Abstract
Congenital erythropoietic porphyria (CEP) is an autosomal recessive disorder of the heme biosynthetic pathway that is characterized by uroporphyrinogen III synthase (UROS) deficiency and the accumulation of non-physiological isomer I porphyrins. These phototoxic metabolites predominantly produced by the erythron result in ineffective erythropoiesis, chronic hemolysis and splenomegaly, but they also disseminate in tissues causing bullous photosensitivity to UV light and skin fragility that may progress to scarring with photo mutilation. Therapeutic management is currently limited to supportive care and bone marrow transplantation is reserved for the most severe cases. We describe here a 26-year-old women previously diagnosed with CEP harbouring two novel UROS gene mutations whose pathogenic mechanism was investigated by extensive molecular analysis. Clinical features included disabling hypertrichosis and skin photosensitivity without hemolysis. The first and rate-limiting 5-aminolevulinate synthase 2 (ALAS2) enzyme controls heme synthesis and porphyrin production in erythroid cells, while iron availability modulates its expression through a post-transcriptional mechanism. We performed iterative phlebotomies over 26 months to induce iron depletion in the patient and investigated the effectiveness and tolerance of this cost-effective approach. We observed a progressive decrease in plasma ferritin and urinary porphyrins upon treatment without inducing anemia. The patient reported improved quality of life and photosensitivity. Our data confirm recent reports highlighting the benefit of iron restriction on the disease phenotype through a reduction in porphyrin accumulation. This new strategy may represent an efficient and well-tolerated treatment for CEP patients with skin involvement and limited hematological component if iron restriction is carefully monitored.
Keywords: Congenital erythropoietic porphyria, Iron deficiency, ALAS2, Therapeutic phlebotomy, UROS, Heme synthesis
1. Introduction
Congenital erythropoietic porphyria (CEP; OMIM 263700) or Günther's disease is a rare inherited disorder of the heme biosynthetic pathway due to uroporphyrinogen III synthase (UROS; EC 4.2.1.75) deficiency [1,2]. The disease is caused by overproduction of non-physiologic type I porphyrin isomers by the erythron, and is characterized by skin photosensitivity and variable degrees of chronic hemolytic anemia [2,3]. Management of CEP is limited to supportive measures and allogeneic bone marrow transplantation (BMT) is the only curative treatment in the most severe cases [[4], [5], [6]]. To date, 51 UROS gene mutations have been reported (Human Gene Mutation Database; http://www.hgmd.cfac.ul/), of which missense and nonsense mutations predominate [7]. Functional evaluation of novel UROS variants is mandatory because we have recently demonstrated that most missense mutations affect protein folding and stability, and may be reactive to treatment with proteasome inhibitors or chemical chaperones [8,9]. Clinical heterogeneity is common in CEP and is due to the metabolic disturbance reflected by porphyrin excess. Other genes of the heme biosynthetic pathway may influence the phenotype, like ALAS2 (EC 2.3.1.37): its overexpression worsened the phenotype of CEP through increased porphyrin production [10]. Since ALAS2 expression is regulated by an iron-dependent post-transcriptional mechanism, it was recently suggested that iron depletion could decrease porphyrin production and improve the clinical phenotype in CEP [[11], [12], [13], [14]]. We report here the pathogenic mechanism of two novel UROS mutations responsible for CEP in a 26-year-old woman whose biological and clinical status improved considerably after iron deprivation induced by prospective iterative phlebotomies.
2. Material and methods
2.1. DNA and RNA analysis
Genomic DNA was isolated from blood samples, after informed consent of the patient and related. Analysis of the UROS gene (Genbank NG_011557.2) was performed by PCR amplification of the isolated DNA followed by bi-directional Sanger sequencing of all exons, exon-intron boundaries (20–30 base pairs from both boundaries), and promoter regions as previously described [10]. Total RNA was isolated from peripheral blood cells of the patient and analysed by RT-PCR using PCR primers located in exon 5 and 10 (Forward 5′- CAAGTCAGTGTATGTGGTTG −3′ and Reverse 5′- CAGCGCTAGGTGGCTGACTCA-3′). RT-PCR products were separated in a 1.5% agarose gel electrophoresis followed by extraction and purification for Sanger sequencing analysis.
