Abstract
Purpose
The novel coronavirus (SARS-CoV-2) caused an acute respiratory illness named COVID-19 and the disease spread all over the World. Fever, cough, fatigue, gastrointestinal infection symptoms form the main clinical symptoms. Pregnants and newborns form a vulnerable population and urgent measures must be addressed. Studies about the effect of COVID-19 on pregnant women, developing fetuses, and infants are limited. Various viral diseases can cause congenital or acquired, unilateral or bilateral hearing loss.
Methods
37 infants whose mother was pregnant between March 2020 and December 2020 and were born after the diagnosis of COVID- 19 during pregnancy and 36 healthy infants were included in the study. Transient evoked otoacoustic emission (TEOAE), distortion product otoacoustic emission (DPOAE) and contralateral suppression of OAE (CLS OAE) tests were performed on all infants.
Results
According to the TEOAE results of patients and controls in the silent a statistically significant difference was observed between the two groups at 3 kHz and 4 kHz (p < 0.05). Contralateral suppression of OAE test results of patients and controls a statistically significant difference was found in all frequencies (p< 0.05). Suppression was much more effective at all frequencies in the normal group than patient group. This difference was found to be more significant at higher frequencies (2,3 and 4 kHz) (p < 0.001).
Conclusions
Our results suggest an insufficiency in medial olivocochlear efferent system in infants exposed to SARS-CoV-2 intrauterine. Cochlear functions should be examined in infants whose mothers had COVID-19.
Keywords: SARS-CoV-2, Infants, Pregnancy, Cochlear functions
1. Introduction
A new type of coronavirus (SARS-CoV-2) caused an acute respiratory disease that spread very quickly throughout China and changed the agenda all over the World. World Health Organization (WHO) announced the COVID-19 epidemic as a public health emergency of international concern on 30 January 2020. The average incubation period of COVID-19 is 2–14 days after contact. Fever, cough, fatigue, gastrointestinal infection symptoms form the main clinical symptoms [1]. COVID-19 infection process needs to be controlled closely among pregnant women. Pregnants and newborns form a vulnerable population and urgent measures must be addressed. Pregnancy is a process that causes partial suppression in the immune system, which makes women prone to viral infections. Especially in winter even seasonal flu increases the morbidity of pregnancy. Therefore, COVID-19 may cause serious health consequences in pregnant women [2].
Studies about the effect of COVID-19 on pregnant women, developing fetuses, and infants are limited. According to the literature, there is no direct relevance a severe course of COVID-19 and pregnancy. Being in third trimester and having co-morbidities put the pregnant into a particular risk of serious COVID-19 disease [3]. There are no findings about the risks such as exact transmission route, mortality rates, preterm birth in pregnant women with COVID-19 [2].
Various viral diseases can cause congenital or acquired, unilateral or bilateral hearing loss. These viral agents can harm inner ear structures directly or activate inflammatory processes that causing hearing loss. Virus-induced hearing loss can be mild, severe to profound and mainly sensorineural. Virus can cause direct destruction to inner ear structures, especially inner ear hair cells and maybe as measles that destroys organ of Corti, or via activation of host immune-mediation devastation [4].
Otoacoustic emissions (OAE) are acoustic featured signals resulting from outer hair cells (OHC) activity in the inner ear. OAEs maintain an objective, exact and easy instrument for evaluating the OHC and the cochlea [5]. OAEs are perfect instruments to obtain the early indication of an even subtle cochlear damage that was not accessible with pure tone audiometry [6]. By taking advantage of these features of OAEs, we aimed to evaluate the effects of COVID-19 on cochlear functions of infants born to mothers exposed to COVID-19 infection during pregnancy by OAEs. To the best of our knowledge this is the first study to examine the cochlear functions of infants exposed to COVID-19 in utero.
