Abstract
Patients with Autism Spectrum Disorder (ASD) may be particularly prone to develop COVID-19. An unusual extended course of COVID-19 disease illness has been reported in one ASD patient and a group of patients have COVID-19 disease in a neurodevelopmental facility. It has been widely reported that many of those with ASD have substantial sleep disorders with low levels of melatonin and various genetic alterations related to melatonin production have been found. Several lines of evidence point to a substantial role of melatonin in the body’s innate defense system including acting as a scavenger, an antioxidant and modulating the immune system. We therefore hypothesize that melatonin deficiency may predispose those ASD patients who have low melatonin output to COVID-19 disease. Potential implications for treatment are discussed.
Keywords: Autism Spectrum Disorder, COVID-19, SARS-CoV-2, Melatonin
Autism Spectrum Disorder (ASD)
The prevalence of Autism Spectrum Disorder (ASD) has been reported as 1 in 132 [1]. It is a devastating illness, first appearing in childhood, with symptoms that include difficulties with social communication and social interaction, often with cognitive deficits and unusual behavior that includes restricted, repetitive activities and interests [2]. Sleep patterns are often disrupted which can lead to major difficulties for families and caregivers as the patients waking during sleep inevitably wakes those caregivers. The severity of sleep problems parallels that of other symptoms and may aggravate those symptoms [3].
COViD-19 disease and ASD
COViD-19 disease is a multiorgan disease due to SARS-CoV-2 viral infection, that become pandemic in early 2020 [4]. It has been hypothesized that those with ASD may be predisposed to COVID-19 disease because of modifications at the immune and genetic levels [5], [6]. Recently, a case series of 16 ASD patients admitted to a neurodevelopmental unit with COVID-19 like symptoms was reported [7]. Eleven of these patients were shown to have COVID-19 disease by reverse transcriptase polymerase chain reaction (RT-PCR) or by serology at follow-up, of these patients only one required oxygen therapy. A recent case report describes a nine-year-old Italian child in a nursing home with exceptionally high viral load and an extended duration of viral shedding Grossi and Teruzzi, [8]. Initial symptoms of cough and nasal discharge disappeared within two days and no clinical signs of lung involvement could be detected. However, quantitative RT-PCR indicated an extremely high viral load. A gradually decreasing load was seen over the following twelve weeks until negative tests were found, but only after 82 days. ASD patients with COVID-19 disease may be a major problem to the health care system; those who are severely intellectually disabled may be unable to communicate symptoms effectively thus monitoring, prevention and testing are doubly important [9].
Melatonin and ASD
A potentially significant factor in ASD is the finding of low urinary levels of the melatonin metabolite 6-sulphatoxymelatonin (aMT6s). In 23 ASD patients aged 4–10 years overall nocturnal aMT6s levels were reduced [10] together with increased N3 sleep, decreased N2 sleep and increased daytime sleepiness [10]. In another study 43 patients with ASD showed lower urinary aMT6s levels (8.26 µg per 24 h vs 18.00 µg per 24 h) both during nighttime and daytime in comparison to 26 controls matched for age, sex and pubertal status [11]. In that study the amount of aMT6s decrease correlated with the severity of illness. Most ASD studies looking for melatonin excretion, except for one [12] reported decreased melatonin output. Another controlled study of 77 children with ASD and 84 controls aged 2.5 to 15.5 years reported that those with ASD had lower nighttime aMT6s than the controls with no difference in the daytime levels [13].
aMT6s levels in the ASD group were associated with sleep disturbance but not with ASD severity. In a study of 83 children with ASD aged 5 to 10, it was reported that aMT6s levels were increased in families with higher income but decreased with increased neurological problems [14]. Manuani and coworkers examined the relationship between the pineal gland volume (PGV) estimated by MRI and early morning melatonin levels in 215 participants, 78 with ASD, 90 unaffected relatives and 47 controls. Although both melatonin and PGV were lower in patients than controls, the melatonin deficit appeared to be more related to group than to PCV. The authors stated that the findings support a deficit in the melatonin synthetic pathway as a possible main cause [15].