2.2. Prokaryotic UROS expression and UROS enzymatic activity
The cDNA for UROS variants were generated by site directed mutagenesis and inserted into the prokaryotic expression vector pET32a. The integrity of the constructs were checked by sequencing. Freshly transformed E. coli BL21 (DE3) cells were cultured in the presence of ampicillin (50 μg/mL) and chloramphenicol (34 μg/mL) and UROS protein expression was induced by adding 1 mM IPTG (isopropyl β-D-1-thiogalactopyranoside, Sigma-Aldrich, USA), during 3 h at 37 °C. Bacterial pellets were harvested by centrifugation and maintained at −80 °C until analysis. UROS activity was measured in bacterial lysates with the coupled-enzyme assay as described previously [10]. Specific activity is expressed as uroporphyrin (μmol/h/g) of total proteins in bacterial lysates.
2.3. UROS expression analysis in eukaryotic erythroid K562 cells
We generated mammalian expression plasmids encoding human UROS (WT, C73R and S197G) fused to the N-terminus end of EGFP in pEGFP-N1 vector (Promega, Inc., USA) as described previously [8]. For stable transfection, 1.106 K562 cells were electroporated (Amaxa™ Nucleofector™ Technology, Lonza, USA) with 5 μg of EGFP-tagged UROS (WT or mutant) encoding plasmids and selected with G418 (0.5 g/L) for 3 weeks. Stably transfected K562 cells were cultured for 16 h in the presence of the proteasome inhibitors MG132 10 μM (Sigma-Aldrich, USA) or bortezomib 100 nM (Velcade®), the lysosomal inhibitor bafilomycin 20 nM (Sigma-Aldrich) or chloroquine 100 μM (Sigma-Aldrich), or di-methyl-sulfoxide (DMSO) 0.1% (Sigma-Aldrich) as a control. Flow cytometry analyses were performed on a ACCURI C6 Plus Flow cytometer (BD Biosciences) and EGFP-tagged UROS protein expression was detected using the FL-1 channel (excitation wave-length 488 nm).
2.4. Molecular dynamic simulation for UROSS197G mutant
The crystal structure of human uroporphyrinogen III synthase (PDB: 1JR2, chain A) was used for MD simulations of UROSS197G as described previously in detail [8].
2.5. Biochemical and hematological parameters analysis
The diagnosis of CEP was determined by classic biochemical analyses of porphyrins in urine and erythrocytes, isomer I/III ratio, enzymatic assays (UROS activity), and UROS gene sequencing according to the European Porphyria Network guidelines and quality control schemes (https://porphyria.eu/). Routinely available biochemical and hematological parameters were determined using standard clinical laboratory tests. We quantified urine porphyrins by reverse-phase HPLC with fluorimetric detection, according to manufacturer recommendations (Porphyrin in urine kit, Chromsystem®, Germany). Total erythrocyte porphyrins, free and Zn-Protoporphyrin were determined as described previously [13,14].
