the possible precipitation of valproic acid-induced hyperammonemic encephalopathy. In most cases, SARS-CoV-2 infection may have precipitated drug toxicity/side effects. The management of toxicity did not diverge from the usually applied principles in the absence of infection.
Conclusions
Due to the limited available evidence and the recent genomic diversity and evolution of the SARS-CoV-2, it is currently not possible to derive evidence-based recommendations for the use of psychotropic drugs in the context of SARS-CoV-2 infection. Nevertheless, we provide some guidance based on the reviewed literature. At the current state of knowledge, there is no contraindication for any psychotropic drug. Caution is warranted regarding the dosing and, in particular, the monitoring of clozapine, lithium and valproate.
Keywords: SARS-CoV-2, COVID-19, Toxicity, Psychotropic drugs, Clozapine, Lithium, Valproic acid
1. Introduction
The current outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first reported in the Chinese city of Wuhan on December 31, 2019 [1]. The spread of SARS-CoV-2 was categorized as a pandemic by the WHO on March 11, 2020; by September 2020, approximately 54 million people had been infected with the virus, and more than 1.3 million people had died [2].
Patients with COVID-19 can be treated with psychotropic drugs for two main reasons. First, antipsychotics are used to treat the neuropsychiatric consequences of SARS-CoV-2 [3]. Second, patients with preexisting disorders who develop COVID-19 will continue their psychotropic treatment during the course of the infection. Given the lack of previous experience with COVID-19, both the prevention of neuropsychiatric symptoms and the management of ongoing psychiatric medications are challenging [4,5]. Herein, we briefly introduce these two situations.
Neuropsychiatric consequences of SARS-CoV-2 are frequently reported, such as confusion and agitation, among patients in intensive care units [6]. Approximately 15% of hospitalized patients meet the criteria for delirium, which is associated with a poorer prognosis, particularly for the elderly [5,7]. Other severe neurological consequences, such as cerebrovascular events and encephalopathy, have also been reported [8,9]. Finally, new-onset psychiatric disorders such as psychosis have also been reported [9].
Despite initial concerns, patients with preexisting psychiatric disorders do not seem to be at higher risk of SARS-CoV-2 infection [10]. One retrospective study suggested that patients treated with clozapine may be at higher risk than patients treated with other antipsychotics, but these results need replication [11]. Overall, given the extremely widespread population, there are numerous patients with ongoing psychiatric treatment who develop COVID-19 [12]. The management of their psychotropic medication in the context of a new infectious illness is based on very limited evidence. Of concern are the commonly abnormal liver and renal functions, in particular for critically ill patients with COVID-19 [13], that can impair the metabolism of psychotropic drugs. Initial reports have suggested an increase in side effects from certain antidepressant and antipsychotic medications [14,15]. In addition, the potential toxicity of clozapine has been discussed [16].
Considering that the COVID-19 pandemic is still expanding across the world, there is an urgent need to summarize available evidence on this subject. Here, we aim to review clinical evidence regarding the potential toxicity/side effects of psychiatric medications in the context of SARS-CoV-2 infection.
2. Methods
2.1. Registration
Our systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [17]. The protocol was published on August 10, 2020 in the International Prospective Register of Systematic Reviews (PROSPERO CRD42020202365).
2.2. Inclusion criteria
Due to the novelty of SARS-COV-2, controlled clinical trials are not available; therefore, we considered all types of empirical studies for inclusion. In a preliminary search, mostly case reports and narrative reviews were found. We decided to distinguish preclinical evidence (e.g., in vivo studies) from clinical evidence (e.g., case reports, clinical trials).
2.3. Search strategy
From the 1st to 15th of August 2020, we searched the MEDLINE, PubMed, Embase, and PsycARTICLE databases as well as the clinicaltrials.gov. and clinicaltrialsregister.eu trial registries. The search terms used for the systematic review combined key words and MeSH terms and are reported in the supplementary material (S1). No restrictions on language or time were applied.
2.4. Selection criteria
In this article, we focus on clinical studies and do not report preclinical findings. For clinical studies concerning the human population, the inclusion criteria were as follows: a) patients with ongoing SARS-CoV-2 infection; b) patients using of psychotropic drugs (e.g., antipsychotics, mood stabilizers, anticonvulsants, antidepressants, benzodiazepines, hypnotics); and c) potential toxicity for the corresponding medication. All articles were retained for full-text review, and the reference lists of retrieved articles were closely examined to identify additional studies.
2.5. Outcomes and data extraction
Two authors (MS and OD) independently inspected the titles, abstracts, and methods of all papers, case reports or clinical trials identified in the electronic searches for relevance. The electronic search was supplemented by examining the reference lists of retrieved articles.
We extracted data using a prespecified data extraction tool designed in Microsoft Excel, with the main data fields extracted being authors, age/sex, treatment suspected to have induced toxicity, dosage, additional treatments, first symptoms of toxicity, diagnosis, and outcome. In addition, we added a case report from our own department to the final list of cases of toxicity.
2.6. Statistical analysis
We calculated descriptive statistics on the included studies using Stata 14.0 software (Stata Corp, College Station, TX, USA).
