Abstract
Background: Despite evidence of the effects of alirocumab on the incidence of acute coronary events, its impact on plaque stabilization remains uncertain. The present study will investigate the effect of alirocumab on fibroatheroma in patients who underwent recent percutaneous coronary intervention (PCI).
Methods and Results: This phase IV, open-label, randomized, blinded near-infrared spectroscopy plus intravascular ultrasound (NIRS-IVUS) analysis, parallel-group, single-center study will enroll Japanese adults recently hospitalized for PCI with suboptimal low-density lipoprotein cholesterol (LDL-C) control (>70 mg/dL) despite stable statin therapy. Thirty patients will be randomized to receive either alirocumab or standard of care. The alirocumab group will receive alirocumab 75 mg every 2 weeks plus 10 mg rosuvastatin per day. The standard-of-care group will receive 10 mg rosuvastatin per day with dose adjustment to achieve LDL-C <70 mg/dL. Post-treatment NIRS-IVUS will be performed at week 36. The primary endpoint is the change in maximum lipid core burden index in 4-mm pullback compartments (maxLCBI[4 mm]) between baseline and week 36. Secondary endpoints include change in LCBI (lesion), angle of lipid core, plaque burden, and serum lipids and biomarkers related to atherosclerosis and inflammation.
Conclusions: The study will clarify the effects of alirocumab on thin-cap fibroatheroma in patients who underwent recent PCI and who have suboptimal LDL-C control with stable statin therapy.
Key Words: Alirocumab, Atherosclerotic plaque, Lipid core plaque, Near-infrared intravascular ultrasound, Thin-cap fibroatheroma
Low-density lipoprotein cholesterol (LDL-C) is an important therapeutic target for the prevention of cardiovascular disease. Previous randomized trials have shown that lipid-lowering therapy (LLT) can significantly reduce the mortality and incidence of cardiovascular events such as myocardial infarction (MI), ischemic stroke, and coronary revascularization.1–3 Multiple randomized trials have reported that intensive LLT can reduce the atheroma volume of non-culprit intermediate stenotic lesions, resulting in the protective effects that are observed.4,5
In addition to the influence of atheroma volume, modification of high-risk rupture-prone plaque features, such as lipid-rich fibroatheroma, can play an important role in the occurrence of cardiovascular events. Several clinical trials have shown that intensive LLT can stabilize vulnerable plaques by reducing the lipid content of the target plaque. This has been evaluated on angioscopy,6 radiofrequency or backscatter intravascular ultrasound (IVUS),7 and optical coherence tomography (OCT).8 Near-infrared spectroscopy plus IVUS (NIRS-IVUS) is unique, because it specifically focuses on quantification of the lipid content of atherosclerotic plaques in vivo. According to a previous study, NIRS-IVUS identified lipid-rich plaques of pathological specimens with a sensitivity of 90%, and a specificity of 93%.9 Therefore, NIRS-IVUS could be a powerful tool to quantify serial changes of the lipid content of atherosclerotic plaques.
Recently, monoclonal antibodies that inhibit proprotein convertase subtilisin/kexin type 9 (PCSK9) have emerged as a novel treatment option for cardiovascular disease.10,11 Randomized multicenter trials have shown that PCSK9 inhibition causes significant reduction in the incidence of adverse clinical events in patients with acute coronary syndrome (ACS).12 Augmenting statin therapy with aggressive LLT significantly reduces the incidence of acute MI and unstable angina, both of which are primarily triggered by the rupture of lipid-rich fibroatheromas.11,12 Therefore, we investigated the effect of treatment with alirocumab and rosuvastatin vs. rosuvastatin alone on lipid-core plaques (LCP) in coronary artery disease (CAD) using NIRS-IVUS (ANTARES).
Methods and Results
Study Design and Objectives
The present study is a phase IV, open-label, randomized, blinded NIRS-IVUS analysis, parallel-group, single-center study. Participants will include Japanese patients recently hospitalized for percutaneous coronary intervention (PCI) with elevated LDL-C undergoing stable statin therapy. Approximately 30 patients with ACS or stable CAD will be enrolled to evaluate the effect of alirocumab added to rosuvastatin on the lipid burden in fibroatheroma, as assessed on NIRS-IVUS evaluation of maximum lipid core burden index in 4-mm pullback compartments (maxLCBI[4 mm]). The protocol has been approved by the institutional review board of Kobe University Hospital (reference number: 290068). All patients provided written informed consent. This study has been registered at clinicaltrials.gov (NCT03529253).