3. Results / discussion
3.1. Molecular and pathogenic analysis of two novel UROS variants identified in a CEP patient
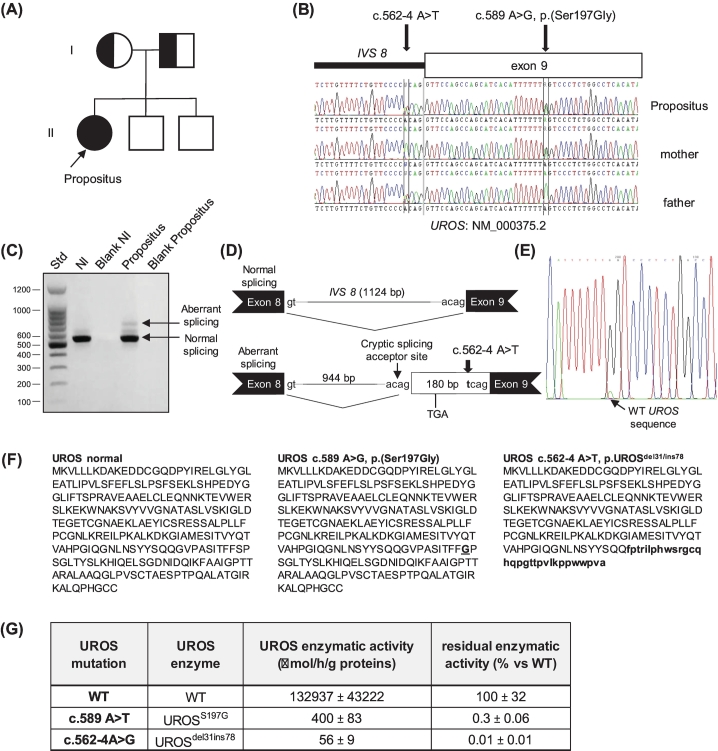
We report the case of a 26-year-old Caucasian female diagnosed with CEP that caused cutaneous bullous eruption and hypertrichosis on sun-exposed areas (hands, feet and face) at age 8 years. She presented elevated urinary excretion of uroporphyrin I (699 nmol/mmol creat, normal <10) and coproporphyrin I (406 nmol/mmol creat, normal <20) isomers and a deficient UROS enzymatic activity in red blood cells (RBCs) (2.4 IU, normal>6). The family had no consanguinity and included two unaffected brothers (Fig. 1A). Molecular analysis of the UROS gene by Sanger sequencing including the erythroid promoter, coding exons 2 to 10, and flanking splice junctions revealed compound heterozygosity combining two novel UROS variants previously unreported in CEP and population databases (Fig. 1B). The paternally transmitted allele was an intronic variant (c.562-4A > T) located in IVS8, resulting in an alternative splicing causing a truncation of the last two coding exons (78 residues) replaced by the in-frame insertion of 31 residues retained from the 3′ end of IVS8, as shown by RT-PCR analysis and sequencing of the mis-spliced mRNA (Fig. 1CD). DNA sequencing of isolated RT-PCR products suggested that some UROS mRNA harbouring the c.562-4A > T variant were normally spliced and contributed to the significant UROS enzymatic activity measured in the patient (Fig. 1E). The maternally transmitted allele was a missense variant - c.589A > G, p.(Ser197Gly) - located in exon 9, leading to the substitution serine to glycine (residue 197), predicted as disease-causing and probably damaging by in silico predicting tools (Mutation Taster and PolyPhen, respectively). The residual activity of UROSdel78/ins31 and UROSS197G mutant proteins expressed in Escherichia coli was 0.01% and 0.3% versus WT respectively (Fig. 1FG).
Fig. 1.
Pedigree and molecular genetic analysis of UROS gene in CEP patient and her family. (A) and (B) Molecular genetic analysis identified compound heterozygosity for two novel UROS gene mutations in the CEP patient: her father and mother transmitted the intronic c.562–4 A > T and the missense c.589 A > G UROS mutations, respectively. (C) and (D) RNA analysis performed on WBC revealed that c.562–4 A > T mutation is responsible for abnormal splicing using a cryptic splicing acceptor site leading to the retention of 180 bp located at the 3’end of intron 8 fragment. This in frame insertion resulted in a premature stop in the aberrant transcript and led to the deletion of the last 78 residues and the insertion of 31 new residues at the C-terminus of the UROS enzyme. (E) Normally spliced RT-PCR products were composed of mixed variant (c.589G black) and WT (c.589A, green) UROS nucleotide sequences, suggesting that some UROS mRNA harbouring the splicing variant escaped abnormal splicing process. (F) Expected amino acid composition of UROSWT, UROSS197G and UROS del78ins31 proteins. (G) Residual intrinsic enzymatic activities from UROSWT, UROSS197G and UROSdel78ins31 variants were determined by prokaryotic expression.