3. Results
3.1. Search results and qualitative description of the included articles
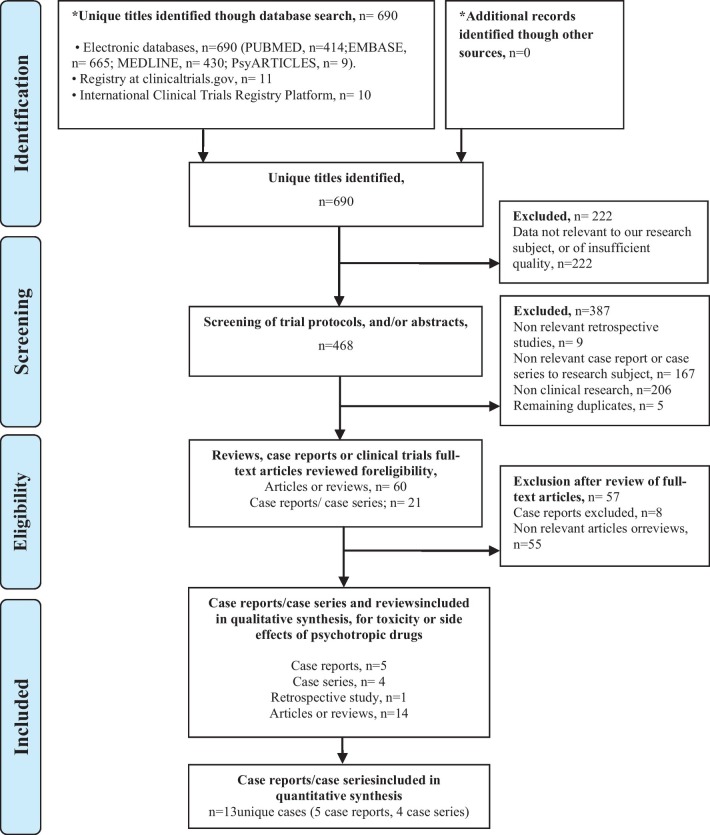
Among the 417 unique articles identified, we selected studies reporting results for SARS-CoV-2 and COVID-19. We identified 8 articles, including one retrospective study, 3 case series, and 4 case reports that reported the toxicity and side effects of psychotropic drugs in the context of SARS-CoV-2 infection (Fig. 1 ). All cases included patients treated with psychotropic drugs for a preexisting psychiatric disorder, albeit two cases.
Fig. 1.
Systematic review PRISMA flowchart.
In the case series, 2 cases of toxicity with lithium were reported, 3 cases of toxicity with clozapine, and 2 cases of serotoninergic syndrome suspicion. In the case reports, 2 cases of intoxication to clozapine, and 2 cases of neuroleptic malignant syndrome, one with risperidone and one with haloperidol were reported. We added a case of valproic acid-induced hyperammonemic encephalopathy that occurred in our department (Table 1 ).
Table 1.
Detailed description of identified cases from case reports and case series reporting toxicity of psychotropic drugs in context of a SARS-CoV-2 infection.
| Authors | Sex, age (years) | Treatment suspected to have induced toxicity or side-effect and its daily dose | Dosage | Additional treatments | First symptoms at presentation | Psychiatric and medical disease | Vital signs at admission, and blood tests | Diagnosis | Management and outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1-Suwanwongse et al.2020 [18] Case 1 |
F, 67 | Lithium 1200 mg/day |
2.28 mEq/L | Quetiapine 800 mg/day Insulin, empagliflozin, metformin, sitagliptin, atorvastatin, irbesartan, and aspirin |
Alteration of consciousness. Behavior changes. Confusion. | Schizoaffective disorder. Diabetes mellitus, hypertension, and hyperlipidemia |
- RR: 18 breaths/min; T: 36.6 °C; HR: 100 bpm; BP: 145/82 mmHg; SpO2: 93%. - Lung exam revealed mild crepitation. A bilateral infiltration at X-ray was visible. - WBC: 7.78 × 10 3/mm3 (lymphocytes 9.4%) - Creatinine: 1.35 mg/dl - ECG was normal. - CT of the head was unremarkable. |
- SARS-CoV-2 pneumonia (positive nasopharyngeal swab) -Acute kidney injury, and lithium toxicity |
The patient benefit from hydroxychloroquine treatment as per hospital protocol; however, her hospital course was complicated with acute hypoxic respiratory failure and she eventually died on day 4 of her hospitalization. |
| 2-Suwanwongse et al.2020 Case 2 |
M, 18 | Lithium 900 mg/day |
2.60 mEq/L | Clozapine 100 mg/day Levothyroxine |
Alteration of consciousness. Fever, nasal congestion, and cough for 7 days. |
Bipolar disorder, autistic spectrum disorder, attention deficit hyperactivity disorder. Hypothyroidism, and mild persistent asthma. |
- RR: 18 breaths/min; T: 101.3 °F; HR: 120 bpm; BP: 120/60 mmHg; SpO2: 98%. - Lung exam was normal. - WBC: 11.79 × 103/mm3 (lymphocytes 17%) - ECG showed sinus tachycardia with an HR of 107 bpm. - CXR was normal. |
- SARS-CoV-2 infection (positive nasopharyngeal swab) -Acute kidney injury, and lithium toxicity |
Intravenous normal saline was administered for the treatment of lithium intoxication and acute kidney injury. The patient did not receive hemodialysis. The patient received symptomatic treatment for the COVID-19 infection. His conditions were resolving, and he was discharged home without any complications on hospital day 11. Lithium was discontinued. |
| 3- Llesuy & Sidelnik, 2020 [19] | F, 50 | Clozapine 300 mg/day |
n.a. | Quetiapine 100 mg/day | At admission, the patient was febrile and in respiratory distress. | Schizophrenia Diabetes, obesity, and active cigarette use |
- T: 38.3 °C; SpO2: 93% under oxygen - C-reactive protein: 330.4 mg/L - Lymphopenia: 900/μL - Hemoglobin A1c of 8% - CXR found bilateral opacities. |
- Acute respiratory distress syndrome on a COVID-19 pneumonia -Implication of clozapine is unclear |
Patient was intubated for hypoxic respiratory failure and admitted to the intensive care unit. On hospital day 6, she had evidence of pulmonary embolus, was started on tissue plasminogen activator with no improvement, and died. Both clozapine and quetiapine were held during the hospitalization. |
| 4 -Cranshaw & Harikumar, 2020 [16] | M, 38 | Clozapine 325 mg/day |
Clozapine 730 ng/mL Norclozapine 310 ng/mL |
Absence | Cough, headache, and reduced oxygen saturation. Followed the next day with drowsiness, markedly increased hypersalivation and myoclonus. | Organic psychosis | - SpO2 94% on room air | - SARS-CoV-2 infection (positive nasopharyngeal swab) - Possible precipitation of clozapine toxicity by SARS-CoV-2 infection |
Clozapine was discontinued during one day due to COVID-19 induced lymphopenia (0.76 × 109/l), and the following day to a transient mild neutropenia (1.26 × 109/l). The patient made an uncomplicated recovery from COVID-19 however experienced a relapse in psychotic symptoms as result of temporary clozapine cessation. |
| 5- Doston et al. 2020 [39] Case 1 |
M, 76 | Clozapine 300 mg/day |
1360 ng/mL | Absence | The patient was admitted to the hospital with COVID-19 and catatonia one month after missing his last ECT treatment. |
Bipolar-type schizoaffective disorder complicated by recurrent catatonia |
