Abstract
Background: Atrial fibrosis and inflammation play important roles in perpetuating and initiating atrial fibrillation (AF). Although the fibrotic area can be visualized as a delayed enhancement area on late gadolinium enhancement magnetic resonance imaging (LGE-MRI), atrial inflammation has not yet been visualized on any imaging modality. We describe the protocol for a feasibility study to visualize atrial inflammation on positron emission tomography/MRI (PET/MRI).
Methods and Results: This is a single-arm, prospective, open-label proof-of concept trial, involving AF patients after cryoballoon ablation (CBA). A total of 30 paroxysmal AF patients will be enrolled and undergo simultaneous PET/MRI for the assessment of regional 18F-fluorodeoxyglucose (18F-FDG) uptake 1 day after the CBA. Furthermore, LGE-MRI will be performed before CBA, and at 1 and 4 weeks after assessing the regional LGE area. The main outcome measures will be (1) the feasibility of imaging inflammation in the left atrium on PET/MRI; and (2) the safety of the intervention.
Conclusions: There are few data on the visualization of atrial inflammation using PET/MRI. Establishing the visualization methodology will contribute to elucidating the fundamental histopathologic findings of the progress to fibrosis, and to the planning and execution of a larger definitive trial to test the usefulness of PET/MRI.
Key Words: Atrial fibrillation, Cryoballoon, Fibrosis, Inflammation, Positron emission tomography/magnetic resonance imaging (PET/MRI)
Inflammation is associated with the initiation, perpetuation, and recurrence of atrial fibrillation (AF).1 Inflammatory biomarkers promote calcium handling abnormalities and provoke abnormal triggering in pulmonary veins (PV) as well as shortening of the atrial action potential duration. Furthermore, myeloperoxidase and heat-shock proteins induce atrial fibrosis, connexin dysregulation, apoptosis, and myolysis, leading to conduction slowing and increased conduction heterogeneity.2,3 The mechanistic links between atrial remodeling and inflammation, however, are complex, and a specific anti-inflammatory intervention is still challenging. Atrial fibrosis, one of the important factors perpetuating AF, can be visualized on late gadolinium enhancement magnetic resonance imaging (LGE-MRI), and a patchy fibrosis pattern is associated with an AF driver.4,5 Atrial fibrosis is considered a surrogate marker of a current AF substrate, and atrial inflammation is another marker. Positron emission tomography/MRI (PET/MRI) is used for the visualization of vessel wall inflammation and evaluation of the viability of the left ventricle, but it has not yet been used for the visualization of atrial inflammation.6,7 Although atrial fluorodeoxyglucose (FDG) uptake in persistent AF has been reported from the whole-body PET/computed tomography database as a retrospective study, no detailed distribution or quantitative analysis of atrial inflammation can be performed due to the thinner atrial wall and unstable cardiac rhythm and respiratory motion.8 We therefore will conduct a proof-of-concept feasibility trial to determine whether acute inflammation around the PV after cryoballoon ablation (CBA) could be visualized on PET/MRI.9
Methods
Objectives
The objectives of this study are therefore to assess (1) the feasibility of PET/MRI in visualizing the atrial inflammation after CBA; and (2) the relationship between severity of inflammation and AF recurrence, unexpected admission and atrial fibrosis on LGE-MRI. This study will be registered with the University Hospital Medical Information Network-Clinical Trial Registry (UMIN-CTR) (UMIN000027417) and Japan Registry of Clinical Trials (jRCT), and the posted information will be updated as needed to reflect the protocol amendments and study progress.
Study Design and Setting
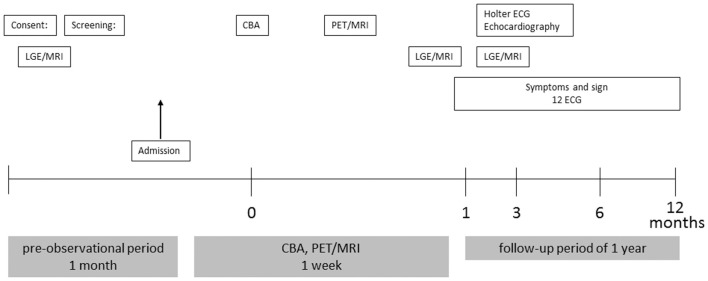
The study is an open-label, single-arm, prospective clinical trial (Figure 1). A total of 30 patients who will undergo CBA will be recruited for this study from Kobe University. The primary endpoint will be whether the inflammation around the PV after CBA is visible or not. The secondary endpoints are the relationship between inflammation and AF recurrence, unexpected admission, and atrial fibrosis on LGE-MRI.
Figure 1.
