Abstract
Numerous randomized double-blind clinical trials have consistently shown that that a single intravenous administration of a subanesthetic dose of ketamine to treatment-resistant depressed patients significantly improved depressive symptomatology rapidly, within two hours, with the effect lasting up to seven days. Despite its very promising effects, ketamine has long been associated with potential for abuse as it can cause psychotropic side effects, such as hallucinations, false beliefs, and severe impairments in judgment and other cognitive processes. Consequently, within the last two decades preclinical research has been carried out aimed at understanding its mechanisms of action and the brain circuits involved in ketamine’s antidepressant effects, both of which are discussed in this review. Furthermore, with the hippocampus being a key target for ketamine’s beneficial antidepressant effects, we and others have begun to examine behavioral and neurochemical effects of drugs that act selectively on the hippocampus due to the preferential location of their receptor targets. Such drugs are negative allosteric modulators (NAMs) and positive allosteric modulator (PAM) of the α5-GABAA receptor. Such compounds are discussed within the framework of how lessons learned with ketamine point to novel classes of drugs, targeting the GABAergic system, that can recapitulate the antidepressant effects of ketamine without its adverse effects.
Keywords: ketamine, depression, ventral hippocampus, α5-containing GABAA receptors, negative allosteric modulators
Introduction
Major depressive disorder (MDD) worldwide is a serious, highly prevalent and disabling mental illness associated with high suicide rates as well as high financial costs [1–3]. Biogenic amine-based antidepressants (AD) have been widely used to treat MDD for over half a century with moderate effectiveness [4]. The major drawbacks associated with such drugs are that (1) optimal, not necessarily initial, beneficial effects can be delayed for weeks if not months [5, 6]; (2) response and remission rates are low, 50% and 30% respectively [4], such that the effect sizes for these drugs are in the modest range of 0.4–0.5 [7]. On average, around 20–30 percent of depressed patients are diagnosed as suffering from treatment resistant depression (TRD) [8]. The definition of TRD lacks a consensus, but generally is that these patients have failed to respond to a minimum of two or three treatment modalities, that may include different class of antidepressants of adequate dose and duration minus or plus electroconvulsive therapy (ECT) [9, 10].
Transcranial magnetic stimulation and vagal nerve stimulation are FDA-approved means of stimulating the brain and are added to treatment as usual as additional options for TRD patients. However, although their clinical efficacy is greater than treatment as usual, such options are not without limitations, given that significant improvement in depressive symptomatology can take many weeks or months to be achieved [11–13]. Therefore, the field has long recognized the need for novel treatment options for MDD and TRD with rapid onset and robust clinical efficacy.
This review will cover the use of subanesthetic doses of ketamine as a rapid and sustained antidepressant for MDD as well as for TRD patients. The mechanisms by which ketamine promotes its beneficial effects will be discussed as well as factors that can limit the clinical utility of ketamine. Finally, a discussion will be presented of how lessons learned with ketamine point to a novel class of drug, targeting the GABAergic system, that can recapitulate the antidepressant effects of ketamine without its adverse effects.
Clinical trials with subanesthetic ketamine in depressed patients
The initial literature regarding the involvement of the glutamate NMDA receptor in antidepressant effects was a consequence of serendipity, as the NMDA receptor modulator D-cyclosporine given to treat tuberculosis was also reported to have antidepressant properties in the 1950’s and 1960’s. In preclinical studies, many years later NMDA receptor antagonists were reported to have antidepressant-like activity [14–16].
It wasn’t until the year 2000, that a clinical trial with a subanesthetic dose of (R, S)-ketamine, an NMDA receptor antagonist, in MDD patients was launched by investigators at Yale University [17]. Since then, a series of randomized double-blind clinical trials have consistently shown that a single intravenous administration of a subanesthetic dose of ketamine to TRD patients significantly improved depressive symptomatology within two hours with the effect lasting up to seven days [18–20]. Further, clinical trials with ketamine have consistently shown its beneficial effects on improving both anhedonia, a core symptom of depression, [21] and suicidal ideation [22–24].
Antidepressant-like effects of ketamine in rodents
The administration of sub-anesthetic doses of ketamine produces antidepressant-like behavioral responses in rodents, such as a decreased immobility in the forced swim test (FST), and this can persist for up to 1 week [25–27]. The sustained effect is of particular interest given the relatively short half-life of the drug in rodents [28]. Further, the sustained antidepressant-like response to a single dose of ketamine does not occur with standard antidepressants, whose effects are noted only while the drug is present. Preclinical studies have also suggested that low-dose ketamine administration acutely induces AMPA-mediated currents [29] leading to activity-dependent rapid release of brain-derived neurotrophic factor (BDNF) and activation of its cognate receptor, TrkB, leading to neuroplastic changes thought to underlie its antidepressant efficacy [25, 26, 30]. Later, we will discuss further the current ideas about the mechanisms of action of ketamine.
Chronic stress has long been associated with behavioral deficits in tasks relevant for the study of depression, as well as with a loss of dendritic spines in brain areas associated with mood regulation [31–33]. In a recent study, a low dose of ketamine reversed the stress-induced spine elimination, restoring coordinated activity within medical prefrontal cortex (mPFC) microcircuits, which in turn was correlated with a positive response in the FST [34].
Sex differences in response to ketamine
In animal models relevant for the study of depression, sex differences in response to ketamine have been reported [35]. Normal cycling female rats appear to be more sensitive to lower doses of ketamine in the forced swim test than male rats and this increased sensitivity is mediated by estrogen and progesterone [36]. However, in male mice, the reported antidepressant-like effects of ketamine are more sustained than that in female mice, namely, in female mice the AD like effect occurs at 24 h but is lost by 7 days [37]. In another study evaluating the effects of repeated ketamine administration, male mice exhibited an antidepressant-like effect whereas female mice exhibited anxiety-like and depression-like behaviors [38].