- Neutropenia with a nadir absolute neutrophil count of 1.100 | -SARS-CoV-2 infection with catatonia -Implication of clozapine is unclear |
The interpretation of the neutropenia is difficult, as the patient received an experimental COVID-19 medication (tocilizumab) that is associated with neutropenia. His absolute neutrophil count gradually rebounded to the 4000 s, and his catatonia resolved with lorazepam and a reduction of clozapine to 200 mg QHS. |
| 6- Doston et al. 2020 Case 2 |
F, 63 | Clozapine 400 mg/day (this treatment was started many years ago) |
1060 ng/mL. | Citalopram 20 mg/day Olanzapine 20 mg/day |
At admission, the patient initially presented with nausea and confusion. She was found to have COVID-19, hyponatremia, and an ileus. | Bipolar-type schizoaffective disorder |
- Hyponatremia: 110 mmol/L - Absolute neutrophil count of 14.970 |
- SARS-CoV-2 infection with an paralytic ileus - Possible precipitation of clozapine side effect by SARS-CoV-2 infection |
Due to the presence of an ileus, clozapine was held for 1 week without adverse consequences, and the drug was gradually reintroduced. |
| 7- Doston et al. 2020 Case 3 |
F, 53 | Clozapine 250 mg/day (this treatment was started many years ago) |
Dosage before infection: 458 ng/mL. Dosage at admission: 2154 ng/mL |
Fluphenazine 15 mg/day | The patient initially presented with delirium, fever, and vomiting. | Schizophrenia | - Absolute neutrophil count of 2200 | - SARS-CoV-2 infection with delirium - Possible precipitation of a clozapine intoxication by SARS-CoV-2 infection |
Clozapine dose was decreased to 50 mg with a temporary increase of fluphenazine to 10 mg twice daily. She tolerated a gradual return to her home dose with normalization in her mental status. |
| 8-Sokolov et al. 2020 [40] | F, 57 | Clozapine 100 mg in the morning and 175 mg at night (this treatment was started many years ago) |
n.a. | Metformin and insulin | The patient presented with shortness of breath and fever. She was transferred to intensive care for intubation and ventilation. She was found to have COVID-19 and a community-acquired pneumonia. | Schizoaffective disorder Depression Chronic obstructive pulmonary disease Pulmonary embolism Type 2 diabetes |
During admission: - SpO2 91% on 40% oxygen; RR:22/min - Type 1 respiratory failure At day ten post-extubation: - MRI brain scan was normal - EEG: Frequent (2.5–4 Hz), florid bilateral, non-synchronous epileptiform discharges were seen over both hemispheres. |
- SARS-CoV-2 pneumonia (positive nasopharyngeal swab) - Acute kidney injury - Nonconvulsive status epilepticus following the reintroduction of clozapine |
After a month of ventilation, the patient was extubated. Clozapine was slowly reintroduced. However, the patient was unable to speak or obey commands and opened her eyes only to pain. Some new onset intermittent right-sided facial jerks were noted. She was diagnosed with non-convulsive status epilepticus on EEG. She was commenced on levetiracetam, and clozapine was abruptly reduced from 275 to 125 mg/day with good effect. |
| 9- Butler et al. 2020 Patient G [41] |
F, 57 | Clozapine 150 mg in the morning and 200 at night. |
n.a. | Aripiprazole 10 mg/day, Lithium carbonate MR 400 mg, Sertraline 200 mg |
The patient was admitted to acute inpatient medical care with hypoxia, hemodynamic instability. On day 2 she was transferred to intensive care and was intubated for mechanical ventilation. | Treatment resistant schizophrenia | - SpO2 94% on 10 L oxygen Supplementation - C-reactive protein: 122 mg/L - WBC: 14.2 × 109/L - Neutrophils: 10.3 × 109/L |
- SARS-CoV-2 pneumonia (positive nasopharyngeal swab) - Type I respiratory failure - Nonconvulsive status epilepticus following the reintroduction of clozapine |
Uptitration of clozapine was started on day 19, and she was extubated on day 32. Clozapine was started at 12.5 mg/day, increasing in steps of 12.5 mg daily, then increasing by 25 mg daily for the following 2 weeks until a dose of 450 mg was reached. Her lithium continued to be paused because of poor renal function. On day 44 she was noted to be ‘twitching’ and a subsequent electroencephalogram confirmed non-convulsive status epilepticus. Clozapine was reduced to 25 mg with good effect. |
| 10- Soh et al. 2020 [14] Case 2 |
M, 44 | Risperidone n.a. |
n.a. | Favipiravir, 1600 mg/day Tazobactam/ piperacillin and azithromycin |
On day 5, risperidone was started for delirium. On day 7 the patient body temperature increased to 40.8 °C. CK level elevated, tachycardia, tachypnea, altered consciousness, and diaphoresis appeared. | No previous psychiatric history | - T: 40 °C - CK level elevated - Tachycardia - Tachypnea |
-Acute respiratory distress syndrome on a SARS-CoV-2 pneumonia with an acute delirium following intensive care - Neuroleptic malignant syndrome possibly precipitated by the SARS-CoV-2 infection |
Neuroleptic malignant syndrome diagnosis was confirmed, and both, favipiravir and risperidone were discontinued on day 8. On the day 8, CK levels decreased, and body temperature normalized on day 9. Later on, the patient's condition stabilized. |
| 11- Serrano et al. 2020 [15] Case 2 |
M, 78 | Risperidone 2 mg/day in combination with lopinavir/ritonavir n.a. |