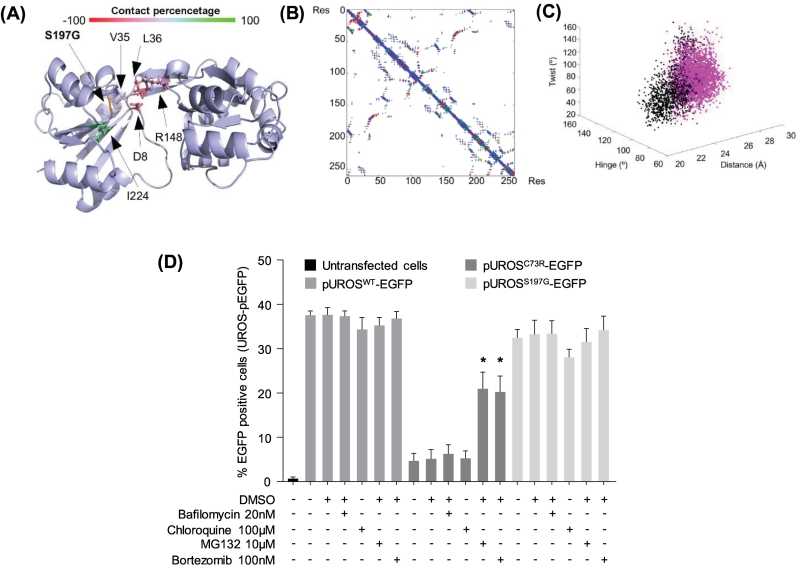
Whereas a tight genotype/phenotype correlation is difficult to establish owing to the heterogeneity of molecular lesions in CEP patients, thorough evaluation of UROS variants is now critical to offer, in the near future, the therapeutic approach that best fits all the recent improvements in the field [7]. We particularly demonstrated that protein unfolding and thermodynamic instability are the leading mechanisms associated with pathogenic UROS missense mutants, which can be overcome by proteostasis drugs or chemical chaperones [8,9]. We further investigated the pathogenicity of the UROSS197G protein by analysing its thermodynamic stability both in vitro and in eukaryotic cell lines, as described previously [8]. We determined that UROSS197G was a Type II mutant, i.e. the mutation produced limited loss of inter-residue contacts (with residues 8, 36, 148) with little oscillation of the open inter-domain distance, resulting in the local unfolding of the protein (Fig. 2A-C). Furthermore, we found that UROSS197G stability was not affected and did not undergo the premature degradation that the UROSC73R mutant underwent in K562 erythroid cells (Fig. 2D). The S197 residue is highly conserved in the cleft between the two major domains of the UROS enzyme that mapped the active site [15]. It is likely that S197 is a contact residue in the active site and that ligand binding is affected in UROSS197G variant. Furthermore, we show here that the thermodynamic stability of UROSS197G is not affected and that treatment with classical drugs that modify protein homeostasis (ex. proteasome inhibitors) did not improve UROSS197G expression (Fig. 2D). Taken together, these results make UROSS197G-targeted chaperone therapy unlikely to be effective [8,9].
Fig. 2.
Investigation of UROSS197Gstability in erythroid cells and molecular dynamics simulation analysis. (A) Protein structure of UROS protein during MS simulation. The colour bar represents the contact-percentage that was obtained by comparing the number of contacts observed below 4 Å in each frame by the total number of frames in the trajectory in the wild type and each mutated residue. Altered residues are represented with sidechains and with their corresponding colour. (B) Contact map of the UROS where the atomic contacts below 4 Å are represented in blue. In the same graph the contacts that are lost in the mutated structure (red) or the contacts that have arisen (green) have been overlaid. (C) Analysis of the inter-domain hinge (°), twist (°) and distance (Å) along the MD simulations (60 ns for WT and 20 ns for the S197G mutant). WT values are represented in black dots and mutant values are plotted in magenta. (D) Human erythroleukemic K562 cells were stably transfected with plasmids expressing EGFP fused to the C-terminus end of WT, C73R, or S197G UROS protein. Stably transfected cells were treated with DMSO or the indicated concentration of lysosome (bafilomycin or chloroquine) or proteasome (MG132 or bortezomib) inhibitors for 16 h. EGFP expression was monitored by flow cytometry analysis. Results are expressed as the mean of four independent experiments; error bars represent SD. * Significant difference (P < 0.001) vs. EGFP-UROS mutant treated with DMSO.