Melatonin synthesis in ASD
Genetic variation in synthesis of melatonin has been reported in ASD. In a unique subgroup of ASD children selected for having sleep onset delay, higher frequencies were reported of variants that decrease acetylserotonin O-methyltransferase (ASMT, the enzyme that determines the quantity of melatonin synthesized), as well as of those that decrease cytochrome P450 1A2 (CYP1A2, the enzyme that metabolizes melatonin) [16]. Two different polymorphisms in the promoter have been reported for ASMT, moreover a splicing mutation was reported in two families [17], [18], [19] In 2065 children, ages 4–18, from the Simons Simplex Collection (SSC) no relationship was found between any gene variants and sleep problems [20]. The authors suggest that sleep problems may be related to translational or posttranslational effects of these genes or to additional ones modifying the sleep system.
Melatonin and COVID-19
Melatonin is a multipotential body substance: it is not just a pineal hormone but is synthesized widely in the body and acts as a potent antioxidant, scavenger, modulates the immune system and modulates macrophage function [21]. It is synthesized in cerebral mitochondria, where it can counteract overproduction of radical oxygen species at the site of their generation during electron transport [22]. In the immune system it supresses innate immunity while facilitating antibody production. Melatonin suppresses NLRP3 inflammasomes, proinflammatory cytokine activation and facilitates switching of highly inflammatory M1 macrophages to anti-inflammatory M2 macrophages [23], [24], [25] In numerous animal viral diseases it has been shown to greatly enhance survival. Moreover, in several instances of human sepsis adjunctive treatment with melatonin has improved patient outcome [26]. The structure and physico-chemical properties of melatonin have been examined using electronic structure methods and molecular-mechanics tools as a predictor of melatonin's bioactivity against the coronavirus 2 proteins. Based on the docking scores obtained, the authors proposed that melatonin could be effective to defend against the viral load in vulnerable populations [27]. Recently it was reported in a case series that 36 to 72 mg of melatonin daily po is a helpful adjuvant in patients with severe pneumonic COVID-19 disease [28]. The analysis of 26,779 records of patients in a COVID-19 database revealed melatonin was associated with an improved outcome [29]. A prospective study of 791 intubated COVID-19 patients showed that melatonin was associated with survival [30]. In the first reported randomly controlled trial of melatonin in COVID-19 patients, 3 mg was administered three times daily to hospitalized patients (24 given melatonin vs 20 none), the melatonin treatment group had significantly less symptoms and were discharged earlier from hospital [31].
Treatment implications
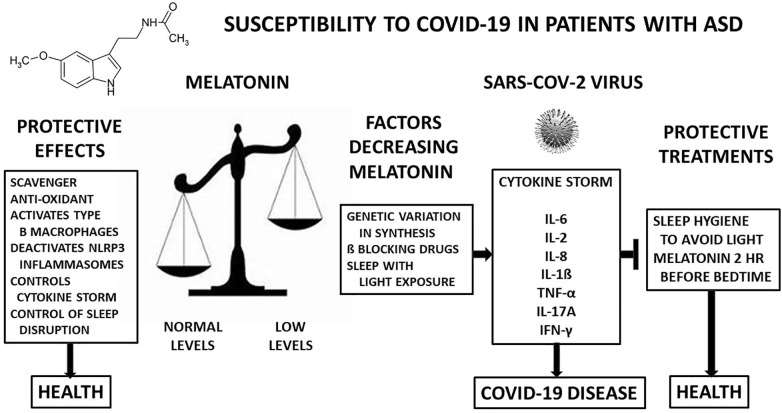
Based on the hypothesis that low melatonin can be a reason for susceptibility to the SARS-CoV-2 virus we postulate that adequate melatonin levels will be preventative. It is known that there is genetic variation in the melatonin synthesis pathway in some ASD patients. In others the sleep irregularity that frequently is seen in ASD may result in a decrease in circulating melatonin because of waking during the night and exposure to light. Light and especially blue light will supress melatonin production by the pineal gland, so it is important to regulate sleeping if it is possible [32]. Two treatments described recently can be of assistance [3]. A comprehensive program of sleep hygiene that improves sleep can be effective in reducing exposure to light at times that would impair melatonin secretion. Another possible treatment is the administration of melatonin. It has often been used to help with sleep disorder [3]. In treatment with melatonin, it should be noted that a minority of individuals develop resistance to its sleep inducing effects after a few days. These people have been shown to be slow metabolizers due to a genetic variation in CYP1A2, the gene that metabolizes melatonin [33] (Fig. 1 ).