Subjects
Inclusion and exclusion criteria are detailed in Table. Eligibility criteria are as follows: patients years who have undergone PCI for any ACS or stable angina pectoris; who have LDL-C >70 mg/dL with or without statin treatment; who have had fibroatheroma detected via calculation of maxLCBI(4 mm) >100 on NIRS-IVUS in non-culprit, angiographically intermediate lesions with 25–75% diameter stenosis; and who have agreed to be enrolled in the trial by written informed consent. Patients who have not previously taken a statin and whose LDL-C is >70 mg/dL at the time of PCI will begin rosuvastatin at 10 mg/day immediately after diagnosis. All patients are eligible for the study if LDL-C remains >70 mg/dL 2–4 weeks after initiation of rosuvastatin therapy, and the physician deems it appropriate (Figure 1).
Table.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| 1. Age ≥20 years old at PCI |
| 2. Undergoing PCI for ACS or stable CAD |
| 3. LDL-C ≥70 mg/dL on daily 10-mg rosuvastatin |
| 4. Fibroatheroma detected on NIRS-IVUS (maxLCBI(4 mm) >100) in non-culprit |
| 5. Angiographically intermediate lesions with diameter stenosis 25–75% |
| 6. Agreement to be enrolled in the trial and signed written informed consent |
| Exclusion criteria |
| 1. Previous treatment with at least 1 dose of any anti-PCSK9 monoclonal antibody |
| 2. Uncontrolled hypertension (SBP >180 mmHg or DBP >110 mmHg) between the time of PCI and randomization visit |
| 3. Known hypersensitivity to alirocumab or rosuvastatin |
| 4. All contraindications to alirocumab and/or rosuvastatin as displayed in the respective national product labeling for these treatments |
| 5. Known history of hemorrhagic stroke |
| 6. Current treatment for cancer |
| 7. Current lipoprotein apheresis |
| 8. Severe liver or renal dysfunction |
| 9. Pregnancy or breast-feeding |
| 10. Recognition as inadequate by the attending physician |
ACS, acute coronary syndrome; CAD, coronary artery disease; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; maxLCBI, maximum lipid core burden index; NIRS-IVUS, near-infrared spectroscopy-intravascular ultrasound; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9; SBP, systolic blood pressure.
Figure 1.
Flow chart of run-in period. CAG, coronary angiography; LDL-C, low-density lipoprotein cholesterol; max LCBI(4 mm), maximum lipid core burden index in the 4-mm section; NIRS-IVUS, near-infrared spectroscopy-intravascular ultrasound.
Study Design and Randomization
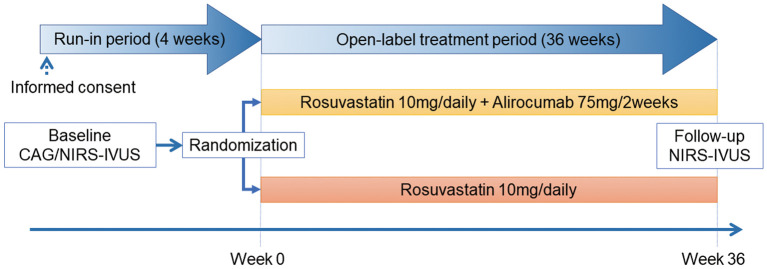
The 36-week, open-label treatment period (including post-treatment NIRS-IVUS) will begin ≤4 weeks after PCI (Figure 2); during which, patients will be randomized to alirocumab or standard-of-care groups (1:1) by permuted-block design. Randomization will be performed by a computer-based allocation system, stratified by gender and baseline serum LDL-C. Participants and investigators were not masked to the allocation, whereas NIRS-IVUS analysis will be blinded.
Figure 2.
Flow chart of the study timeline. CAG, coronary angiography; NIRS-IVUS, near-infrared spectroscopy–intravascular ultrasound.