Taken together, data from preclinical studies support the assessment of possible sex differences in response to ketamine in humans and potential roles of estrogen and progesterone in those differences. However, clinical trials in humans fail to support sex differences in response to ketamine in TRD, namely, both sexes showed a comparable response as well as tolerability [39]. Moreover, there are no significant differences observed in response to ketamine between TRD pre- and post-menopause women [39]. It is worth noting that this clinical trial was not powered to determine the effects of menstrual cycle at time of ketamine injection and did not account for external hormonal treatments. It is possible that further clinical trials with larger sample sizes might still reveal sex differences in response to some aspects of the response to ketamine.
Potential adverse effects of ketamine
Despite its very promising effects, ketamine has long been associated with potential for abuse as it often causes psychotropic side effects, such as hallucinations, false beliefs, and severe impairments in judgment and other cognitive processes [40, 41]. Under its nickname “Special K,” ketamine is often sold as a street drug. Long-term effects associated with repeated use of ketamine are also a concern. Preclinical studies have shown that repeated administration of ketamine promotes neuronal adaptations that are associated with development of addiction [42, 43].
Earlier in 2019, the Food and Drug Administration approved the use of intranasal S-ketamine, namely, esketamine spray (Spravato®) for TRD. This approval was granted as in clinical trials Spravato® was shown to be efficacious in significantly improving depressive symptomatology including a positive outcome with respect to suicidal ideation [44, 45]. Although this approval of esketamine represents the genesis of a glutamatergic-targeting approach for TRD, some concerns remain. There have been reports raising concerns about rapid relapse after discontinuation of esketamine (40% after about 3–4 months) and potential risk for suicide [46, 47].
Pioneering studies led by Hashimoto and colleagues revealed that a single injection of R-ketamine (arketamine), but not esketamine, to mice pretreated with dexamethasone, promoted sustained antidepressant-like effects, measured by a significant reduction in time spent immobile in the FST and tail suspension test (TST) [48]. The same group have also shown (1) a greater magnitude of response for arketamine in comparison to esketamine in normalizing social defeat stress-induced decreases in sucrose consumption, and (2) that arketamine, but not esketamine, reversed inescapable shock-induced deficits in the learned helplessness paradigm [49]. In the same study, it was shown that esketamine disrupted prepulse inhibition of the acoustic startle response, whereas arketamine did not, which would imply that arketamine is not associated with psychotomimetic-like effects [49]. Similarly, esketamine administration, in a dose dependent manner, has been shown to induce conditioned place preference in mice, whereas arketamine administration failed to do so [49], which could imply that arketamine has less abuse-like properties. More recent studies have replicated and expanded these studies and demonstrated antidepressant-like effects of arketamine [50, 51]. Data from clinical trials with arketamine in depressed patients have not been published to date.
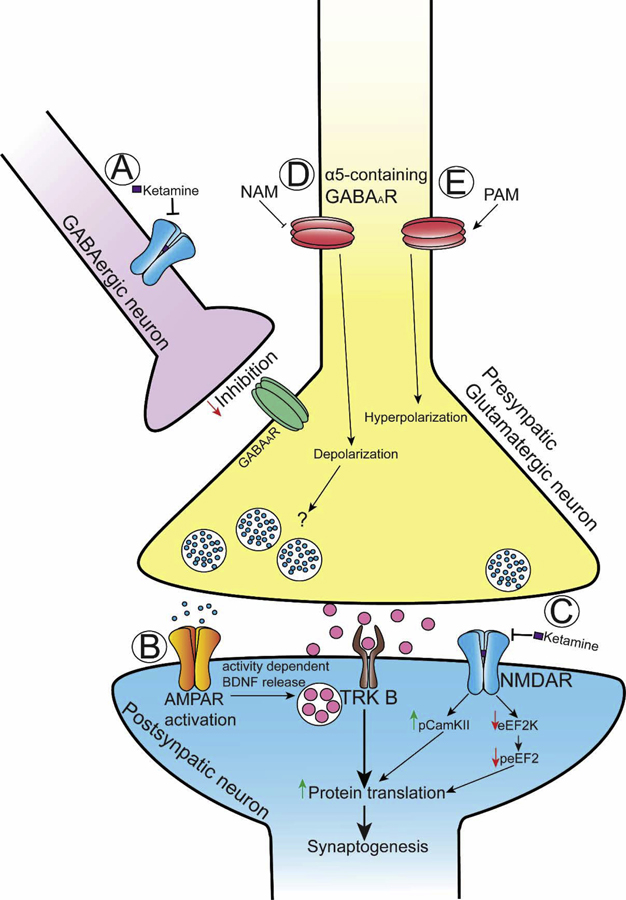
Further understanding of the mechanisms by which ketamine produces its beneficial versus adverse effects should be useful in developing drugs without its adverse effect profile. As such, a diagram illustrating many, but not all, of the mechanisms by which ketamine promotes its effects reviewed within the next sections are shown in Figure 1.
Figure 1:
(A) Ketamine blockade of NMDA receptors onto GABAergic interneurons decreases GABAergic tone onto glutamatergic neurons, thereby causing glutamatergic neuronal activation (“disinhibition hypothesis”) and glutamate release. (B) Glutamate -induced AMPA receptor activation leads to activity-dependent Brain Derived Neurotrophic Factor (BDNF) release, with consequently activation of the BDNF receptor, TrKB, and a downstream signaling activation that is linked to synaptogenesis. (C) ketamine blockade of NMDA receptors that are normally activated by spontaneous, rather than evoked glutamate release, causes phosphorylation of CamKII (pCamKII) and inhibition of the elongation factor 2 kinase (eEF2K). The inhibition of eEF2K decreases the levels of the phosphorylated form of the elongation factor 2 (peEF2). Both decreases in peEF2 and increases in pCamKII are associated with increased protein translation. (D) Negative allosteric modulators of α5-containing GABAA receptors (NAM) causes depolarization and activity-dependent glutamate release. (E) Positive allosteric modulator of the α5-containing GABAA receptors (PAM) causes hyperporalization.