n.a. | Lopinavir/ritonavir, 400/100 mg and hydroxychloroquine 200 mg, twice daily. Two doses of interferon beta-1b on days 3 and 4 Single administration of tocilizumab on day-9 Risperidone, 2 mg/day (started during hospitalization) Morphine 3 mg/day |
By day-10 the patient developed acute delirium that required 1 mg of risperidone twice daily for the next 48 h and a single administration of 3 mg of morphine for dyspnea control. Subsequently, the patient's level of consciousness worsened, and he developed tachycardia, diaphoresis, and hyperthermia that was unresponsive to antipyretics. | No previous psychiatric history. Hypertension, diabetic chronic kidney disease, and prior colorectal cancer |
- CK level 802 U/L - C-reactive protein: 1.06 mg/dL |
- SARS-CoV-2 infection (positive nasopharyngeal swab) -Acute delirium possibly induced by the association of antivirals drugs -Serotoninergic syndrome possibly precipitated by the several combinations of drug, or the SRAS-CoV-2 infection |
Due to serotoninergic syndrome suspicion, lopinavir/ritonavir and risperidone were immediately discontinued, instead adding fluid therapy, active cooling, and 0.25 mg of clonazepam every 6 h. The symptoms rapidly improved and resolved within the next several days. |
| 12- Kajani et al. 2020 [42] | M, (35 to 58) | Haloperidol long-lasting injection n.a. |
n.a. | Cefepime, linezolid, ampicillin, acyclovir, and hydroxychloroquine | The patient was admitted for an altered mental status. On admission, he was febrile, tachycardic, and tachypneic. On exam, the patient had a rigid posture and could not respond to painful stimuli. | Schizophrenia Hepatitis C |
- RR: 18 breaths/min; T: 36.6 °C; HR: 122 bpm; BP: 109/71; SpO2: 93%. - Mild leukocytosis (12.3 K/mm3) with a neutrophilic predominance (87.2%) and lymphopenia (5.1%). Cerebral spinal fluid (CSF) analysis revealed 0 WBC, 0 RBC and normal protein. - C-reactive protein: 5.8 mg/dl - EEG showed slow background and occasional right hemispheric discharge associated with some left-hand tremor - CXR revealed bilateral peripheral infiltrates |
- SARS-CoV-2 infection (positive PCR test) - Acute kidney failure - Neuroleptic malignant syndrome possibly precipitated by the SARS-CoV-2 infection |
The patient was admitted to the ICU for ventilator management and treatment of his altered mental status with associated rhabdomyolysis and acute kidney failure. Maximum accumulated dose of dantrolene of 10 mg/kg IV was administered over a two-day period followed by bromocriptine 5 mg loading dose then 2.5 mg three times daily. He was initiated on hemodialysis for acute renal failure. Hydroxychloroquine treatment was continued for a planned duration of five days. His CK began to downtrend on hospital day 4, and rigidity also improved at around the same time. He remained intubated and sedated, with no improvement in mentation. |
| 13- Serrano et al. 2020 Case 1 |
M, 66 | Duloxetine in combination with lithium and lopinavir/ ritonavir n.a. |
n.a. | Lithium (800 mg/day) and duloxetine (120 mg/day) Haloperidol 2 mg/day Lopinavir/ritonavir, 400/100 mg and hydroxychloroquine 200 mg, twice daily |
By day-3, the patient developed delirium, and 1 mg of haloperidol twice daily was added. For the next 4 days, his level of consciousness progressively declined, in association with high blood pressure, tachycardia, diaphoresis, and urinary retention. |
Bipolar disorder Cervical spinal stenosis |
- CK level: 767 U/L - Creatinine level: 1.47 mg/dL |
- SARS-CoV-2 bilateral pneumonia (positive nasopharyngeal swab) -Acute delirium possibly induced by the association of antivirals drugs -Serotoninergic syndrome possibly precipitated by the several combinations of drug, or the SRAS-CoV-2 infection |
Due to serotoninergic syndrome suspicion, duloxetine, lithium, haloperidol, and lopinavir/ritonavir were discontinued. Cyproheptadine at 8 mg every 6 h was started. Over the next 10 days, the myoclonus disappeared and his neurological status improved steadily. |
Abbreviations: beats per minute (bpm); blood pressure (BP); breaths per minute (breaths/min); cells per cubic millimeter (/mm3); chest X-Ray (CXR); computed tomography (CT); creatine kinase (CK); degrees Celsius (°C); electrocardiogram (ECG); electroencephalogram (EEG); heart rate (HR); not available (n.a.); oxygen saturation percentage on room air (SpO2); RR: respiratory rate (RR); temperature (T); white blood cells (WBC).
Among the included cases, a total of 63.6% of patients were males. The patients' ages ranged from 18 to 67 with a median of 55 years. A history of psychiatric disorders was present in all cases, with the exception of two case reports on the management of acute delirium following intensive care [14,15]. Two patients eventually died due to COVID-19.
In addition to case reports, we retrieved 10 narrative reviews providing general recommendations for the management of psychotropic drugs in the context of SARS-CoV-2 infection (supplementary material. S1). Most articles examined lithium, valproic acid and clozapine, which are psychotropic drugs that can induce toxicity and severe side effects and therefore require monitoring of plasma levels. These reviews were considered for developing recommendations for the management of the potential toxicity of psychotropic drugs.