Patients in the alirocumab group will receive 75 mg alirocumab every 2 weeks (Q2 W) in addition to statin therapy (rosuvastatin 10 mg/day). Patients in the standard-of-care group will receive rosuvastatin 10 mg/day, with initiation and/or dose adjustment of non-statin LLT to achieve LDL-C <70 mg/dL, based on the 2016 ESC/EAS guidelines for the management of dyslipidemia.13 Any non-statin LLT will be continued at the same dosage determined after PCI. Post-treatment NIRS-IVUS of the same vessels imaged at PCI will be conducted at the end of the treatment period (week 36±2 weeks). Image analysis will be performed by an independent imaging core lab (Kobe University Core Analysis Laboratory: KCAL) who are blinded to the treatment group.
NIRS Data Acquisition and Analysis
Combined NIRS and greyscale IVUS will be performed using the TVC Imaging SystemTM with a 2.4-Fr InsightTM Catheter (Infraredx, Burlington, MA, USA). The region of interest (ROI) is defined as the longest coronary segment between 2 clearly identifiable landmarks (side branches). Calculation of LCBI will be performed automatically, and the maximum LCBI will be estimated in 4-mm pullback compartments for every analyzed lesion to calculate maxLCBI(4 mm). Quantitative greyscale IVUS measurements will be performed using echoPlaque (Indec Systems, Santa Clara, CA, USA, Ver 4.0.27) as previously described.14
Blood Sample Analysis
Serum total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), triglyceride, non-HDL-C, and high-sensitivity C-reactive protein will be measured at weeks 0, 4, 12, 24, and 36, as reported previously.15 Serum apolipoprotein A-1, apolipoprotein B, lipoprotein (a), remnant-like particle cholesterol, and free PCSK9 will be measured at weeks 0 and 36 to investigate the potential relationship between lipid profile modification and plaque stabilization. Serum interleukin-1β, interleukin-6, tumor necrosis factor-α, monocyte chemoattractant protein-1, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, matrix metalloproteinase-2, and matrix metalloproteinase-9 will be measured at weeks 0 and 36.
Endpoints
The primary endpoint will be the absolute change of maxLCBI(4 mm) between baseline and at week 36. Secondary endpoints will be the change in maximum and average angles of the lipid core in the maxLCBI(4 mm) segment, average angle of the lipid core in the maxLCBI(4 mm) segment, LCBI in the ROI, total atheroma volume, percent atheroma volume, and the minimum lumen area between baseline and week 36. Additionally, cardiac death, MI,16 ischemia-driven target lesion revascularization (TLR), target vessel revascularization, major adverse coronary events (defined as the composite of death, MI, and TLR), and adverse drug reactions will be assessed during follow-up.
Sample Size Calculation
The study is expected to enroll approximately 30 patients. Sample size calculations are based on the primary efficacy variable of the change in maxLCBI(4 mm) between baseline and week 36. According to a previous study,17 the difference in the change of maxLCBI(4 mm) from baseline between the 2 groups (rosuvastatin/alirocumab combination therapy or rosuvastatin monotherapy) is assumed to be −151, with an SD of 126. Considering this assumption, a sample size of 22 patients (11 in each group) would be required to detect significant differences between treatments with a power of 80%, assuming a 2-sided significance level of 5%. Estimating a dropout rate of 30%, 30 patients (15 in each group) will be needed.
Discussion
The development of intravascular imaging has clarified the time course of atherosclerotic progression, enabling the efficacy of drug intervention aimed at slowing this progression in human coronary arteries to be evaluated. Of these imaging techniques, IVUS has played a central role in evaluating quantitative changes of coronary plaques. The accuracy of gray-scale IVUS in plaque characterization, however, is limited, especially for the detection lipid content, which is one of the most important characteristics of rupture-prone vulnerable plaques.
Recently, NIRS-IVUS has become clinically available for daily practice. Two pivotal studies have validated the accuracy of the NIRS catheter for detection of lipid-rich coronary plaques.18,19 The primary endpoint of the latter study was that spectral data of coronary arteries could be agreed with those gathered from autopsy specimens, to verify the feasibility of invasive detection of coronary lipidic plaques using the NIRS system.