Acute effects of ketamine – initial targets
1). Disinhibition hypothesis
As mentioned previously, ketamine is an antagonist of the NMDA receptor of the excitatory transmitter glutamate. This would imply that the actions of ketamine would result in a reduction of excitatory transmission. However, in various brain imaging studies in humans, NMDA antagonism was consistently associated with a rather robust increase in cortical activity [52–55]. Microdialysis measurement of extracellular glutamate levels in the PFC of rats administered different sub-anesthetic doses of ketamine revealed a transient (up to 100 min) enhancement of glutamate release [56]. Electrophysiological recordings in behaving rodents receiving systemic dosing with an NMDA receptor antagonist showed enhancement of cortical activity [57]. A follow-up mechanistic study demonstrated that NMDA antagonism by MK-801 preferentially decreased interneuron function and augmented pyramidal cell firing [58]. This paradox associated with elevation of extracellular glutamate following NMDA receptor antagonism in vivo led to the so-called “disinhibition hypothesis” for the acute action of ketamine, whereby low doses of ketamine selectively block NMDA receptors on GABAergic interneurons that inhibit glutamate transmission [31, 59]. This selectivity occurs because GABAergic interneurons are fast firing, which results in removal of the Mg2+ block from the NMDA receptor channel, allowing ketamine to enter, bind, and block the channel. A further corroboration of the disinhibition hypothesis of ketamine acute actions is from a study by Widman and McMahon [60] whereby they demonstrated using electrophysiology that subanesthetic doses of ketamine decreased inhibition onto pyramidal neurons leading to an increase in the excitatory drive of hippocampal pyramidal cells. Consistent with the idea that ketamine is resulting in an enhancement of glutamatergic transmission are the preclinical observations that AMPA receptor antagonists inhibit its AD-like effect [29, 61].
2). NMDAR-independent mechanism
A preclinical study by Zanos and colleagues [61] demonstrated that the metabolism of (R, S)-ketamine to (2S,6S;2R,6R)-hydroxynorketamine (HNK) is crucial to ketamine’s antidepressant effects, and that the (2R,6R)-HNK enantiomer exerts rapid antidepressant effects without ketamine’s adverse effects and abuse potential in rodents. Pre-treatment with the AMPA receptor inhibitor NBQX abolished both ketamine’s and HNK’s antidepressant-like effects in animal models [61], thus further corroborating that AMPA receptor (AMPAR)-mediated maintenance of synaptic potentiation is crucial to ketamine’s antidepressant mechanism as previously demonstrated by Maeng and colleagues [29]. It is worth noting that a study by Yamaguchi and colleagues showed that mice pretreated with cytochrome P450 inhibitors to prevent the generation of (2R,6R)-HNK, did not prevent the antidepressant-like effects of arketamine, suggesting that metabolism of arketamine to (2R,6R)-HNK might not be necessary for the antidepressant-like effects of arketamine [62].
3). Mu-opioid-related effects
A study showed that acute antidepressant effects of ketamine are blocked when patients are pretreated with oral naltrexone [63], indicating that ketamine’s antidepressant effects are mediated, at least in part, by mu opioid agonism. The study did not address whether the effect was due to release of endogenous opioids or via direct binding of ketamine to the mu opioid receptor (MOR), although its affinity for the MOR [64] is on average 50–60 times less than its affinity for the NMDA receptor [65, 66], varying upon species, and other factors such as the type of assay utilized to determine the affinity, and the tissue [66].
By contrast, in depressed patients with comorbid alcohol use disorder, pretreatment with naltrexone failed to block the antidepressant effects of ketamine [67]. These contradictory effects of naltrexone upon ketamine’s antidepressant effects should be interpreted carefully and further randomized larger clinical studies should be launched in order to elucidate the involvement of the opioid system in ketamine’s antidepressant effect.
Because of these clinical findings, preclinical research has been initiated to test whether mu opioid activation is associated with the antidepressant-like effects of ketamine. A preclinical study in mice by Zhang and Hashimoto revealed that pretreatment with naltrexone did not prevent the antidepressant-like effects of ketamine in two different stress-induced behavioral deficits relevant for the study of depression, namely, the chronic social defeat stress and the lipopolysaccharide-induced inflammation model [68]. Very recently though, Klein and colleagues showed, in rats, that systemic blockade of opioid receptors by naltrexone prevented the antidepressant-like effects of ketamine in the congenitally learned helpless (cLH) rat model [69]. In addition to the behavioral deficits measured, cLH rats showed hyperactive firing of the lateral habenula (LHb), which was then normalized back to control wild type values by ketamine [69], in agreement with the original findings by Yang and colleagues [70]. Pretreatment with naltrexone or the specific MOR antagonist CTAP prevented the effects of ketamine on normalizing LHb hyperactivity [69]. Most interestingly, activation of the opioid system by morphine did not replicate the behavioral antidepressant-like effects of ketamine on cLH rats [69]. Again, like the behavioral data, activation of the opioid system by morphine or the MOR agonist DAMGO was not sufficient to replicate ketamine’s effects upon normalization of LHb hyperactivity in cLH rats. Therefore, it seems that opioid system may be necessary, but not sufficient for the antidepressant-like effects of ketamine. Further research continues to be necessary to assess the involvement of MOR in ketamine’s antidepressant effects.