3.2. Phlebotomy improves both biological and clinical outcome in CEP patient
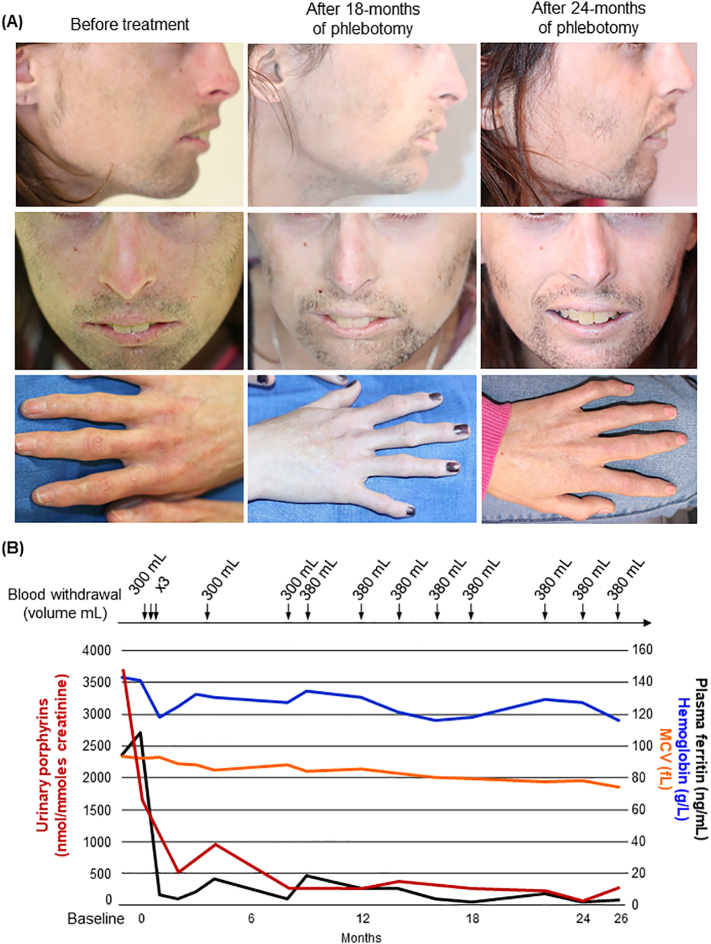
The patient presented photosensitivity with blistering and crusted erosions following sun exposure. Her photosensitivity worsened with progressive scarring and sclerodermiform modifications of the sun-exposed skin areas and face (Fig. 3A). She also complained of disabling facial hypertrichosis. She never presented anemia or biological indicators of chronic hemolysis (slight increase in LDH but normal levels of haptoglobin and total bilirubin) and showed baseline elevation of urinary porphyrins (total uroporphyrin 3698 nmol/mmol creat) considered as moderate as observed in non-transfusion-dependent patient [5] (Supplementary Table 1). Treatment options are scarce in CEP and the patient was treated by unsatisfactory conservative treatment essentially based on sun avoidance. Recent reports indicate the benefit of substrate reduction therapy by modulating ALAS2 activity with iron restriction to reduce porphyrin production [[12], [13], [14]]. As we recently described a major improvement in porphyrin accumulation and hemolysis by iron deprivation in CEP patient, we prospectively treated our patient by iterative phlebotomies for more than 26 months [13]. Treatment started with 300 ml blood removal, three times at weekly interval, followed by bleeding every two months (350–400 mL/session) (Fig. 3B and Supplementary Table 2). We observed progressive iron depletion over time and adjusted the bleeding rate to maintain low ferritin levels (<10 μg/L) (Supplementary Table 1). There was a concomitant decrease in urinary porphyrin excretion (270 nmol/mmol creat at 26 months post-treatment) corresponding to a 93% reduction compared to baseline (Supplementary Table 1), a level close to that obtained after hematopoietic-stem cell transplantation in CEP patients [5,16]. Whereas this metabolic profile is still abnormal and could cause cutaneous blistering in patients with porphyria cutanea tarda (PCT), it is usually associated with a major skin photosensitivity improvement in CEP patients treated by BMT [5,16]. Hemoglobin levels remained close to 12 g/dl over the 26 months of treatment with progressively decreasing MCV, which reflected iron restriction in the patient (Fig. 3B and Supplementary Table 1). She experienced significant improvement in her photosensitive skin lesions (disappearance of blistering and erythematous eruptions) and quality of life with progressive attenuation of the reddish urine colour.