2. Materials and methods
2.1. Patients and data collection
This cross-sectional study was conducted on infants who applied to Malatya Training and Research Hospital neonatal hearing screening service and passed the screening ABR. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was conducted after obtaining approval of the Ministry of Health in Turkey and approval of the ethics committee in Malatya Clinical Research Ethics (Ethical number 2020/207) before the study. Thirty-seven infants whose mother was pregnant between March 2020 and December 2020 and were born after the diagnosis of COVID- 19 during pregnancy and 36 healthy infants whose mother had no diagnosis of COVID-19 or other disease during her pregnancy were included in the study. Birth weight, maternal age, gender, type of delivery were recorded for all participants and the trimester of pregnancy of the mother during the period of COVID-19, fever, cough, shortness of breath, olfactory dysfunctions, diarrhea, myalgia, headache, runny nose, sore throat symptoms were questioned in the patient group. A detailed ear, nose and throat examination was performed for all infants included in the study before the test. Tympanogram and acoustic reflexes were obtained for both ears with 226 Hz probe tone frequency in all participants. Infants with diseases related to the outer ear and middle ear, those who have not undergone neonatal hearing screening, subjects who have received postnatal intensive care treatment, participants who have hereditary hearing loss in their family, those whose mother had TORCH group infection during pregnancy, infants with auricular and external auditory canal anomalies, those with a birth weight below 1500 g, babies born before 37th week and with hyperbilirubinemia requiring hospitalization were excluded from the study. Transient evoked otoacoustic emission (TEOAE), Distortion Product Otoacoustic Emission (DPOAE) and Contralateral Suppression of OAE (CLS OAE) tests were performed on all infants. All audiologic measurements were performed in a sound-proof room. The present study was carried out in a blind manner that the audiologist who performed the OAEs was not informed whether the babies were patient or healthy control. Inclusion criteria were infants with type A tympanogram, normal neonatal hearing screening ABR bilaterally, and had no additional disease. Written informed consent was obtained from all participating parents.
2.2. TEOAE test
The test was carried out by using the EP15 Eclipse Module device and OtoAccess™ database software program (Interacoustics, Middelfart Denmark) binaurally. Signal, noise, signal to noise ratio (S/N-R) values obtained from TEOAE tests were used as study parameters. TEOAE measurements were carried out at 1000–15000–2000–3000–4000 Hz frequencies. A linear click TEOAE stimulus with an intensity of 80 ± 3 dB SPL was delivered for one ear. The presence of TEOAE measurement method is the normal case of standard; reconstructed reproducibility value was accepted only when better than 70%. Stimulus stability was >80%.
2.3. DPOAE test
The test was carried out by using the EP15 Eclipse Module device and OtoAccess™ database software program (Interacoustics, Middelfart Denmark) binaurally. DPOAE test was applied at frequencies of 1000–2000–4000-6000 Hz. Signal, noise, signal to noise ratio (S/N-R) values obtained from DPOAE tests were used as study parameters. For DPOAEs two simultaneous pure-tone signals were presented to the ear at two different frequencies (f1 and f2, where f2 > f1). Recordings were obtained with a frequency ratio f2/f1 fixed at 1.22.
2.4. Contralateral suppression of OAE (CLS OAE) tests
TEOAE test parameters were used for the measurement of CLS with TEOAE test. Measurement was done bilaterally. A linear click TEOAE stimulus with an intensity of 80 ± 3 dB SPL was given from one ear, and a 60 dB SPL white noise was delivered to the contralateral ear in linear stimulus mode for efferent auditory system suppression and TEOAE recording repeated. A contralateral white noise stimulus was formed by the same audiometer and delivered by insert earphones. TEOAE responses were compared with the contralateral suppression responses by measuring them binaurally. Signal, noise and S/N-R values of both tests were examined as study parameters. Signal and S/N-R suppression amplitude were calculated by subtracting the TEOAE results detected in the presence of noise from the TEOAE responses recorded under silent condition. Those with positive suppression amplitudes were classified as ‘there is suppression’ and those with negative values were classified as ‘no suppression’. The frequency of the signal suppressions for each patient was calculated.
3. Statistical analysis
Data were summarized by mean ± standard deviation, median (min-max), and count (percentage). Conformity to normal distribution was made using the Shapiro-Wilk test. In statistical analysis, the Mann-Whitney U test, Independent samples t-test, Continuity Correction, Fisher's exact, and Pearson chi-square test were used where appropriate. The p-value of <0.05 was considered statistically significant. IBM SPSS Statistics 26.0 program was used for analysis.