3.2. Lithium toxicity
Acute lithium intoxication was reported in a case series consisting of 2 cases [18], but a causal association between COVID-19 and lithium intoxication was not certain (cases 1 & 2). Severe lithium toxicity with neurologic symptoms was present in both patients (plasma level > 2.0 mEq/L). Lithium (900 and 1200 mg/day) was discontinued for both patients. One of the patients, a 67-year-old woman, initially presented an acute kidney injury associated with lithium intoxication. She also developed pneumonia and died of acute hypoxic respiratory failure. For the second patient, an acute kidney injury appeared after lithium intoxication, and prompt treatment led to a favourable outcome.
3.3. Valproic acid toxicity
We added a new case of encephalopathy to valproic acid in the context of a SARS-CoV-2 infection that occurred in our department (case 12) (supplementary material. S2, S3). A week after the introduction of valproic acid (1500 mg/day), the patient presented spatial disorientation, mild confusion with a stupor state and severe psychomotor retardation associated with bilateral and symmetrical negative myoclonia in both upper arms. Hyperammonaemia was observed. Treatment with valproic acid was only discontinued for a day following a rapid improvement in consciousness following one dose of lactitol (osmotic laxative). We considered the case as possible precipitation of encephalopathy due to SARS-CoV-2 infection.
3.4. Clozapine toxicity
We retrieved five cases that reported clozapine side effects in the context of SARS-CoV-2 infection [16,19,20]. In all cases, the clozapine dosage was in a medium range (250–400 mg/day). For cases 5, 6 and 7, clozapine was used for many years without complications.
In case 3, the patient died of acute respiratory distress syndrome due to COVID-19 pneumonia. For this patient, a causal role of clozapine in severe infection remains difficult to determine. Clozapine levels were not obtained, but clozapine was given in conjunction with quetiapine, which may raise the risk of pneumonia [21]. In case 4, the patient developed neutropenia but was in comedication with an experimental COVID-19 medication (tocilizumab) that is associated with neutropenia. For these two cases, the role of clozapine is uncertain.
We found three cases in which clozapine levels were very high (1360, 1060 and 2154 ng/mL, respectively), and a causal role of clozapine is probable. For case 5, precipitation of clozapine side effects was seen with drowsiness, markedly increased hypersalivation, myoclonus with lymphopenia and neutropenia. For case 6, a clozapine-induced paralytic ileus occurred, and for the last case (case 7), delirium associated with fever and vomiting was present.
3.5. Other antipsychotic side effects
Regarding other antipsychotics, three case reports on the toxicity of risperidone and haloperidol were found. For risperidone, we found two case reports on the toxicity of risperidone used for the treatment of delirium. We identified other reports of side effects of antipsychotics with the case of a neuroleptic malignant syndrome due to the introduction of risperidone and favipiravir, an antiviral medication (case 8). This patient did not suffer from a preexisting psychiatric disorder. Risperidone was added for the treatment of delirium in the context of intensive care for COVID-19 pneumonia. Neuroleptic malignant syndrome appeared two days after the introduction of the antipsychotic.
Another report presents the occurrence of a serotoninergic syndrome occurring after the introduction of risperidone for the treatment of delirium occurring in a patient receiving a combination of several antiviral agents (case 9) [15]. These included lopinavir/ritonavir, hydroxychloroquine and two doses of interferon beta-1b.
For haloperidol, we found one case of neuroleptic malignant syndrome with a haloperidol long-acting injection (case 10) that occurred three weeks after the last injection in the context of a SARS-CoV-2 infection.
3.6. Antidepressant side effects
Only one case reported the occurrence of a serotoninergic syndrome in the presence of an antidepressant. In this case (case 11), the patient was treated with duloxetine (120 mg/day) combined with lithium (800 mg/day), lopinavir/ritonavir was added during hospitalization [15].
In both cases 9 and 11, the serotoninergic syndrome disappeared when treatments were stopped.
4. Discussion
The current SARS-CoV-2 pandemic requires urgent research on the safe management of psychotropic drugs for patients suffering from mental illness who develop COVID-19 and for patients with neuropsychiatric complications of COVID-19. Our systematic review has identified several case reports in the context of SARS-CoV-2 infection, mostly concerning patients with preexisting psychiatric disorders. Only two cases of toxicity of antipsychotic treatment for delirium as a neuropsychiatric consequence of COVID-19 were found. In most cases, the SARS-CoV-2 infection context may have precipitated drug toxicity and side effects with different mechanisms, e.g., dehydration, drug-drug interactions, acute kidney injury, and cytokine implications. In the retrieved reports, the management of toxicity did not differ from that applied in the absence of infection. Deaths were not directly related to the toxicity of psychotropic drugs.
4.1. Drug toxicity and SARS-CoV-2 infection
Our systematic review shows that clinical evidence for drug toxicity in the context of COVID-19 remains scarce. Lithium, valproic acid and clozapine, which are three drugs with a narrow therapeutic index, also present specific mechanisms of toxicity. Below, we discuss potential mechanisms that could lead to an increase in toxicity for lithium, valproic acid and clozapine in the context of SARS-CoV-2 infection.
4.1.1. Lithium toxicity and SARS-CoV-2 infection
Lithium is usually prescribed for the treatment of mood disorders and is generally well tolerated [22]. Our systematic review identified one article that reports lithium toxicity in 2 COVID-19 patients associated with poor oral intake, dehydration, and decreased renal function [18]. Furthermore, hypotension, hypoxemia, sepsis, and nephrotoxic drugs can also arise in the context of COVID-19 and increase the severity of lithium toxicity. Since lithium is mostly secreted by the kidneys, the risk of acute kidney injury in the context of coronavirus infection is a major problem [23,24]. Uribarri and colleagues found that renal failure on admission in patients with SARS-CoV-2 infection is frequent and is associated with a greater number of complications and in-hospital mortality [25].