Molecular targets of ketamine leading to sustained synaptic effects
Numerous studies have been carried out in vivo to examine the sustained plastic effects of ketamine in brain areas relevant to mood regulation. Two regions consistently implicated in depression and antidepressant efficacy are the prefrontal cortex (PFC) and the hippocampus, cortical regions intimately associated with the regulation of mood and higher order cognitive processing [71–75]. Ketamine induces plasticity in the hippocampus and mPFC. For example, ketamine increased GluA1 in the mPFC 24h after a single administration with this increase required for its sustained behavioral effects [27]. Initial events that trigger such plasticity had previously been reported to be related to activation of the BDNF receptor, TrkB as well as deactivation of eEF2, which in turn leads to increases in global protein synthesis [25].
Adaikkan and colleagues [76], using an eEF2 kinase knockout mouse, further elucidate the importance of eEF2 in the effects of ketamine originally reported by Autry and colleagues [25] and show also that ketamine induces phosphorylation of CamKII at distinct residues. Ketamine induced a rather transient phosphorylation of CamKII at T305 which causes its inactivation. This was followed by CamK phosphorylation at T286 in both mPFC and hippocampus. The ketamine-induced CamKII activation returned to basal levels at 180 min. Using a CamK II specific inhibitor (tatCN21) the authors showed that blockade of ketamine-induced CamKII phosphorylation blunted the increase in the AMPA subunit, GluA1, seen 24h following a single ketamine injection, and it also prevented ketamine-induced behavioral effects in the FST. Moreover, inhibition of CamKII phosphorylation prevented the anxiolytic-like effects of ketamine in the Novelty Suppressed Feeding Test (NSFT). The results of this study provide a new molecular target, namely CamKII, for novel antidepressants.
In the PFC, ketamine induces activation of the mTOR pathway, which is also necessary for ketamine’s antidepressant-like effects. This pathway eventually increases the expression of synaptic proteins that strengthen synapses in a region-specific way, reducing symptoms of depression [27].
Synaptic effects of ketamine in PFC and hippocampus seems to require activation of different molecular cascades (mTor and eEFK, respectively) to promote the increase of synaptic proteins and plasticity that underly its behavioral effects [27, 77, 78]. In addition to sex differences with regard to behavioral effects of ketamine in rodents, there are also reports regarding sex differences in response to ketamine-induced increases in synaptic proteins and spine density [38, 79]. Moreover, the increased sensitivity of female rats to a low dose of ketamine is mechanistically independent of phosphorylation of mTOR or eEF2 [36].
Neurobiological effects of ketamine (brain circuits targeted by ketamine)
Although preclinical studies have consistently provided a basis for the molecular mechanisms of action of ketamine as discussed above, less has been done with respect to the brain circuits in which such effects occur. Ventral regions of the hippocampus are connected to the limbic system with afferents to key regions including the prefrontal cortex [80, 81], nucleus accumbens [82–84], hypothalamus [85] and indirectly to the midbrain dopamine system [86]. Furthermore, the hippocampus has been extensively implicated in the actions of stress, depression, and antidepressant actions and represents a site of convergence whereby the effects of stress and antidepressant drugs may act to regulate HPA axis function and mood via connections with the hypothalamus and limbic forebrain, respectively [87].
We reported that a circuit from the vHipp to the mPFC is both necessary and sufficient for the sustained AD-like effects of ketamine in rats, with the vHipp being an initial target for its effects [26]. Also supporting the disinhibition hypothesis of ketamine’s mechanism of action is a study by Donegan and Lodge [88] showing that chemical ablation of the perineuronal net surrounding hippocampal fast-firing parvalbumin (PV)-containing interneurons blunted the sustained antidepressant-like response to ketamine in the FST.
The ventral hippocampus sends unidirectional glutamatergic inputs to the mPFC [89]. Jett and colleagues [90] demonstrated that brief high frequency stimulation of the ventral hippocampus-induced plasticity in the mPFC and that such plasticity facilitated behavior dependent on mPFC function, i.e, cognitive flexibility. Consequently, we hypothesized that acute pharmacological augmentation of ventral hippocampus activity due to ketamine will enhance glutamatergic transmission to the mPFC and that this will induce plasticity in the mPFC that accounts for its antidepressant-like effects. We provided evidence in favor of this hypothesis [26]. Ketamine also induces plasticity in a pathway from the ventral hippocampus to the nucleus accumbens shell and such ketamine-induced enduring changes in this circuit are thought to mediate resilience to stress in the learned helplessness paradigm [91].
Lessons learned with ketamine and novel targets to treat depression by means of targeting the hippocampus
By understanding specific brain circuits responsible for discrete dimensions of an antidepressant response, such circuits can be targeted to develop novel antidepressant medications while hopefully minimizing off target effects [92, 93]. Given ideas about the mechanisms associated with ketamine’s antidepressant-like effects and the notion that altered excitatory/inhibitory balance is associated with mood disorders, the field has focused on glutamatergic [94, 95] and/ or GABAergic systems as novel targets for treatment of MDD and TRD [92]. Based on our study [26], we hypothesized that if we could find a drug that did what ketamine did in the vHipp, namely remove GABAergic inhibition of glutamatergic pyramidal neurons, but not have direct effects outside the hippocampus, this might produce an antidepressant without the adverse effects of ketamine.
Although this idea seems feasible and supported by preclinical data (as detailed in the subsequent paragraphs below), it is worth noting that there have also been clinical trials suggesting that the dissociative effects of ketamine are mechanistically linked to its antidepressant effects as ketamine-induced dissociative symptoms can predict its antidepressant response [96, 97]. Therefore, accordingly to this theory, one could argue that eliminating ketamine’s side effects would also eliminate its beneficial antidepressant effects. Further studies on this topic are clearly needed, but it is also worth mentioning that the association between ketamine’s dissociative effects and subsequent antidepressant response may also reflect nothing more than the fact that those who have an initial dissociative response have received sufficient drug to produce both effects. By contrast, those not having a dissociative response may, for individual reasons, not have been administered a sufficient does to produce either response.