Fig. 3.
Cutaneous features and follow-up of urinary porphyrins, ferritin, mean corpuscular volume (MCV) and hemoglobin in CEP patient over 26 months of phlebotomy. (A) Cutaneous features in the CEP patient before and after treatment by phlebotomy (pictures were collected after 18 and 24 months of treatment). Scaring lesions were observed on nose and hands as well as erythematous skin, which improved upon treatment. Hypertrichosis mainly affected malar area and chin and improved discretely upon treatment. (B) Follow-up of urinary porphyrins, ferritin, MCV and hemoglobin over 26 months of treatment by phlebotomy.
This is actually the third report of a successful metabolic and clinical improvement of CEP disease by iron depletion therapy (data summarised in Table 1). We recently demonstrated that iron chelation therapy with deferiprone led to a significant drop of porphyrin accumulation and iron overload as well as a complete correction of both skin photosensitivity and hemolytic anemia in CEP mice [14]. Egan and colleagues treated a CEP patient with off-label use of deferasirox for 8 months to obtain iron deficiency [12]. Total urine porphyrins decreased by approximately 95% while markers of hemolysis normalized and that her photosensitivity improved. However, the chronic delivery of iron chelators in CEP patients devoted to iron overload is questionable due to safety concerns and economical consideration. Phlebotomy is a less-expensive alternative that is effective in decreasing hepatic iron in PCT [17]. We recently reported a significant biological and clinical improvement in a CEP patient treated by iterative phlebotomies [13]. The patient presented skin photosensitivity and chronic hemolysis without anemia and received therapeutic phlebotomies every month (150–200 mL blood removal per session) from more than 3 years now [13]. In this new prospective study, we alleviated the bleeding process and performed phlebotomies every two months (~400 mL blood removal per session) over 26 consecutive months, which led to a similar reduction in porphyrin overload and iron store depletion. The frequency of repeated bleeds should be carefully adapted to avoid acute anemic syndrome and long-term iron-deficient anemia. Finally, the clinical tolerance was excellent for both patients without any adverse effect to report.
Table 1.
Demographic, biological and clinical findings in three reported cases of CEP patients treated by iron depletion therapy.
| Characteristics | Case #1 | Case #2 | Case #3 | |
|---|---|---|---|---|
| Reference | 12 | 13 | present CEP patient | |
| Sex | Female | Female | Female | |
| Age at diagnosis | 12 months | 25 years | 8 years | |
| UROS genotype | c.217 T > C / c.311C > T | c.172G > A / c.172G > A | c.598A > T / c.562-4A > G | |
| UROS variant | p.Cys73Arg / p.Ala104Val | p.Gly58Ser | p.Ser197Gly / p.Gln187_Ter266delins31 | |
| UROS enzymatic activity * | N.R. | 1.7 U/mg Hb/h (N > 6) | 2.4 U/mg Hb/h (N > 6) | |
| Clinical and biological findings before treatment | Skin photosensitivity | + | + | + |
| Hemolysis | + | + | − | |
| Anemia | + (blood transfusion) | − | − | |
| Red urine | + | + | +/− | |
| Splenectomy | + (25 years old) | − | − | |
| Erythrodontia | + | + | +/− | |
| Total urine porphyrins | 108,364 μg/24 h § | 2642 nmol/mmol creat (N < 25) | 3698 nmol/mmol creat (N < 25) | |
| Erythrocyte porphyrins | N.R. | 8.7 μmol/L RBC (N < 1.2) | N.D. | |