4.1.2. Valproic acid toxicity and SARS-CoV-2 infection
The toxicity of valproic acid is closely linked to impaired liver function but remains complex [26,27]. Fan and colleagues reported on a small sample of COVID-19 patients who one-third of patients presented abnormal liver function [28]. These patients were more likely to be male and had higher levels of procalcitonin and C-reactive protein. The elevation of liver enzymes was subnormal to the usual three-fold level. Schaefer and colleagues described several combined mechanisms in response to SARS-CoV-2 infections that could explain the impact on liver function [29]: the direct viral effect of SARS-COV-2 on the liver, a localized and systemic inflammatory response, muscle injury, ischaemia and cardiomyopathy, and drug-induced liver injury, as is possible with valproic acid.
In the new case report from our department, the patient had a dosage of 20 mg/kg/day but developed possible valproic acid-induced hyperammonemic encephalopathy more than a week after the introduction of the drug at a stable dose. We hypothesize that cytokine release in the context of SARS-CoV-2 infection may have precipitated hyperammonemia throughout a perturbation of the urea cycle.
4.1.3. Clozapine toxicity and SARS-CoV-2 infection
Our retrieved cases identified two different mechanisms for clozapine toxicity in the context of SARS-CoV-2 infection.
First, three cases reported typical clozapine side effects in association with increased plasma levels during SARS-CoV-2 infection (cases 5, 6 and 7). Indeed, an increase in clozapine levels could be the result of severe inflammation with a massive release of cytokines responsible for the decrease in the expression and activity of CYP1A2 and CYP3A4, two cytochromes implicated in clozapine metabolism [[30], [31], [32]].
In one case, the patient developed severe COVID-19 pneumonia and eventually died (case 4). However, the causal role of clozapine and comedication with quetiapine in this case is uncertain. One study suggested an increased risk for SARS-CoV-2 infection [11] in patients treated with clozapine but does not report the risk for COVID-19 pneumonia. Earlier studies conducted before the SARS-CoV-2 pandemic have suggested an increased risk for pneumonia, probably more concerning bacterial pneumonia [21,30]. This risk seems to be increased for combination treatments. Potential mechanisms include hypersalivation and sedation as well as immunosuppressive effects. At present, it is premature to conclude on an increased risk for COVID-19 pneumonia and bacterial superinfection in patients taking clozapine. However, certain precautionary measures are recommended for clozapine use in the context of SARS-CoV-2 infection, as outlined below.
4.1.4. Serotonergic and neuroleptic malignant syndromes
We identified a case series reporting the occurrence of two cases of serotoninergic syndrome. In both cases, patients were treated with lopinavir/ritonavir combined with lithium/duloxetine and risperidone. Ritonavir has in vitro antiviral activity against SARS-CoV-1 and MERS-CoV [33] but is no longer recommended for use in patients with COVID-19. The drug can trigger a serotoninergic syndrome in patients with concomitant treatment with SSRIs, mainly due to diminished elimination [34].
We have also found a description of two neuroleptic malignant syndrome with the association of risperidone and favipiravir, and with a haloperidol long-acting injection. Since evidence is limited to case reports, we cannot conclude that there is an increase in the risk of occurrence of both syndromes in the context of SARS-CoV-2 infection; however, caution is warranted when associating antipsychotics and antidepressants with antivirals.
4.1.5. Combination of psychotropic drug and anti-COVID-19 drugs
As reported in several cases retrieved, drug-drug interactions can occur when combining psychotropic drugs with antiviral treatments, with a potential increase in the occurrence of side effects. Ostuzzi and colleagues have recently reviewed interactions between psychotropic medications and drugs to treat COVID-19 [5]. They describe the special warnings and precautions for use according to the British National Formulary and the European Medicines Agency. In addition, Asadi-Pooya and colleagues described drug-drug interactions for antiepileptic drugs and anti-COVID-19 drugs [35]. Almost all widely used psychotropic medications have a risk of drug-drug interaction with anti-COVID-19 drugs. Some antipsychotics are concerned with a risk of psychotropic-related respiratory depression. Indeed, in addition to the risk of increasing plasmatic levels of drugs to toxic levels, sedative, anticholinergic and QTc prolongation properties of psychotropic drugs can interact with the properties of anti-COVID-19 drugs. Caution should be taken when combining psychotropic and anti-COVID-19 drugs to avoid drug-drug interactions [36].
4.2. Recommendations for the management of psychotropic drugs in the context of the COVID-19 pandemic
As outlined above, the evidence for the incidence, diagnosis and management of psychopharmacologic drug toxicity in the context of SARS-CoV-2 infection remains very limited. Although it is not possible to formulate evidence-based recommendations at this point, we believe that there is an urgent need for guidance on this issue. Several recommendations have been published on the management of clozapine and other psychotropic drugs during the COVID-19 pandemic [4,5,37]. Based on these reviews, the evidence reviewed in this article and general recommendations for the management of psychopharmacologic drugs, we developed a set of pragmatic recommendations for the management of psychopharmacologic drugs and, more specifically, lithium, valproic acid, and clozapine in the context of SARS-CoV-2 infection. These recommendations should be employed in the context of a personalized approach taking into account the patient's history combined with monitoring his clinical status.
4.2.1. General recommendations
Recommendation 1 - In the case of a SARS-CoV-2 infection, close clinical and blood test monitoring is warranted to ensure adequate efficacy and limit the toxicity of psychotropic drugs (plasma level of the psychotropic drugs and other relevant tests such as full blood count, dosage of renal and/or hepatic function).
Recommendation 2 - Patients with COVID-19 may require adjustment of their treatments to prevent drug-drug interactions between COVID-19 medical treatments and psychotropic medications.
Recommendations 3 - Patients with severe COVID-19 may require adjustment of their treatments because metabolism and elimination may be reduced due to the frequently abnormal liver and renal functions in critically ill patients [13].