In the hippocampus, about 25% of the GABAA receptors contain the α5-subunits [98–100]. The hippocampal α5-containg GABAA receptors have been reported as mainly extrasynaptic [101–104], where it mediates tonic inhibition [98, 105–107], and are located abundantly in the dendritic fields of hippocampal CA1 and CA3 pyramidal cells in rats [105, 108], as well as in humans [109]. Furthermore, α5-containg GABAA receptors have also been found in inhibitory synapses modulating phasic inhibition [110–114]. Given the relatively specific localization of the α5 subunit of the GABAA receptor to the hippocampus and their inhibitory control over hippocampal output, it is an ideal candidate as a target for drugs that could act selectively on the hippocampus and recapitulate ketamine’s effect there. L-655,708 is a selective negative allosteric modulator (NAM) of the benzodiazepine (BZ) site of the α5-GABAA receptor (Fig 1D); its selectivity is based on preferential affinity for the α5 subunit in comparison with its affinity for other α subunits [115]. As shown by us [116] and by Thompson and colleagues [117], systemic administration of L-655,708 produces sustained AD-like effects in rodents and this effect is dependent on activation of the ventral hippocampus [116]. Both L-655,708 and MRK-16, another α5-GABA-NAM promote sustained antidepressant-like effects in the absence of abuse-like properties [116, 118]. Moreover, studies by Thompson and colleagues have shown that dysregulation of excitatory synapses in the hippocampus and nucleus accumbens in response to chronic stress can contribute to anhedonia-like behavior and that treatment with an α5-GABAA-NAM rapidly restores synaptic function and behavioral deficits in response to stress [118]. This is in agreement with studies showing that ketamine-induced plasticity in a pathway from the hippocampus to the accumbens mediates resilience to stress [91].
In addition to this growing work with the α5-GABA-NAM discussed above, there has been substantial work showing that positive allosteric modulators of the α5-GABAA receptor (α5-GABA-PAM; Fig 1E) also produce antidepressant-like effects in the FST, as well as anxiolytic-like effects [119, 120]. Moreover, such α5-GABA-PAMs were able to reverse stress-induced deficits in working memory as well as the detrimental effects of age upon working memory [120]. It is worth noting that the beneficial effects of α5-GABA-PAMs require the presence of the drugs, whereas the antidepressant-like effects of α5-GABA-NAMs are sustained long after the drugs have been cleared. Further works needs to be done in order to identify the mechanisms by which both α5-GABA-NAM and α5-GABA-PAM are eliciting the same behavioral result. One possible theory by which both PAMs and NAMs would cause the same end-result, namely, be useful to treat MDD, is that both manipulations are hypothesized to increase the signal-to-noise ratio of hippocampal transmission, albeit via different mechanisms. With regards to the α5-GABA-PAM, chronic administration would produce sustained decreases in tonic hippocampal activity (noise), without dramatically altering phasic activation of pyramidal neurons (the signal), resulting in an increase in signal-to-noise ratio. Conversely, the α5-GABA-NAMs acutely decrease hippocampal GABAergic transmission, which is accompanied by a massive glutamatergic surge that in turn leads to glutamatergic plasticity. This idea is like the disinhibition hypothesis promoted by subanesthetic ketamine administration presented earlier. Such glutamatergic plasticity results in a greater signal and, by extension, an increased signal-to-noise ratio. Thus, it is likely that both PAMs and NAMs increase hippocampal signal-noise; however, this remains to be established. Indeed, direct side-by-side comparisons of the antidepressant-like effects of both α5-GABA-PAMs and α5-GABA-NAMs in the same paradigm is crucial.
By identifying the mechanisms by which systemic administration of compounds targeting novel system and/or circuits it is possible recapitulate the therapeutic effects of ketamine without its psychotomimetic and abuse-related effects with the hopes to provide novel, safe, and effective approaches for treating patients suffering from depression.
Acknowledgment
FRC is supported by the NIH (MH 113899), DJL is supported by the NIH (MH090067) and AF is supported by the VA (01 BX000559). We thank Robert Hammack for assistance in creating the art work for figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors report no conflicts of interest.
References
- [1].Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ, What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies, Journal of affective disorders 116(1–2) (2009) 4–11. [DOI] [PubMed] [Google Scholar]
- [2].Kessler RC, Avenevoli S, McLaughlin KA, Green JG, Lakoma MD, Petukhova M, Pine DS, Sampson NA, Zaslavsky AM, Merikangas KR, Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A), Psychological medicine 42(9) (2012) 1997–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mrazek DA, Hornberger JC, Altar CA, Degtiar I, A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013, Psychiatric services 65(8) (2014) 977–87. [DOI] [PubMed] [Google Scholar]
- [4].Rush AJ, Limitations in efficacy of antidepressant monotherapy, The Journal of clinical psychiatry 68 Suppl 10 (2007) 8–10. [PubMed] [Google Scholar]
- [5].Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A, Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29(3) (2004) 566–79. [DOI] [PubMed] [Google Scholar]
- [6].Stassen HH, Delini-Stula A, Angst J, Time course of improvement under antidepressant treatment: a survival-analytical approach, European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 3(2) (1993) 127–35. [DOI] [PubMed] [Google Scholar]
- [7].Leucht S, Hierl S, Kissling W, Dold M, Davis JM, Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses, The British journal of psychiatry : the journal of mental science 200(2) (2012) 97–106. [DOI] [PubMed] [Google Scholar]
- [8].Demyttenaere K, Van Duppen Z, The Impact of (the Concept of) Treatment-Resistant Depression: An Opinion Review, The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 22(2) (2019) 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M, Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach, Journal of affective disorders 156 (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [10].Sackeim HA, Aaronson ST, Bunker MT, Conway CR, Demitrack MA, George MS, Prudic J, Thase ME, Rush AJ, The assessment of resistance to antidepressant treatment: Rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF), Journal of psychiatric research 113 (2019) 125–136. [DOI] [PubMed] [Google Scholar]
- [11].Bewernick B, Schlaepfer TE, Update on Neuromodulation for Treatment-Resistant Depression, F1000Research 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carreno FR, Frazer A, Vagal Nerve Stimulation for Treatment-Resistant Depression, Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 14(3) (2017) 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A, Vagus nerve stimulation in psychiatry: a systematic review of the available evidence, J Neural Transm (Vienna) (2016). [DOI] [PubMed]