Study flow. CBA, cryoballoon ablation; ECG, electrocardiography; LGE-MRI, late gadolinium enhancement magnetic resonance imaging; PET/MRI, positron emission tomography/magnetic resonance imaging.
Subjects
Patients must fulfill the following criteria to be included in this study: (1) presence of AF and ability to undergo CBA; (2) age 20–80 years; and (3) ability to provide written voluntary consent to participate in this study. Exclusion criteria are as follows: (1) pregnancy; unwillingness to practice contraception during the study; or currently lactating; (2) severe renal or liver dysfunction (dialysis patients, estimated glomerular filtration rate <30 mL/min/1.73 m2; (3) allergies; (4) epileptic convulsions; or (5) any other reasons deeming the patient to be inappropriate as a participant.
CBA
The patients will be studied under deep propofol sedation while breathing spontaneously. Unfractionated heparin will be given in bolus form before trans-septal puncture to maintain an activated clotting time >300 s. If AF occurs, internal electrical cardioversion will be performed to restore sinus rhythm.10
Mapping and ablation will be performed using the NavX system (St. Jude Medical, St. Paul, MI, USA). A single trans-septal puncture will be performed. Complete occlusion will be confirmed by injecting contrast medium. PV isolation will be performed with a second-generation cryoballoon (Arctic Front Advance, Medtronic, Minneapolis, MN, USA) with a freeze cycle of 180 s. If PV potentials remain, the operator will decide whether additional CB ablation or touch-up radiofrequency ablation will be applied. The procedural endpoint will be electrophysiologically proven bidirectional block confirmed using a circular mapping catheter (Optima, St. Jude Medical).
18F-FDG PEF/MRI and Analysis
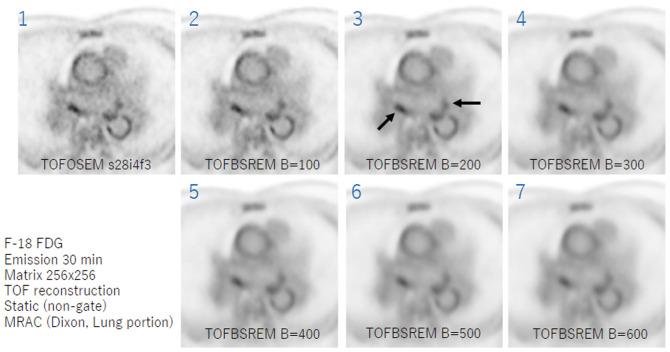
18F-FDG PET/MRI will be performed on a simultaneous PET/MR scanner (Signa PET/MR, GE Medical Systems, Waukesha, WI, USA). Subjects will fast for >18 h before the infusion of 3.5 MBq/kg 18F-FDG (0.9 mCi/kg). PET will be initiated 60 min after the 18F-FDG and continued for 30 min with the simultaneous acquisition of MRI. Non-contrast-enhanced MRI (2-point Dixon 3-D volumetric interpolated fast spoiled gradient echo, LAVA-Flex) will be carried out for attenuation correction and anatomic co-registration (slice thickness, 5.2 mm; gap, 2.6 mm). Additionally, MRI will be used to assess the anatomy of the left atrium (LA) and PV, including 2D-cine- and 3D-fast imaging employing a steady-state acquisition (FIESTA) and 3D-spoiled gradient recalled acquisition in the steady-state (SPGR). All PET and MRI will be performed simultaneously under respiratory and cardiac gating using a navigator-echo technique and vectorcardiogram. The PET reconstruction parameters will be as follows: ordered subset expectation maximization method with time of flight (TOF); matrix, 512×512; slice thickness, 2.8; field of view, 60 cm; iterations, 3; subsets, 16, Gaussian filter width, 4 mm. To correct for any background noise from the18F-FDG in the blood pool, the PET will also be reconstructed using the TOF-block sequential regularized expectation maximization (Q.Clear®) method with a β-value 200–300. Figure 2 shows PET reconstructed with different β-values. The blood pool noise can be reduced by the TOF-block sequential regularized expectation maximization (Q.Clear®) method with a higher β-value, while the sensitivity can dramatically decrease and the 18F-FDG uptake in the LA wall may be underestimated. Considering the optimal blood pool noise reduction without reducing the sensitivity, β-value 200–300 is considered optimal. The images will be analyzed using commercially available workstations (Advantage Workstation ver. 4.7, GE Medical Systems, Waukesha, WI, USA; and Ziostation ver. 2.4.2.3., Ziosoft, Tokyo, Japan). The 18F-FDG uptake will be assessed on the LA wall. From each region of interest, the standardized uptake values (SUV) will be measured. The SUV represents the 18F-FDG activity adjusted for the 18F-FDG dose, corrected for any decay, and divided by the body weight according to the following formula: SUV=tissue tracer activity (MBq/mL)/injected dose per patient body weight (MBq/kg). In the active segment analysis, the degree of the SUV will be categorized by color-coded scaling, whereas non-inflamed segments will be excluded. A 3-D reconstruction of the color-coded FDG uptake and volume-rendered LA and PV will be fused semi-automatically on the workstation. The volume-rendered LA and PV image will be generated from the LAVA-Flex sequence. The area of the LA and PV will be determined by a consensus reading performed by a cardiologist with years of experience and a cardiovascular radiologist (Y.W.) with 10 years of experience.