| Plasma porphyrins | N.R. | 289 nmol/L (N < 20) | N.D. | |
| Therapeutic management | Sun avoidance, supportive measures Splenectomy, transfusion | Sun avoidance, supportive measures | Sun avoidance, supportive measures | |
| Clinical and biological findings upon iron depletion therapy | Age at treatment initiation | 32 years | 49 years | 26 years |
| Lenght of follow-up | 8 months (iron chelation) | 23 months | 26 months | |
| Skin photosensitivity | − | − | − | |
| Hemolysis | − | − | − | |
| Anemia (transfusion) | +/− (no transfusion) | − | - (MCV↓) | |
| Red urine | N.R. | − | − | |
| Erythrodontia | N.R. | N.R. | +/− | |
| Total urine porphyrins | 5896 μg/24 h § | 271 nmol/mmol creat (N < 25) | 270 nmol/mmol creat (N < 25) | |
| Erythrocyte porphyrins | N.R. | 7.3 μmol/L RBC (N < 1.2) | 6.8 μmol/L RBC (N < 1.2) | |
| Plasma porphyrins | N.R. | 26 nmol/L (N < 20) | N.D. | |
| Clinical outcome | Reduced skin photosensitivity Improved QoL | Reduced skin photosensitivity Improved QoL | Reduced skin photosensitivity Improved QoL |
N.R. not reported; N.D. not determined; * UROS enzymatic activity determined in red blood cells; § Normal values are not provided in ref. [12] but are usually <200-300 mg/24 h; MCV mean corpuscular volume, + positive; − negative; QoL Quality of Life (reported by the patient, not formerly measured in all three studies).
In summary, we deciphered the pathogenic molecular mechanism associated with two novel UROS mutations identified in a CEP patient. Our data support that the c.562-4A > T variant causes aberrant splicing and truncation of the UROS protein while the missense UROS c.589A > G mutation induce protein unfolding without thermodynamic instability responsible for profound loss of catalytic activity. In this context, we show that iron depletion therapy based on iterative phlebotomies efficiently reduced porphyrin accumulation and significantly improved the clinical outcome. We propose that phlebotomy is a convenient treatment that can be implemented in patients with minor hematological involvement and could become an efficient and well-tolerated therapeutic approach in CEP.
Biochemical and hematological parameters in CEP patient upon treatment by phlebotomy.
Clinical schedule template.
Funding sources
This study was supported by grants from Laboratory of Excellence GR-Ex (reference ANR-11-LABX-0051). The Labex GR-Ex is funded by the programme “Investissements d'avenir” of the French National Research Agency (reference ANR-11-IDEX-0005-02). This study was supported in part by recurrent research funding from Institut National de la Santé et de la Recherche médicale (INSERM) and the University of Bordeaux. The funding sources were not involved in any may in conducting the study, writing the report or submitting the publication.
Human subject study
This work has been carried out in accordance with The Code of ethics of the World Medical Association (declaration of Helsinki) for experiments involving humans. The study was approved by the institutional review board of Labex GR-Ex, Institut IMAGINE (Institut des Maladies Génétiques), Paris (Patient Identification # 15-371-P). The patient gave informed consent to participate in the study and authorization to the publication of related data.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Mr. Ray Cooke, an independent medical writer provided editorial assistance and language help during preparation of this manuscript.