4.2.2. Recommendations specific to lithium
Considering the narrow therapeutic index and high risk of toxicity associated with lithium therapy and its documented interactions with several commonly used drugs, we developed three recommendations for the prevention of renal toxicity of lithium in the context of SARS-CoV-2 infection:
Recommendation 1 - When SARS-CoV-2 infection is suspected in a patient treated with lithium, a plasmatic dosage of lithium and an assessment of kidney functioning, including serum urea, creatinine, electrolytes, glomerular filtration rate and proteinuria, should be obtained immediately.
Recommendation 2 - If the patients become symptomatic in the context of suspicion of SARS-CoV-2 infection (in particular in case of dehydration, fever, nausea, vomiting, neurologic symptoms), a pause in lithium administration should be considered until a plasmatic dosage is available.
Recommendation 3 - Clinicians should consider pausing lithium if the patient's kidney function becomes moderately impaired (defined as a GFR of 45-59 mL/min/1.73 m2) with or without evidence of kidney damage or if creatinine clearance is <60 mL/min.
4.2.3. Recommendations specific to valproic acid
For lithium, an increased risk for sedation and confusion is present with the use of valproic acid in the context of SARS-CoV-2 infection, and liver function can be impaired [29]. We suggest the following recommendations for valproic acid in the context of SARS-CoV-2 infection:
Recommendations 1 - In case of suspicion of a SARS-CoV-2 infection in a patient treated with valproic acid, a plasmatic dosage of valproic acid and liver function (alanine aminotransferase, alanine aminotransferase, gamma glutamyl), ammonaemia, amylase and lipase should be measured, as well as kidney function.
Recommendation 2 - If the patients become symptomatic (in particular in case of dehydration, fever, nausea, vomiting, oedema or neurologic symptoms), discontinuation of valproic acid should be considered until a plasmatic dosage is available.
Recommendation 3 - If moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment is observed, the dosage of valproic acid should be adjusted.
Recommendation 4 - In cases of suspicion of valproic acid-induced encephalopathy with or without evidence of liver damage, clinicians should consider discontinuing valproic acid.
4.2.4. Recommendations for clozapine
Clozapine is the psychotropic drug that has received the most attention in the context of the COVID-19 pandemic. It can lead to clozapine-associated neutropenia and agranulocytosis and may therefore increase the risks of superinfection in the lungs compared to other antipsychotics.
Recommendations 1 - When SARS-CoV-2 infection is suspected, a plasmatic dosage of clozapine and a complete blood count (with white cell count and absolute neutrophil counts) should be conducted immediately.
Recommendation 2 - If the patient presents severe respiratory symptoms (dyspnoea, hypoxia) and SARS-CoV-2 infection is suspected, lowering clozapine by as much as half should be considered until a plasmatic dosage is available.
Recommendation 3 - If symptoms of clozapine toxicity (severe drowsiness, seizures, symptoms of anticholinergic toxicity) emerge, lowering clozapine by as much as half should be considered until a plasmatic dosage is available.
Recommendation 4 - Even though some authors report that clozapine can be introduced during a SARS-CoV-2 infection [38], in the case of a severe SARS-CoV-2 infection, we recommend postponing clozapine introduction if possible.
5. Conclusions
Evidence of the precipitation of toxicity and the increase in side effects of psychotropic drugs with SARS-CoV-2 is mostly limited to the case-report level. Naturalistic studies on the risk or severity of SARS-CoV-2 infection in patients already receiving psychotropic drugs could provide indirect evidence of their possible toxicity. The current state of knowledge does not contraindicate the use of any psychotropic drug for patients with a SARS-CoV-2 infection. Nevertheless, for patients receiving psychotropic drugs with narrow therapeutic indices, such as lithium, valproic acid, and especially clozapine, clinicians should conduct close monitoring to ensure adequate efficacy and limit the precipitation of toxicity or the increase in potential side effects. Patients should be informed of these potential risks in the context of the COVID-19 pandemic.
Funding
Internal funding only.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.genhosppsych.2021.02.006.
Appendix A. Supplementary data
Prisma checklist
Search terms and retreived articles.
Quality of case reports and case series (Murad & colleagues tool)
Quality of case reports and case series (Naranjo scale)
Data availability
This is a systematic review, all data used is already published online and available
References
- 1.Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12 doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JHU . In: Medicine. JHU, editor. 2020. Coronavirus ressource center. editor. [Google Scholar]
- 3.García C.A.C., Sánchez E.B.A., Huerta D.H., Gómez-Arnau J. Covid-19 treatment-induced neuropsychiatric adverse effects. Gen Hosp Psychiatry. 2020;S0163-8343(20):30077–3–‐3. doi: 10.1016/j.genhosppsych.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostuzzi G., Gastaldon C., Papola D., Fagiolini A., Dursun S., Taylor D., et al. Pharmacological treatment of hyperactive delirium in people with COVID-19: rethinking conventional approaches. Ther Adv Psychopharmacol. 2020;10 doi: 10.1177/2045125320942703. 2045125320942703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., et al. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaze M., Attali D., Petit A.C., Blatzer M., Simon-Loriere E., Vinckier F., et al. Repurposing chlorpromazine to treat COVID-19: the reCoVery study. L’Encéphale. 2020;46:169–172. doi: 10.1016/j.encep.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govind R., Fonseca de Freitas D., Pritchard M., Hayes R.D., JH MacCabe. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study. Br J Psychiatry. 2020:1–7. doi: 10.1192/bjp.2020.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melamed O.C., Hahn M.K., Agarwal S.M., Taylor V.H., Mulsant B.H., Selby P. Physical health among people with serious mental illness in the face of COVID-19: concerns and mitigation strategies. Gen Hosp Psychiatry. 2020;66:30–33. doi: 10.1016/j.genhosppsych.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020:1–13. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soh M., Hifumi T., Isokawa S., Shimizu M., Otani N., Ishimatsu S. Neuroleptic malignant syndrome in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243.e1–2243.e3. doi: 10.1016/j.ajem.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mas Serrano M., Pérez-Sánchez J.R., Portela Sánchez S., De La Casa-Fages B., Mato Jimeno V., Pérez Tamayo I., et al. Serotonin syndrome in two COVID-19 patients treated with lopinavir/ritonavir. J Neurol Sci. 2020;415:116944. doi: 10.1016/j.jns.2020.116944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cranshaw T., Harikumar T. COVID-19 infection may cause clozapine intoxication: case report and discussion. Schizophr Bull. 2020;46:751. doi: 10.1093/schbul/sbaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwanwongse K., Shabarek N. Lithium toxicity in two coronavirus disease 2019 (COVID-19) patients. Cureus. 2020;12:e8384. doi: 10.7759/cureus.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llesuy J.R., Sidelnik S.A. Death from COVID-19 in a patient receiving clozapine: factors involved and prevention strategies to consider. Prim Care Companion CNS Disord. 2020;22(4):20l02699. doi: 10.4088/PCC.20l02699. [DOI] [PubMed] [Google Scholar]
- 20.Dotson S., Hartvigsen N., Wesner T., Carbary T.J., Fricchione G., Freudenreich O. Clozapine toxicity in the setting of COVID-19. Psychosomatics. 2020;61:577–578. doi: 10.1016/j.psym.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo C.-J., Yang S.-Y., Liao Y.-T., Chen W.J., Lee W.-C., Shau W.-Y., et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2013;39:648–657. doi: 10.1093/schbul/sbr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybakowski J.K. Challenging the negative perception of lithium and optimizing its long-term administration. Front Mol Neurosci. 2018;11:349. doi: 10.3389/fnmol.2018.00349. PMID: 30333722; PMCID: PMC6175994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian J.-Y., Wang B., Liu B.-C. Acute kidney injury in the 2019 novel coronavirus disease. Kidney Dis (Basel) 2020;323:1–6. doi: 10.1159/000509086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uribarri A., Núñez-Gil I.J., Aparisi A., Becerra-Muñoz V.M., Feltes G., Trabattoni D., et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) registry. J Nephrol. 2020;33:737–745. doi: 10.1007/s40620-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstner T., Buesing D., Longin E., Bendl C., Wenzel D., Scheid B., et al. Valproic acid induced encephalopathy – 19 new cases in Germany from 1994 to 2003 – a side effect associated to VPA-therapy not only in young children. Seizure. 2006;15:443–448. doi: 10.1016/j.seizure.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Gayam V., Mandal A.K., Khalid M., Shrestha B., Garlapati P., Khalid M. Valproic acid induced acute liver injury resulting in hepatic encephalopathy- a case report and literature review. J Commun Hosp Int Med Perspect. 2018;8:311–314. doi: 10.1080/20009666.2018.1514933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Z., Chen L., Li J., Cheng X., Yang J., Tian C., et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer E.A.K., Arvind A., Bloom P.P., Chung R.T. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis. 2020;15:175–180. doi: 10.1002/cld.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leon J., Ruan C.-J., Verdoux H., Wang C. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatry. 2020;33 doi: 10.1136/gpsych-2019-100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark S.R., Warren N.S., Kim G., Jankowiak D., Schubert K.O., Kisely S., et al. Elevated clozapine levels associated with infection: a systematic review. Schizophr Res. 2018;192:50–56. doi: 10.1016/j.schres.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 32.Prior T.I., Baker G.B. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci JPN. 2003;28:99–112. [PMC free article] [PubMed] [Google Scholar]
- 33.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-a possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSilva K.E., Le Flore D.B., Marston B.J., Rimland D. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS (London, England) 2001;15:1281–1285. doi: 10.1097/00002030-200107060-00010. [DOI] [PubMed] [Google Scholar]
- 35.Asadi-Pooya A.A., Attar A., Moghadami M., Karimzadeh I. Management of COVID-19 in people with epilepsy: drug considerations. Neurol Sci. 2020;41:2005–2011. doi: 10.1007/s10072-020-04549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.English B.A., Dortch M., Ereshefsky L., Jhee S. Clinically significant psychotropic drug-drug interactions in the primary care setting. Curr Psychiatry Rep. 2012;14:376–390. doi: 10.1007/s11920-012-0284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siskind D., Honer W.G., Clark S., Correll C.U., Hasan A., Howes O., et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci JPN. 2020;45:200061. doi: 10.1503/jpn.200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouaziz N., Ben Rejeb H., Ateb S., Fourati T., Chammas F., Baha D., et al. Thoughts on a favourable evolution of a COVID-19 in a patient with resistant schizophrenia and on a combination of clozapine and paliperidone palmitate. Encephale. 2020;46:S126–s127. doi: 10.1016/j.encep.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dotson S., Hartvigsen N., Wesner T., Carbary T.J., Fricchione G., Freudenreich O. Clozapine toxicity in the setting of COVID-19. Psychosomatics. 2020;61:577–578. doi: 10.1016/j.psym.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokolov E., Hadavi S., Mantoan Ritter L., Brunnhuber F. Non-convulsive status epilepticus: COVID-19 or clozapine induced? BMJ Case Reports. 2020;13 doi: 10.1136/bcr-2020-239015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler M., Bano F., Calcia M., McMullen I., Sin Fai Lam C.C., Smith L.J., et al. Clozapine prescribing in COVID-19 positive medical inpatients: a case series. Ther Adv Psychopharmacol. 2020;10 doi: 10.1177/2045125320959560. 2045125320959560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kajani R., Apramian A., Vega A., Ubhayakar N., Xu P., Liu A. Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav Immun. 2020;88:28–29. doi: 10.1016/j.bbi.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prisma checklist
Search terms and retreived articles.
Quality of case reports and case series (Murad & colleagues tool)
Quality of case reports and case series (Naranjo scale)
Data Availability Statement
This is a systematic review, all data used is already published online and available