Figure 2.
Positron emission tomography (PET) according to β-value. Panel 1, original PET without noise reduction; Panels 2–7, PET noise reduction using the time-of-flight (TOF)-block sequential regularized expectation maximization (BSREM; Q.Clear®) method with increasing β-value. Of note, the blood pool noise can be dramatically reduced with a higher β value. Black arrows, strong 18F-fluorodeoxyglucose uptake around the pulmonary vein. OSEM, ordered subset expectation maximization.
LGE-MRI and Analysis
All patients will undergo contrast-enhanced MRI using a 1.5-T MR system (Achieva, Philips Medical, Best, The Netherlands) equipped with a 5-channel cardiac coil before, 1 week, and 1 month after CBA. This scan technique is established, and acquired images have been used for AF ablation procedures.11 Contrast-enhanced magnetic resonance angiography (CE-MRA) of the PV-LA anatomy will be acquired using a breath-hold 3-D fast field echo (FFE) sequence in the coronal plane during the first pass of a contrast agent (gadobutrol, Gadovist; Bayer Yakuhin, Osaka, Japan) injection at a dose of 0.1 mmol/kg. LGE-MRI of the LA will be acquired using a 3-D inversion recovery, respiration-navigated, electrocardiogram-gated, T1-FFE sequence in the transverse plane 15 min after the contrast injection, which has been previously reported.12 The typical parameters are as follows: repetition time/echo time, 4.7/1.5 ms; voxel size, 1.43×1.43×2.40 mm (reconstructed to 0.63×0.63×1.20 mm); flip angle, 15; SENSE, 1.8; and 80 reference lines. The inversion time (TI) will be set at 280–320 ms, using a Look-Locker scan. The data acquisition will be performed during the mid-diastolic phase of the left ventricle. The typical scan time for LGE-MRI will be 7–12 min depending on heart rate and respiration pattern. CE-MRA, and LGE-MRI will be transferred to a workstation (Ziostation ver. 2.4.2.3., Ziosoft) for further image post-processing and image analysis.
Post-Interventional Management and Follow-up
The 24-h Holter electrocardiograms will be performed 3, 6, and 12 months after the procedure. If symptoms occur outside the recording period, patients will be requested to contact the institution or the referring physician to obtain electrocardiography documentation. AF episodes lasting >30 s will be considered recurrences.
Outcome Measures
There are no prior empiric data on the feasibility of PET/MRI to visualize atrial inflammation after CBA. The primary endpoint will be whether the inflammation around the PV after CBA is visible on PET/MRI or not. If the FDG uptake in the respective analyzed segment is ≥50% of the previously determined threshold, the respective segment will be defined as “FDG visible”. Furthermore, if the FDG distribution around the PV corresponds with the lesion distribution assessed on LGE-MRI, the FDG uptake will be defined as “inflammation visible”. The secondary endpoint will be the proportion of patients with AF recurrence and unexpected admissions during the 1-year follow-up in the inflammation visible group after CBA. Finally, the distribution of inflammation and lesions assessed on LGE-MRI will be compared.
Measurements
Patient characteristics, laboratory data, and echocardiographic data will be collected. CBA data, PET/MRI, and LGE-MRI data will be collected. The maximum inflammation and lesion width around the PV will be calculated.
Sample Size and Analytical Plan
According to the pilot study, the incidence of inflammation around the PV in the enrolled subjects who underwent CBA was 65%, but no prior data on the incidence of inflammation around the PV before CBA have been reported. The incidence of a low-voltage area around the PV before ablation was 35% in the pilot study, which was used as the surrogate value. To test the null hypothesis that there is no difference in the inflammation incidence between that before and after CBA using a paired t-test with a 2-sided significance level of 5%, and 80% power for the analysis of the primary endpoint, the required number of subjects was calculated to be 27. To allow for 3 dropouts, the target sample size of this study has been set at 30.
Management and Patient Safety
For the data summarization or analysis, in principle the data will be handled as follows. When any doubt arises, the responsible biostatistician and study representative will discuss how to handle it. Monitoring of the study will be performed in order to periodically check whether this study is conducted safely in accordance with the protocol and whether the data are properly collected. The reporting of the results will follow the CONSORT statement-extension for Pilot and Feasibility Studies.