- [14].Papp M, Moryl E, Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression, European journal of pharmacology 263(1–2) (1994) 1–7. [DOI] [PubMed] [Google Scholar]
- [15].Pilc A, Wieronska JM, Skolnick P, Glutamate-based antidepressants: preclinical psychopharmacology, Biological psychiatry 73(12) (2013) 1125–32. [DOI] [PubMed] [Google Scholar]
- [16].Trullas R, Skolnick P, Functional antagonists at the NMDA receptor complex exhibit antidepressant actions, European journal of pharmacology 185(1) (1990) 1–10. [DOI] [PubMed] [Google Scholar]
- [17].Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, Antidepressant effects of ketamine in depressed patients, Biological psychiatry 47(4) (2000) 351–4. [DOI] [PubMed] [Google Scholar]
- [18].Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression, Biological psychiatry 74(4) (2013) 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L, A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression, The American journal of psychiatry 173(8) (2016) 816–26. [DOI] [PubMed] [Google Scholar]
- [20].Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression, Archives of general psychiatry 63(8) (2006) 856–64. [DOI] [PubMed] [Google Scholar]
- [21].Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr., Neural correlates of change in major depressive disorder anhedonia following open-label ketamine, Journal of psychopharmacology 29(5) (2015) 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, Brutsche NE, Ameli R, Furey ML, Zarate CA Jr., Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety, Journal of psychiatric research 58 (2014) 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS, Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial, Psychological medicine 45(16) (2015) 3571–80. [DOI] [PubMed] [Google Scholar]
- [24].Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, Soleimani L, Charney DS, Foulkes AL, Mathew SJ, Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression, Depression and anxiety 31(4) (2014) 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM, NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses, Nature 475(7354) (2011) 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ, Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine, Molecular psychiatry 21(9) (2016) 1298–308. [DOI] [PubMed] [Google Scholar]
- [27].Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS, Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure, Biological psychiatry 69(8) (2011) 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P, Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats, Journal of the American Association for Laboratory Animal Science : JAALAS 52(5) (2013) 567–70. [PMC free article] [PubMed] [Google Scholar]
- [29].Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors, Biological psychiatry 63(4) (2008) 349–52. [DOI] [PubMed] [Google Scholar]
- [30].Duman RS, Li N, Liu RJ, Duric V, Aghajanian G, Signaling pathways underlying the rapid antidepressant actions of ketamine, Neuropharmacology 62(1) (2012) 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Duman RS, Aghajanian GK, Sanacora G, Krystal JH, Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants, Nature medicine 22(3) (2016) 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM, Dendritic Spines in Depression: What We Learned from Animal Models, Neural plasticity 2016 (2016) 8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vose LR, Stanton PK, Synaptic Plasticity, Metaplasticity and Depression, Current neuropharmacology 15(1) (2017) 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C, Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation, Science 364(6436) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wright KN, Kabbaj M, Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability, Curr Opin Behav Sci 23 (2018) 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carrier N, Kabbaj M, Sex differences in the antidepressant-like effects of ketamine, Neuropharmacology 70 (2013) 27–34. [DOI] [PubMed] [Google Scholar]
- [37].Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress, Neuroscience 290 (2015) 49–60. [DOI] [PubMed] [Google Scholar]
- [38].Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM, Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice, Behavioural brain research 312 (2016) 305–12. [DOI] [PubMed] [Google Scholar]
- [39].Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew S, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH, Fava M, Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression, Journal of psychiatric research 110 (2019) 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP, Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations, Anesthesiology 88(1) (1998) 82–8. [DOI] [PubMed] [Google Scholar]
- [41].Short B, Fong J, Galvez V, Shelker W, Loo CK, Side-effects associated with ketamine use in depression: a systematic review, The lancet. Psychiatry 5(1) (2018) 65–78. [DOI] [PubMed] [Google Scholar]
- [42].Bates MLS, Trujillo KA, Long-lasting effects of repeated ketamine administration in adult and adolescent rats, Behavioural brain research 369 (2019) 111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, Bates M, The neurobehavioral pharmacology of ketamine: implications for drug abuse, addiction, and psychiatric disorders, ILAR journal / National Research Council, Institute of Laboratory Animal Resources 52(3) (2011) 366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC, Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial, JAMA psychiatry 75(2) (2018) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB, Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study, The American journal of psychiatry 176(6) (2019) 428–438. [DOI] [PubMed] [Google Scholar]
- [46].Perez-Esparza R, Kobayashi-Romero LF, Garcia-Mendoza AM, Lamas-Aguilar RM, Fonseca-Perezamador A, Promises and concerns regarding the use of ketamine and esketamine in the treatment of depression, Acta psychiatrica Scandinavica 140(2) (2019) 182–183. [DOI] [PubMed] [Google Scholar]
- [47].Schatzberg AF, A Word to the Wise About Intranasal Esketamine, The American journal of psychiatry 176(6) (2019) 422–424. [DOI] [PubMed] [Google Scholar]
- [48].Zhang JC, Li SX, Hashimoto K, R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine, Pharmacology, biochemistry, and behavior 116 (2014) 137–41. [DOI] [PubMed] [Google Scholar]
- [49].Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K, R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects, Translational psychiatry 5 (2015) e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zanos P, Gould TD, Intracellular Signaling Pathways Involved in (S)- and (R)-Ketamine Antidepressant Actions, Biological psychiatry 83(1) (2018) 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang K, Hashimoto K, An update on ketamine and its two enantiomers as rapid-acting antidepressants, Expert review of neurotherapeutics 19(1) (2019) 83–92. [DOI] [PubMed] [Google Scholar]