Fig. 1.
It is hypothesized that melatonin, well documented for its protective effects in various human and animal studies will protect ASD patients who have are known to have low melatonin levels and may be susceptible to COVID-19 disease. Furthermore, restoration of melatonin levels will be protective against the SARS-CoV-2 viral infection. Melatonin’s protective actions in the left box are conducive to health. SARS-CoV-2 virus with low melatonin levels result in a cytokine storm that causes COVID-19 disease. Raising melatonin levels may help prevent illness due to SARS-CoV-2. SARS-CoV-2 figure is from Pixaby.
Conclusion
We hypothesize that a low melatonin output, found in those with ASD due either to genetic variation in the synthetic enzyme pathway or to frequent nighttims with exposure to light that suppresses melatonin synthesis by the pineal gland, may lead to susceptibility to COVID-19 disease. Further we propose that treatment with sleep hygiene to correct nighttime waking and treatment with melatonin are both treatments that may prevent COVID-19 disease or reduce its severity in ASD patients.
Sources of funding
No funding is declared.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baxter A.J., Brugha T.S., Erskine H.E., Scheurer R.W., Vos T., Scott J.G. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 2.Association AP. Diagnostic and statistical manual of mental disorders, 5th e. Vol. 5. Arlington, VA: American Psychiatric Association; 2013.
- 3.Karthikeyan R., Cardinali D.P., Shakunthala V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Understanding the role of sleep and its disturbances in autism spectrum disorder. Int J Neurosci. 2020:1–14. doi: 10.1080/00207454.2019.1711377. [DOI] [PubMed] [Google Scholar]
- 4.Hu B., Guo H., Zhou P., Shi Z.L. Nature Reviews Microbiology; Nature Research: 2020. Characteristics of SARS-CoV-2 and COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman G. COVID-19 and autism. Med Hypotheses. 2020;1:142. doi: 10.1016/j.mehy.2020.109797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima ME de S, Barros LCM, Aragão GF. Could autism spectrum disorders be a risk factor for COVID-19? Medical Hypotheses. 2020 Nov 1;144. [DOI] [PMC free article] [PubMed]
- 7.Nollace L., Cravero C., Abbou A., Mazda-Walter B., Bleibtreu A., Pereirra N., et al. Autism and COVID-19: a case series in a neurodevelopmental unit. J Clin Med. 2020;9(9):2937. doi: 10.3390/jcm9092937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossi E., Teruzzi V. Exceptionally high COVID-19 viral load and a very long duration of shedding in a young pauci-symptomatic child with autism resident in an Italian nursing home. J Infect. 2020;81(3) doi: 10.1016/j.jinf.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baghdadli A., Picot M.C., Miot S., Munir K. A call to action to implement effective COVID-19 prevention and screening of individuals with severe intellectual developmental and autism spectrum disorders. J Autism Develop Disorders. 2020 doi: 10.1007/s10803-020-04719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leu R.M., Beyderman L., Botzolakis E.J., Surdyka K., Wang L., Malow B.A. Relation of melatonin to sleep architecture in children with autism. J Autism Dev Disord. 2011;41(4):427–433. doi: 10.1007/s10803-010-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biological psychiatry. 2005;57(0006–3223; 2):134–8. [DOI] [PubMed]
- 12.Ritvo E.R., Ritvo R., Yuwiler A., Brothers A., Freeman B.J., Plotl S. Elevated daytime helatonin concentrations in autism: a pilot study. Eur Child Adolesc Psychiatry. 1993;2 doi: 10.1007/BF02098862. [DOI] [PubMed] [Google Scholar]
- 13.Babinska K., Siklenkova L., Stebelova K., Waczulikova I., Celusakova H., Vidosovicova M., et al. Urinary levels of 6-sulphatoxymelatonin and their associations with sleep disorders and behavioural impairments in children with autism spectrum disorder. bratisl Med J. 2019;120(11):849–855. doi: 10.4149/BLL_2019_141. [DOI] [PubMed] [Google Scholar]