4. Results
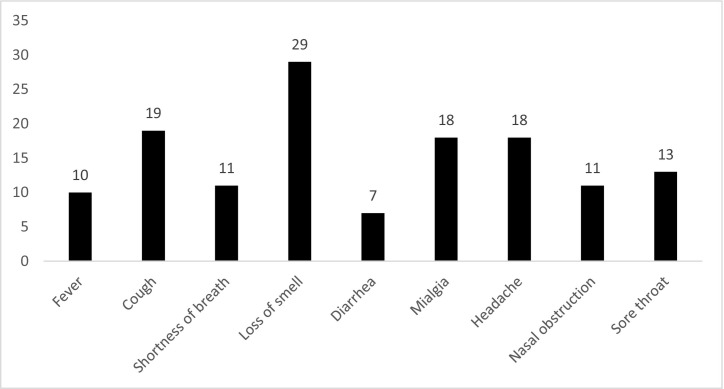
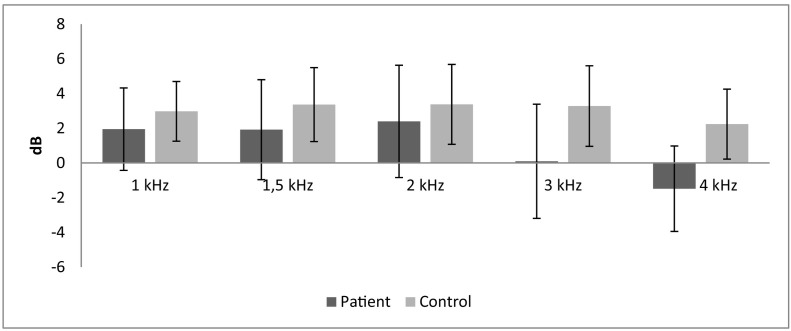
Demographics including birth weight, gender, maternal age, etc. are summarized in Table 1 . Demographic data were similar for both groups. We observed that 67.6% of the mothers had COVID-19 during their third trimester of pregnancy and the rest of them (32.4%) were in the second trimester while they had the disease in the patient group. Loss of smell (78.4%) and cough (51.4%) were the most frequent maternal COVID-19 symptoms in the patient group (Table 2 ). According to the TEOAE results of patients and controls in the silent, a statistically significant difference was observed between the two groups at 3 kHz and 4 kHz (p < 0.05). When the two groups were compared, amplitude ratios in both groups were close to each other at low frequencies in silence, while the amplitude ratios were lower in the patient group at high frequencies than controls (Table 3 ). DPOAE results of patients and controls are seen in Table 4 . A statistically significant difference was observed between the two groups only at the frequency of 1 kHz (p > 0.001). Statistical significance at a single frequency in DPOAE was not considered as the differential effect of the disease. We think that COVID-19 does not affect this level. Contralateral Suppression of OAE test results of patients and controls a statistically significant difference was found in all frequencies (p < 0.05) (Table 5 ). Suppression was much more effective at all frequencies in the normal group than patient group. This difference was found to be more significant, especially at higher frequencies (2,3 and 4 kHz) (p < 0.001). The contralateral suppression amplitudes of patients and controls are given in the Table 6 and Fig. 1 .There was a difference in the contralateral suppression amplitudes of all frequencies between groups (p < 0.05). This difference was found to be more significant, especially at higher frequencies (3 and 4 kHz) (p < 0.001). TEOAE measurements were also performed in noise for both groups. Accordingly, we found a statistically significant difference between the patient and control groups only at 4 kHz (p < 0.05) which supports that COVID-19 may affect outer hair cells at high frequencies. When the TEOAE, contralateral suppression OAE, DPOAE amplitudes were compared within the patient group according to the trimester variable, we did not observe a statistically significant difference (p > 0.05).
Table 1.
Demographics.
| Group |
||||||
|---|---|---|---|---|---|---|
| Patients |
Controls |
p Value | ||||
| Mean ± SD | Median (min- max) | Mean ± SD | Median (min- max) | |||
| Birth weight | 3167,84 ± 364,8 | 3100 (2500–4160) | 3017,78 ± 466,07 | 3000 (2000–4060) | 0,063 | |
| Maternal age | 29,54 ± 3,98 | 29 (22–36) | 29,22 ± 4,02 | 29,5 (21–36) | 0,782 | |
| Number | Percent | Number | Percent | |||
| Gender | Female | 18 | 48,6% | 15 | 41,7% | 0,716 |
| Male | 19 | 51,4% | 21 | 58,3% | ||
| Delivery | Cesarean | 15 | 40,5% | 11 | 30,6% | 0,518 |
| Normal | 22 | 59,5% | 25 | 69,4% | ||
Table 2.