- [52].Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D, Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers, The American journal of psychiatry 154(6) (1997) 805–11. [DOI] [PubMed] [Google Scholar]
- [53].Lahti AC, Holcomb HH, Medoff DR, Tamminga CA, Ketamine activates psychosis and alters limbic blood flow in schizophrenia, Neuroreport 6(6) (1995) 869–72. [DOI] [PubMed] [Google Scholar]
- [54].Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y, Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats, Neuroscience 114(3) (2002) 769–79. [DOI] [PubMed] [Google Scholar]
- [55].Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J, Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 16(5) (1997) 357–72. [DOI] [PubMed] [Google Scholar]
- [56].Moghaddam B, Adams B, Verma A, Daly D, Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex, The Journal of neuroscience : the official journal of the Society for Neuroscience 17(8) (1997) 2921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jackson ME, Homayoun H, Moghaddam B, NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex, Proceedings of the National Academy of Sciences of the United States of America 101(22) (2004) 8467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Homayoun H, Moghaddam B, NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 27(43) (2007) 11496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Duman RS, Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections, Dialogues in clinical neuroscience 16(1) (2014) 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Widman AJ, McMahon LL, Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy, Proceedings of the National Academy of Sciences of the United States of America 115(13) (2018) E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, NMDAR inhibition-independent antidepressant actions of ketamine metabolites, Nature 533(7604) (2016) 481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, Mizuno-Yasuhira A, Chaki S, (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(9) (2018) 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF, Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism, The American journal of psychiatry 175(12) (2018) 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, Crisp T, Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro, Neuropharmacology 26(9) (1987) 1253–60. [DOI] [PubMed] [Google Scholar]
- [65].Kornhuber J, Mack-Burkhardt F, Kornhuber ME, Riederer P, [3H]MK-801 binding sites in post-mortem human frontal cortex, European journal of pharmacology 162(3) (1989) 483–90. [DOI] [PubMed] [Google Scholar]
- [66].Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms, Pharmacological reviews 70(3) (2018) 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yoon G, Petrakis IL, Krystal JH, Association of Combined Naltrexone and Ketamine With Depressive Symptoms in a Case series of Patients With Depression and Alcohol Use Disorder, JAMA psychiatry 76(3) (2019) 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang K, Hashimoto K, Lack of Opioid System in the Antidepressant Actions of Ketamine, Biological psychiatry 85(6) (2019) e25–e27. [DOI] [PubMed] [Google Scholar]
- [69].Klein ME, Chandra J, Sheriff S, Malinow R, Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents, Proceedings of the National Academy of Sciences of the United States of America (2020). [DOI] [PMC free article] [PubMed]
- [70].Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H, Ketamine blocks bursting in the lateral habenula to rapidly relieve depression, Nature 554(7692) (2018) 317–322. [DOI] [PubMed] [Google Scholar]
- [71].Cook SC, Wellman CL, Chronic stress alters dendritic morphology in rat medial prefrontal cortex, Journal of neurobiology 60(2) (2004) 236–48. [DOI] [PubMed] [Google Scholar]
- [72].Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM, Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression, Journal of psychiatric research 44(13) (2010) 799–807. [DOI] [PubMed] [Google Scholar]
- [73].Garrett JE, Wellman CL, Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence, Neuroscience 162(1) (2009) 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Squire LR, Stark CE, Clark RE, The medial temporal lobe, Annual review of neuroscience 27 (2004) 279–306. [DOI] [PubMed] [Google Scholar]
- [75].Videbech P, Ravnkilde B, Hippocampal volume and depression: a meta-analysis of MRI studies, The American journal of psychiatry 161(11) (2004) 1957–66. [DOI] [PubMed] [Google Scholar]
- [76].Adaikkan C, Taha E, Barrera I, David O, Rosenblum K, Calcium/Calmodulin-Dependent Protein Kinase II and Eukaryotic Elongation Factor 2 Kinase Pathways Mediate the Antidepressant Action of Ketamine, Biological psychiatry 84(1) (2018) 65–75. [DOI] [PubMed] [Google Scholar]
- [77].Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists, Science 329(5994) (2010) 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K, Mechanistic Target of Rapamycin-Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model, Biological psychiatry 83(1) (2018) 18–28. [DOI] [PubMed] [Google Scholar]
- [79].Sarkar A, Kabbaj M, Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats, Biological psychiatry 80(6) (2016) 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ishikawa A, Nakamura S, Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat, Journal of neurophysiology 96(4) (2006) 2134–8. [DOI] [PubMed] [Google Scholar]
- [81].Jay TM, Witter MP, Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin, The Journal of comparative neurology 313(4) (1991) 574–86. [DOI] [PubMed] [Google Scholar]
- [82].French SJ, Totterdell S, Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats, Neuroscience 119(1) (2003) 19–31. [DOI] [PubMed] [Google Scholar]
- [83].Friedman DP, Aggleton JP, Saunders RC, Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain, The Journal of comparative neurology 450(4) (2002) 345–65. [DOI] [PubMed] [Google Scholar]
- [84].Miller EJ, Saint Marie LR, Breier MR, Swerdlow NR, Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat, Neuroscience 165(2) (2010) 601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Meibach RC, Siegel A, Efferent connections of the hippocampal formation in the rat, Brain research 124(2) (1977) 197–224. [DOI] [PubMed] [Google Scholar]
- [86].Lodge DJ, Grace AA, Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia, Trends in pharmacological sciences 32(9) (2011) 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Duman RS, Malberg J, Thome J, Neural plasticity to stress and antidepressant treatment, Biological psychiatry 46(9) (1999) 1181–91. [DOI] [PubMed] [Google Scholar]
- [88].Donegan JJ, Lodge DJ, Hippocampal Perineuronal Nets Are Required for the Sustained Antidepressant Effect of Ketamine, The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 20(4) (2017) 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Swanson LW, A direct projection from Ammon’s horn to prefrontal cortex in the rat, Brain research 217(1) (1981) 150–4. [DOI] [PubMed] [Google Scholar]