References
- 1.J. To-Figueras, Millet O., Herrero C. In: Handbook of Porphyrin Science (Chapter 29) Kadish K.M., Smith K.M., Guilard R., editors. 2013. pp. 151–217. [DOI] [Google Scholar]
- 2.Di Pierro E., Brancaleoni V., Granata F. Advances in understanding the pathogenesis of congenital erythropoietic porphyria. Br. J. Haematol. 2016;173:365–379. doi: 10.1111/bjh.13978. [DOI] [PubMed] [Google Scholar]
- 3.Katugampola R.P., Badminton M.N., Finlay A.Y., Whatley S., Woolf J., Mason N. Congenital erythropoietic porphyria: a single-observer clinical study of 29 cases. Br. J. Dermatol. 2012;167:901–913. doi: 10.1111/j.1365-2133.2012.11160.x. [DOI] [PubMed] [Google Scholar]
- 4.Dawe S.A., Peters T.J., Du Vivier A., Creamer J.D. Congenital erythropoietic porphyria: dilemmas in present day management. Clin. Exp. Dermatol. 2002;27:680–683. doi: 10.1046/j.1365-2230.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 5.Katugampola R.P., Anstey A.V., Finlay A.Y., Whatley S., Woolf J., Mason N. A management algorithm for congenital erythropoietic porphyria derived from a study of 29 cases. Br. J. Dermatol. 2012;167:888–900. doi: 10.1111/j.1365-2133.2012.11154.x. [DOI] [PubMed] [Google Scholar]
- 6.Martinez Peinado C., Díaz de Heredia C., J. To-Figueras, Arias-Santiago S., Nogueras P., Elorza I. Successful treatment of congenital erythropoietic porphyria using matched unrelated hematopoietic stem cell transplantation. Pediatr. Dermatol. 2013;30:484–489. doi: 10.1111/pde.12117. [DOI] [PubMed] [Google Scholar]
- 7.Erwin A.L., Desnick R.J. Congenital erythropoietic porphyria: recent advances. Mol. Genet. Metab. 2019;128:288–297. doi: 10.1016/j.ymgme.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blouin J.M., Bernardo-Seisdedos G., Sasso E., Esteve J., Ged C., Lalanne M. Missense UROS mutations causing congenital erythropoietic porphyria reduce UROS homeostasis that can be rescued by proteasome inhibition. Hum. Mol. Genet. 2017;26:1565–1576. doi: 10.1093/hmg/ddx067. [DOI] [PubMed] [Google Scholar]
- 9.Urquiza P., Laín A., Sanz-Parra A., Moreno J., Bernardo-Seisdedos G., Dubus P. Repurposing ciclopirox as a pharmacological chaperone in a model of congenital erythropoietic porphyria. Sci. Transl. Med. 2018;10:7467. doi: 10.1126/scitranslmed.aat7467. [DOI] [PubMed] [Google Scholar]
- 10.J. To-Figueras, Ducamp S., Clayton J., Badenas C., Delaby C., Ged C. ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood. 2011;118:1443–1451. doi: 10.1182/blood-2011-03-342873. [DOI] [PubMed] [Google Scholar]
- 11.Manceau H., Gouya L., Puy H. Acute hepatic and erythropoietic porphyrias: from ALA synthases 1 and 2 to new molecular bases and treatments. Curr. Opin. Hematol. 2017;24:198–207. doi: 10.1097/MOH.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 12.Egan D.N., Yang Z., Phillips J., Abkowitz J.L. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood. 2015;126:257–261. doi: 10.1182/blood-2014-07-584664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirmiran A., Poli A., Ged C., Schmitt C., Lefebvre T., Manceau H. Phlebotomy as an efficient long-term treatment of congenital erythropoietic porphyria. Haematologica. 2020;(Jan 9) doi: 10.3324/haematol.2019.228270. haematol.2019.228270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blouin J.M., Ged C., Lalanne M., Lamrissi-Garcia I., Morice-Picard F., Costet P. Iron chelation rescues hemolytic anemia and skin photosensitivity in congenital erythropoietic porphyria. Blood. 2020;136:2457–2468. doi: 10.1182/blood.2020006037. [DOI] [PubMed] [Google Scholar]
- 15.Cunha L., Kuti M., Bishop D.F., Mezei M., Zeng L., Zhou M.M., Desnick R.J. Human uroporphyrinogen III synthase: NMR-based mapping of the active site. Proteins. 2008;71:855–873. doi: 10.1002/prot.21755. [DOI] [PubMed] [Google Scholar]
- 16.Besnard C., Schmitt C., Galmiche-Rolland L., Debray D., Fabre M., Molina T. Bone marrow transplantation in congenital erythropoietic porphyria: sustained efficacy but unexpected liver dysfunction. Biol Blood Marrow Transplant. 2020;26:704–711. doi: 10.1016/j.bbmt.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Salameh H., Sarairah H., Rizwan M., Kuo Y.F., Anderson K.E., Singal A.K. Relapse of porphyria cutanea tarda after treatment with phlebotomy or 4-aminoquinoline antimalarials: a meta-analysis. Br. J. Dermatol. 2018;179:1351–1357. doi: 10.1111/bjd.16741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biochemical and hematological parameters in CEP patient upon treatment by phlebotomy.
Clinical schedule template.