Pharmacological local effects of specific agents used for plaque regression and/or stabilization can be tracked using NIRS. The prospective randomized YELLOW trial involves patients with multi-vessel CAD who are undergoing PCI.17 After baseline assessment with NIRS and IVUS, patients were randomized to a treatment of either rosuvastatin (40 mg/day) or LLT. After 6–8 weeks of short-term intensive statin therapy, NIRS IVUS showed a significant reduction in the plaque lipid content with maxLCBI(4 mm). Although a multinational trial demonstrated that evolocumab caused significant reduction in plaque and LDL-C,20 the study was limited to quantitative evaluation, meaning that potential qualitative plaque modifications afforded by the PCSK9 inhibitor remain unclear. The present ANTARES study will provide insights into the ability of alirocumab to slow or reduce the progression of coronary atheroma volume in Japanese patients who have recently experienced ACS and who, despite statin therapy, fail to achieve LDL-C target levels defined by the JAS guidelines (<70 mg/dL).21
Limitations of the ANTARES study include the relatively small number of patients and short duration for follow-up NIRS-IVUS. It is well known, however, that qualitative plaque changes appear earlier than quantitative changes. Thus, if the addition of alirocumab 75 mg Q2 W has strong anti-atherosclerotic effects, we can consider that evaluation of these effects will be possible in terms of the primary or secondary endpoints.
Disclosures
The authors declare no conflicts of interest. No grants were received from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgments
Not applicable.
References
- 1. Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL, et al.. Value of primordial and primary prevention for cardiovascular disease: A policy statement from the American Heart Association. Circulation 2011; 124: 967–990. [DOI] [PubMed] [Google Scholar]
- 2. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al.. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58: 2432–2446. [DOI] [PubMed] [Google Scholar]
- 3. Heart Protection Study Collaborative Group.. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high-risk individuals: A randomised controlled trial. Lancet 2011; 378: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, et al.. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010; 55: 2399–2407. [DOI] [PubMed] [Google Scholar]
- 5. Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al.. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 6. Ueda Y, Matsuo K, Nishimoto Y, Sugihara R, Hirata A, Nemoto T, et al.. In-stent yellow plaque at 1 year after implantation is associated with future event of very late stent failure: The DESNOTE Study (detect the event of very late stent failure from the drug-eluting stent not well covered by neointima determined by angioscopy). JACC Cardiovasc Interv 2015; 8: 814–821. [DOI] [PubMed] [Google Scholar]
- 7. Hattori K, Ozaki Y, Ismail TF, Okumura M, Naruse H, Kan S, et al.. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging 2012; 5: 169–177. [DOI] [PubMed] [Google Scholar]
- 8. Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, et al.. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: The EASY-FIT study. J Am Coll Cardiol 2014; 64: 2207–2217. [DOI] [PubMed] [Google Scholar]
- 9. Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O’Connor WN, Muller JE.. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 2002; 105: 923–927. [DOI] [PubMed] [Google Scholar]
- 10. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al.. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al.. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097–2107. [DOI] [PubMed] [Google Scholar]
- 13. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al.. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 14. Otake H, Kubo T, Takahashi H, Shinke T, Okamura T, Hibi K, et al.. Optical Frequency Domain Imaging Versus Intravascular Ultrasound in Percutaneous Coronary Intervention (OPINION Trial): Results from the OPINION imaging study. JACC Cardiovasc Imaging 2018; 11: 111–123. [DOI] [PubMed] [Google Scholar]
- 15. Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al.. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 2009; 54: 293–302. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al.. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60: 1581–1598. [DOI] [PubMed] [Google Scholar]
- 17. Kini AS, Baber U, Kovacic JC, Limaye A, Ali ZA, Sweeny J, et al.. Changes in plaque lipid content after short-term intensive versus standard statin therapy: The YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol 2013; 62: 21–29. [DOI] [PubMed] [Google Scholar]
- 18. Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, et al.. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging 2008; 1: 638–648. [DOI] [PubMed] [Google Scholar]
- 19. Waxman S, Dixon SR, L’Allier P, Moses JW, Petersen JL, Cutlip D, et al.. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: Initial results of the SPECTACL study. JACC Cardiovasc Imaging 2009; 2: 858–868. [DOI] [PubMed] [Google Scholar]
- 20. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al.. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316: 2373–2384. [DOI] [PubMed] [Google Scholar]
- 21. Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al.. Executive summary of the Japan Atherosclerosis Society guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan–2012 version. J Atheroscler Thromb 2013; 20: 517–523. [DOI] [PubMed] [Google Scholar]