Maternal COVID-19 symptoms of patient group.
Table 3.
TEOAE results.
| Group |
|||||
|---|---|---|---|---|---|
| Patients |
Controls |
p Value | |||
| Mean ± SD | Median (min- max) | Mean ± SD | Median (min- max) | ||
| 1 kHzH | 18,7 ± 5,85 | 18 (9–31) | 18,83 ± 4,68 | 20 (7–25) | 0,746 |
| 1,5 kHz | 18,82 ± 6,19 | 19 (5–34) | 19,99 ± 3,35 | 20 (12–29) | 0,121 |
| 2 kHz | 17,31 ± 5,25 | 16,5 (8–29) | 17,85 ± 5,3 | 18 (2−31) | 0,285 |
| 3 kHz | 16,03 ± 4,14 | 15 (9–29) | 18,13 ± 4,29 | 19 (10–27) | 0,002 |
| 4 kHz | 8,74 ± 3,73 | 8 (−3−22) | 10,75 ± 4,99 | 11 (−1−22) | 0,004 |
The amplitudes of the transient evoked otoacoustic emissions (TEOAEs) of patients and controls (TEOAE) in the silent are seen in the table above.According to the results, a statistically significant difference was observed between the two groups at 3 kHz and 4 kHz (p < 0.05).
Table 4.
DPOAE results.
| Group |
|||||
|---|---|---|---|---|---|
| Patient |
Control |
p Value | |||
| Mean ± SD | Median (min- max) | Mean ± SD | Median (min- max) | ||
| 1 kHz | 4,04 ± 7,7 | −8,9 (19,7 − 10,04) | 3,04 ± 9,3 | 5,2 (23,3−0) | <0,0001 |
| 2 kHz | 5,47 ± 9,55 | 3,1 (29–12,12) | 4,93 ± 10,1 | 4,3 (22,7–0) | 0,097 |
| 4 kHz | 5,96 ± 11,1 | 2,8 (29,7 − 11,68) | 5,55 ± 10,7 | 3,4 (28,6–0) | 0,688 |
| 6 kHz | 4,75 ± 10,2 | 2,5 (23,5-12,03) | 4,95 ± 9,6 | 3,5 (27,2–0) | 0,451 |
The Distortion Product Otoacoustic Emission (DPOAE) results of patients and controls are seen in the table above. Accordingly, a statistically significant difference was observed between the two groups only at the frequency of 1 kHz (p < 0.001).
Table 5.
Contralateral Supression of OAE test results.
| Suppression | Group |
|||
|---|---|---|---|---|
| Patient |
Control |
p Value | ||
| Count (%) | Count (%) | |||
| 1 kHz | No | 11 (14,86%) | 2 (2,78%) | 0,023 |
| Yes | 63 (85,14%) | 70 (97,22%) | ||
| 1,5 kHz | No | 13 (17,57%) | 2 (2,78%) | 0,008 |
| Yes | 61 (82,43%) | 70 (97,22%) | ||
| 2 kHz | No | 18 (24,32%) | 1 (1,39%) | <0,0001 |
| Yes | 56 (75,68%) | 71 (98,61%) | ||
| 3 kHz | No | 45 (60,81%) | 2 (2,78%) | <0,0001 |
| Yes | 29 (39,19%) | 70 (97,22%) | ||
| 4 kHz | No | 66 (89,19%) | 7 (9,72%) | <0,0001 |
| Yes | 8 (10,81%) | 65 (90,28%) | ||
Contralateral Supression of OAE (CLS OAE) test results of patients and controls are given in the table above. A statistically significant difference was found in all frequencies (p < 0.05). Suppression was much more effective at all frequencies in the normal group than patient group. This difference was found to be more significant especially at higher frequencies (2,3,4 kHz) (p < 0.001).
Table 6.
Contralateral Supression Amplitudes.
| Amplitudes | Group |
||||
|---|---|---|---|---|---|
| Patient |
Control |
p Value | |||
| Mean ± SD | Median (min- max) | Mean ± SD | Median (min- max) | ||
| 1 kHz | 1,95 ± 2,38 | 2 (−8–8) | 2,97 ± 1,72 | 3 (−2–8) | 0,002 |
| 1,5 kHz | 1,92 ± 2,88 | 2 (−7–10) | 3,36 ± 2,13 | 3 (0−11) | 0,005 |
| 2 kHz | 2,39 ± 3,23 | 2 (−7–12) | 3,38 ± 2,3 | 3 (0–11) | 0,026 |
| 3 kHz | 0,09 ± 3,29 | 0 (−9–11) | 3,28 ± 2,32 | 3 (−1−10) | <0,0001 |
| 4 kHz | -1,49 ± 2,46 | -1 (−9–6) | 2,24 ± 2,02 | 2 (−1−11) | <0,0001 |
Fig. 1.