Results
Although the results are currently under investigation, a representative case is shown in Figure 2 (Supplementary Movie), in which strong18F-FDG uptake is seen around the PV.
Discussion
18F-FDG PET is able to visualize the inflammation in the left ventricle and arterial wall, but visualization of the inflammation in the LA has not been reported due to the thinner atrial wall and unstable cardiac and respiratory motions. To improve the imaging quality, the PET acquisition will be focused on the LA, and we will preserve a longer acquisition time (30 min) and require a completely controlled fasting period >18 h. If successful, this approach will be applied for visualizing pre-existing inflammation in the LA. Establishing the methodology of visualizing the inflammation in the LA will facilitate assessment of any further AF substrate and the innervation at an early stage. We hope that intervention for atrial inflammation will reduce the initiation, perpetuation, and recurrence of AF.
Conclusions
This is a new approach to visualize the inflammation induced by CBA in patients with AF. This study result will facilitate the further elucidation of the AF mechanism and early intervention for any further AF substrate.
Funding
This study is partly supported by JSPS KAKENHI Grant Number JP18K08036.
Disclosures
The Section of Arrhythmia is supported by an endowment from Medtronic JAPAN and Abbott JAPAN. The authors declare no conflicts of interest.
Supplementary Files
Supplementary Movie. Reconstructed positron emission tomography (PET) of the left atium using the TOF-block sequential regularized expectation maximization (Q.Clear®) method with a β-value 200–300. Notably, 18F-fluorodeoxyglucose uptake around the pulmonary vein could be visible.
Acknowledgments
We would like to thank Mr. John Martin for English-language assistance.
References
- 1. Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T.. Inflammation and atrial fibrillation: A comprehensive review. J Arrhythm 2018; 34: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kondo H, Abe I, Gotoh K, Fukui A, Takanari H, Ishii Y, et al.. Interleukin 10 treatment ameliorates high-fat diet-induced inflammatory atrial remodeling and fibrillation. Circ Arrhythm Electrophysiol 2018; 11: e006040. [DOI] [PubMed] [Google Scholar]
- 3. Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, et al.. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 2018; 15: 1717–1727. [DOI] [PubMed] [Google Scholar]
- 4. Hansen BJ, Zhao J, Fedorov VV.. Fibrosis and atrial fibrillation: Computerized and optical mapping; a view into the human atria at submillimeter resolution. JACC Clin Electrophysiol 2017; 3: 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honarbakhsh S, Schilling RJ, Orini M, Providencia R, Keating E, Finlay M, et al.. Structural remodeling and conduction velocity dynamics in the human left atrium: Relationship with reentrant mechanisms sustaining atrial fibrillation. Heart Rhythm 2019; 16: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rischpler C, Langwieser N, Souvatzoglou M, Batrice A, van Marwick S, Snajberk J, et al.. PET/MRI early after myocardial infarction: Evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur Heart J Cardiovasc Imaging 2015; 16: 661–669. [DOI] [PubMed] [Google Scholar]
- 7. van der Valk FM, Verweij SL, Zwinderman KA, Strang AC, Kaiser Y, Marquering HA, et al.. Thresholds for arterial wall inflammation quantified by 18F-FDG PET imaging: Implications for vascular interventional studies. JACC Cardiovasc Imaging 2016; 9: 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie B, Chen BX, Wu JY, Liu X, Yang MF.. Factors relevant to atrial (18)F-fluorodeoxyglucose uptake in atrial fibrillation. J Nucl Cardiol, doi:10.1007/s12350-018-1387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiuchi K, Fukuzawa K, Mori S, Watanabe Y, Hirata KI.. Feasibility of imaging inflammation in the left atrium post AF ablation using PET technology. JACC Clin Electrophysiol 2017; 3: 1466–1467. [DOI] [PubMed] [Google Scholar]
- 10. Kiuchi K, Kircher S, Watanabe N, Gaspar T, Rolf S, Arya A, et al.. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol 2012; 5: 667–675. [DOI] [PubMed] [Google Scholar]
- 11. Kiuchi K, Okajima K, Shimane A, Yokoi K, Teranishi J, Aoki K, et al.. Visualization of the radiofrequency lesion after pulmonary vein isolation using delayed enhancement magnetic resonance imaging fused with magnetic resonance angiography. J Arrhythm 2015; 31: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al.. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014; 7: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie. Reconstructed positron emission tomography (PET) of the left atium using the TOF-block sequential regularized expectation maximization (Q.Clear®) method with a β-value 200–300. Notably, 18F-fluorodeoxyglucose uptake around the pulmonary vein could be visible.