- 14.Bridgemohan C., Cochran D.M., Howe Y.J., Pawlowski K., Zimmerman A.W., Anderson G.M., et al. Investigating potential biomarkers in autism spectrum disorder. Front Integr Neurosci. 2019;2:13. doi: 10.3389/fnint.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruani A., Dumas G., Beggiato A., Traut N., Peyre H., Cohen-Freoua A., et al. Morning plasma melatonin differences in autism: beyond the impact of pineal gland volume. Frontiers. Psychiatry. 2019;10(FEB) doi: 10.3389/fpsyt.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veatch O.J., Pendergast J.S., Allen M.J., Leu R.M., Johnson C.H., Elsea S.H., et al. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J Autism Dev Disord. 2015;45(1):100–110. doi: 10.1007/s10803-014-2197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsäter H, et al. Abnormal melatonin synthesis in autism spectrum disorders. Molecular psychiatry. 2008;13(1359–4184; 1):90–8. [DOI] [PMC free article] [PubMed]
- 18.Jonsson L., Anckarsäter H., Zettergren A., Westberg L., Walum H., Lundström S., et al. Association between ASMT and autistic-like traits in children from a Swedish nationwide cohort. Psychiatric Genetics. 2014;24(1):21–27. doi: 10.1097/YPG.0000000000000010. Available from http://journals.lww.com/00041444-201402000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson L., Ljunggren E., Bremer A., Pedersen C., Landén M., Thuresson K., et al. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med Genomics. 2010 doi: 10.1186/1755-8794-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson A.E.E., Dorman J.S., Chasens E.R., Feeley C.A., Devlin B. Variations in genes related to sleep patterns in children with autism spectrum disorder. Biol Res Nurs. 2019;21(3):335–342. doi: 10.1177/1099800419843604. [DOI] [PubMed] [Google Scholar]
- 21.Cardinali D.P., Brown G.M., Pandi-Perumal S.R. Can melatonin be a potential “silver bullet” in treating COVID-19 patients? Diseases. 2020;8(4):44. doi: 10.3390/diseases8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardeland R. Melatonin and the electron transport chain. Cell Mol Life Sci. 2017;74(21):3883–3896. doi: 10.1007/s00018-017-2615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Wang G, Ai L, Shi J, Zhang J, Chen YX. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Scientific Reports [Internet]. 2018;8(1):15579. Available from: http://dx.doi.org/10.1038/s41598-018-34011-8. [DOI] [PMC free article] [PubMed]
- 24.Xia Y., Chen S., Zeng S., Zhao Y., Zhu C., Deng B., et al. Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res. 2019 doi: 10.1111/jpi.12547. [DOI] [PubMed] [Google Scholar]
- 25.Favero G., Franceschetti L., Bonomini F., Rodella L.F., Rezzani R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/1835195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biancatelli R.M.L.C., Berrill M., Mohammed Y.H., Marik P.E. Melatonin for the treatment of sepsis: the scientific rationale. J Thoracic Disease. 2020;2(Suppl 1):S54–S65. doi: 10.21037/jtd.2019.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Zaqri N., Pooventhiran T., Alsalme A., Warad I., John A.M., Thomas R. Structural and physico-chemical evaluation of melatonin and its solution-state excited properties, with emphasis on its binding with novel coronavirus proteins. J Mol Liq. 2020;15:318. doi: 10.1016/j.molliq.2020.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo R.R., Quizon G.R.A., Juco M.J.M., Roman A.D.E., de Leon D.G., Punzalan F.E.R., et al. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): a case series. Melatonin Res. 2020;3(Mv):297–310. [Google Scholar]
- 29.Zhou Y, Hou Y, Shen J, Mehra R, Kallianpur A, Culver DA, et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020 Nov 6;18(11). [DOI] [PMC free article] [PubMed]
- 30.Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv : the preprint server for health sciences [Internet]. 2020 Oct 18; Available from: http://www.ncbi.nlm.nih.gov/pubmed/33083812.
- 31.Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, double-blind clinical trial. Available from: https://doi.org/10.22541/au.160734344.45295921/v1. [DOI] [PMC free article] [PubMed]
- 32.Gooley J.J. Light-induced resetting of circadian rhythms in humans. J Sci Technol Light. 2017;41:69–76. [Google Scholar]
- 33.Braam W., van Geijlswijk I., Keijzer H., Smits M.G., Didden R., Curfs L.M. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J Intellectual Disability Res: JIDR. 2010;54(6):547–555. doi: 10.1111/j.1365-2788.2010.01283.x. [DOI] [PubMed] [Google Scholar]