Contralateral supression amplitudes.
The contralateral supression amplitudes of patients and controls are given in the table and figure above. There was a difference in the amplitudes of all frequencies between groups (p < 0.05). This difference was found to be more significant especially at higher frequencies (3,4 kHz) (p < 0.001).
5. Discussion
In this present study according to TEOAE results of patients and controls in the silent, a statistically significant difference was observed between the two groups at 3 kHz and 4 kHz (p < 0.05). When the two groups were compared, amplitude ratios in both groups were close to each other at low frequencies in silence, while the amplitude ratios were lower in the patient group at high frequencies than controls. Contralateral suppression of OAE test results of patients and controls showed a statistically significant difference in all frequencies (p < 0.05). Suppression was much more effective at all frequencies in the normal group than patient group. This difference was found to be more significant, especially at higher frequencies (2,3 and 4 kHz) (p < 0.001). According to these results, we think that SARS-CoV-2 may affect the infant's efferent system, especially at high frequencies. There was a difference in the contralateral suppression amplitudes of all frequencies between groups (p < 0.05). This difference was found to be more significant, especially at higher frequencies (3 and 4 kHz) (p < 0.001). The amplitudes of normal infants were more elevated than patients, particularly at higher frequencies. This suggests that the efferent system works better in normal infants and can suppress noise more accurately than patients.
Viruses including herpes simplex, HIV, hepatitis, measles, rubella, mumps, lassa, enteroviruses can affect cranial nerves, causing sudden sensorineural hearing loss (SSNHL), peripheral facial paralysis, olfactory and/or taste impairments. Neuritis resulting from viral invasion of the cochlear nerves, cochleitis caused by viral invasion of both cochlea and perilymphatic tissues and the stress occurred due to the cross interaction of the inner ear antigen to viral diseases, are the main mechanisms of virus induced SSNHL. Autopsy appears to the sole way to ensure exact proof for clear understanding of the neural injury caused by the virus. Autopsy of patients infected with SARS-Cov-2 have demonstrated hyperemic and edematous brain tissue with neuronal degeneration [[7], [8], [9], [10]].
According to the past corona virüs endemics, there are studies that focused on abortion, prematüre birth and intrauterine growth retardation in pregnancy. There is no scientific data on vertical transmission of the virus. Therefore the complications are thought to be originated from the systemic effects of the disease on mother. The cytokine storm is characterized by the increase of IL-2, IL-7, IL-10 GCSF, Gamma MCP-1, TNF-α that is developing in patients with severe COVID-19 infection. Due to the proinflammatory period seen in the first and third trimester of the pregnancy, it is considered that cytokine storm in pregnants may be much more severe. The cytokine storm is thought to cause neuronal defects in fetuses. Although the SARS-COV-2 has not been shown to pass to the baby, abnormally increased TNF-alpha levels in maternal blood have a toxic effect on embryos in the early embryo period [11,12]. All mothers had standard COVID-19 treatment protocol in our study and none of them required hospitalization and/or intensive care. 67.6% of the mothers had COVID-19 during their third trimester of pregnancy.
The organ of Corti, located at the basilar membrane, is the sensory organ of hearing and is formed by sensory hair cells. There are two types of sensory hair cells; inner and outer. The transmission of electrical inputs from the cochlea to the auditory nervous system and electrical signals to the cochlea from the nervous system takes place through afferent and efferent neural pathways, respectively. The efferent auditory system is essential for human auditory sensation. The medial efferent olivocochlear bunch plays a critical role in auditory attention [13].
Otoacoustic emissions (OAE) are formed by the outer hair cells and detected in the external ear canal. OAE amplitudes can be affected from a sound introduced ipsilateral or contralaterally to abnormal ear. Sound-induced OAE suppression mechanism is carried out by the efferent auditory system. Contralateral acoustic stimulation in normal auditory system is result in inhibition of cochlear activity. Absence of contralateral suppression is an indicator of efferent auditory system dysfunction [14].