- [90].Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA, Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway, Psychopharmacology 232(17) (2015) 3123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Belujon P, Grace AA, Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity, Biological psychiatry 76(12) (2014) 927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Duman RS, Shinohara R, Fogaca MV, Hare B, Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function, Molecular psychiatry (2019). [DOI] [PMC free article] [PubMed]
- [93].Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS, Ketamine: A Paradigm Shift for Depression Research and Treatment, Neuron 101(5) (2019) 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Amidfar M, Woelfer M, Reus GZ, Quevedo J, Walter M, Kim YK, The role of NMDA receptor in neurobiology and treatment of major depressive disorder: Evidence from translational research, Progress in neuro-psychopharmacology & biological psychiatry 94 (2019) 109668. [DOI] [PubMed] [Google Scholar]
- [95].Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr., Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments, The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 22(2) (2019) 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA, Do the dissociative side effects of ketamine mediate its antidepressant effects?, Journal of affective disorders 159 (2014) 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Niciu MJ, Shovestul BJ, Jaso BA, Farmer C, Luckenbaugh DA, Brutsche NE, Park LT, Ballard ED, Zarate CA Jr., Features of dissociation differentially predict antidepressant response to ketamine in treatment-resistant depression, Journal of affective disorders 232 (2018) 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G, GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain, Neuroscience 101(4) (2000) 815–50. [DOI] [PubMed] [Google Scholar]
- [99].Sur C, Quirk K, Dewar D, Atack J, McKernan R, Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric AcidA receptors have alpha5 beta3 gamma2 pharmacological characteristics, Molecular pharmacology 54(5) (1998) 928–33. [DOI] [PubMed] [Google Scholar]
- [100].Wisden W, Laurie DJ, Monyer H, Seeburg PH, The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon, The Journal of neuroscience : the official journal of the Society for Neuroscience 12(3) (1992) 1040–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Brunig I, Scotti E, Sidler C, Fritschy JM, Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro, The Journal of comparative neurology 443(1) (2002) 43–55. [DOI] [PubMed] [Google Scholar]
- [102].Christie SB, Miralles CP, De Blas AL, GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 22(3) (2002) 684–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U, Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors, Proceedings of the National Academy of Sciences of the United States of America 99(13) (2002) 8980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fritschy JM, Johnson DK, Mohler H, Rudolph U, Independent assembly and subcellular targeting of GABA(A)-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo, Neuroscience letters 249(2–3) (1998) 99–102. [DOI] [PubMed] [Google Scholar]
- [105].Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA, Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors, Proceedings of the National Academy of Sciences of the United States of America 101(10) (2004) 3662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Glykys J, Mody I, Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice, Journal of neurophysiology 95(5) (2006) 2796–807. [DOI] [PubMed] [Google Scholar]
- [107].Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL, Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain, The Journal of comparative neurology 499(3) (2006) 458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sur C, Fresu L, Howell O, McKernan RM, Atack JR, Autoradiographic localization of alpha5 subunit-containing GABAA receptors in rat brain, Brain research 822(1–2) (1999) 265–70. [DOI] [PubMed] [Google Scholar]
- [109].Wainwright A, Sirinathsinghji DJ, Oliver KR, Expression of GABA(A) receptor alpha5 subunit-like immunoreactivity in human hippocampus, Brain research. Molecular brain research 80(2) (2000) 228–32. [DOI] [PubMed] [Google Scholar]
- [110].Ali AB, Thomson AM, Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex, Cerebral cortex 18(6) (2008) 1260–71. [DOI] [PubMed] [Google Scholar]
- [111].Salesse C, Mueller CL, Chamberland S, Topolnik L, Age-dependent remodelling of inhibitory synapses onto hippocampal CA1 oriens-lacunosum moleculare interneurons, The Journal of physiology 589(Pt 20) (2011) 4885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schulz JM, Knoflach F, Hernandez MC, Bischofberger J, Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear alpha5-GABAA receptors, Nature communications 9(1) (2018) 3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zarnowska ED, Keist R, Rudolph U, Pearce RA, GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus, Journal of neurophysiology 101(3) (2009) 1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Magnin E, Francavilla R, Amalyan S, Gervais E, David LS, Luo X, Topolnik L, Input-Specific Synaptic Location and Function of the alpha5 GABAA Receptor Subunit in the Mouse CA1 Hippocampal Neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 39(5) (2019) 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM, [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit, Neuropharmacology 35(9–10) (1996) 1331–5. [DOI] [PubMed] [Google Scholar]
- [116].Carreno FR, Collins GT, Frazer A, Lodge DJ, Selective Pharmacological Augmentation of Hippocampal Activity Produces a Sustained Antidepressant-Like Response without Abuse-Related or Psychotomimetic Effects, The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 20(6) (2017) 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM, Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 40(11) (2015) 2499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, Thompson SM, A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-Like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice, eNeuro 4(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Prevot TD, Li G, Cook JM, Sibille E, Insight into Novel Treatment for Cognitive Dysfunctions across Disorders, ACS chemical neuroscience 10(5) (2019) 2088–2090. [DOI] [PubMed] [Google Scholar]
- [120].Prevot TD, Li G, Vidojevic A, Misquitta KA, Fee C, Santrac A, Knutson DE, Stephen MR, Kodali R, Zahn NM, Arnold LA, Scholze P, Fisher JL, Markovic BD, Banasr M, Cook JM, Savic M, Sibille E, Novel Benzodiazepine-Like Ligands with Various Anxiolytic, Antidepressant, or Pro-Cognitive Profiles, Mol Neuropsychiatry 5(2) (2019) 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]