Studies on the effects of COVID-19 on hearing are limited in the literature. M.W.M Mustafa showed in his study that COVID-19 had harmful effects on the hair cells in the cochlea even if the disease was asymptomatic. He showed the damage to the outer hair cells by reduced TEOAE amplitudes in COVID-19 positive patients compared with control group. [4]. Kilic et al. reported five COVID-19 positive male patients who presented with the sole complaint of unilateral sudden sensorineural hearing loss [10].
In conclusion, OAEs have become a more frequently used method to measure injury to outer hair cells and seem to be a highly sensitive method to cochlear insult. OAEs suppression is an important clinical instrument for detecting central auditory system inhibitory efferent role on cochlear processes that play a critical task in speech perception. We found low TEOAE amplitudes in patients at high frequencies (3–4 kHz) and weak contralateral suppression activity of patients, especially at higher frequencies (2,3,4 kHz). Our results suggest an insufficiency in medial olivocochlear efferent system in infants exposed to SARS-CoV-2 intrauterine. Cochlear functions should be examined in infants whose mothers had COVID-19, even if they are asymptomatic during their pregnancy. In our literature search, we could not find any publication examining the effects of COVID-19 on cochlear functions of newborns exposed to SARS-CoV-2 intrauterine. In this respect, although our patient population is small we believe that our article will contribute to the literature.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was conducted after obtaining approval of the Ministry of Health in Turkey and approval of the ethics committee in Malatya Clinical Research Ethics (Ethical number 2020/207).
Informed consent
Informed consent was obtained from all participants included in this study.
Declaration of competing interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;13(7(1)):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozcan H., Elkoca A., Yalçın O. COVID-19 infection and its effects on pregnancy. Anatolian Clinic Journal of Medical Sciences. 2020;25(special issue on COVID-19):43–50. [Google Scholar]
- 3.Turan O., Hakim A., Dashraath P., WJL Jeslyn, Wright A., Abdul-Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: a systematic review. Int J Gynaecol Obstet. 2020;151(1):7–16. doi: 10.1002/ijgo.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41(3) doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimatore G., Cavagnaro M., Skarzynski P.H., Fetoni A.R., Hatzopoulos S. Detection of age-related hearing losses (ARHL) via transient-evoked otoacoustic emissions. Clin Interv Aging. 2020;15:927–935. doi: 10.2147/CIA.S252837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shupak A., Tal D., Sharoni Z., Oren M., Ravid A., Pratt H. Otoacousticemissions in early noise-induced hearing loss. Otol Neurotol. 2007;28(6):745–752. doi: 10.1097/MAO.0b013e3180a726c9. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy P.G. Herpes simplex virus type 1 and Bell’s palsy-a current assessment of the controversy. J Neurovirol. 2010;16(1):1–5. doi: 10.3109/13550284.2010.522868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals.Trends Hear 2014;18:2331216514541361. [DOI] [PMC free article] [PubMed]
- 9.Mateer E.J., Huang C., Shehu N.Y., Paessler S. Lassa fever-induced sensorineural hearing loss: a neglected public health and social burden. PLoS Negl Trop Dis. 2018;12(2) doi: 10.1371/journal.pntd.0006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilic O., Kalcioglu M.T., Cag Y., Tuysuz O., Pektas E., Caskurlu H., et al. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis. 2020;97:208–211. doi: 10.1016/j.ijid.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dursun P. Maternal and fetal effects of SARS-CoV-2 (COVID-19) Journal of Health Science Yuksek Ihtisas University. 2020;1:73–77. [Google Scholar]
- 13.Joseph J., Suman A., Jayasree G.K., Prabhu P. Evaluation of contralateral suppression of otoacoustic emissions in Bharatanatyam dancers and non-dancers. J Int Adv Otol. 2019;15(1):118–120. doi: 10.5152/iao.2018.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugur A.K., Kemaloglu Y.K., Ugur M.B., Gunduz B., Saridogan C., Yesilkaya E. Otoacoustic emissions and effects of contralateral white noise stimulation on transient evoked otoacoustic emissions in diabetic children. Int J Pediatr Otorhinolaryngol. 2009;73(4):555–559. doi: 10.1016/j.ijporl.2008.12.002. [DOI] [PubMed] [Google Scholar]