Highlights
-
•
Re-irradiation of the brain is feasible with an encouraging overall survival.
-
•
Treatment related toxicity was low within the reported dose range.
-
•
EQD2 cumulative dose distributions were calculated using rigid registration.
-
•
Dose-Response-Modelling with logistic regression showed a correlation of the D1cc brain with any grade of acute toxicity.
Keywords: Re-irradiation, Brain neoplasms, Organs at risk, Glioblastoma, Cranial irradiation
Abstract
Introduction
The use of cranial re-irradiation is growing with improving overall survival and the advent of high-precision radiotherapy techniques. Still the value of re-irradiation needs careful evaluation regarding safety and efficacy. We analyzed dosimetric and clinical data of patients receiving cranial re-irradiation using EQD2 sum plans.
Methods and material
We retrospectively analyzed the data of 76 patients who received repeated cranial radiotherapy from 02/2013 to 09/2016. 34 patients suffered from recurrent primary brain tumors, 42 from brain metastases. Dosimetric analysis was performed accumulating EQD2 dose distributions based on rigid image registration. Clinical and radiological data was collected at follow-ups including toxicity, local control and overall survival.
Results
In total 76 patients had at least 2 courses of intracranial radiotherapy. The median accumulated prescription EQD2 dose was 96.5 Gy2 for all radiation courses combined. The median D(0.1 cc) of the brain for patients receiving more than 100 Gy2 was 114 Gy2 with a highest dose of 161.5 Gy2. 74% of patients suffered from low grade (G1–G2) acute toxicity, only two high grade (>G3) toxicities were recorded.
Median overall survival from the time of first re-irradiation was 57 weeks (range 4–186 weeks). The median time to local failure for patients with a primary brain tumor was not reached and 24 weeks (range 1–77 weeks) for patients with brain metastases.
Conclusion
Repeated radiotherapy appears both safe and efficient in patients with recurrent primary or secondary brain tumors with doses to the brain up to 120 Gy2 EQD2, doses below 100 Gy2 for brainstem and doses below 75 Gy2 EQD2 to chiasm and optic nerves.
1. Introduction
Intracranial recurrence either in-field or in the vicinity after an initial local radiotherapy treatment is observed in a significant portion of patients treated for primary brain tumors and with longer follow-up also in patients with brain metastases [1], [2], [3]. Re-irradiation is considered a valuable salvage option in these challenging situations. With the development of stereotactic and image-guided radiotherapy techniques re-irradiation for localized volumes has become possible while simultaneously optimally sparing organs at risk (OAR) [4]. Mostly retrospective clinical data has been reported and published over the past two decades with generally good efficacy and acceptable toxicity [5], [6], [7], [8], [9], [10]. Only few prospective studies have been performed in the field of cranial re-irradiation [11], [12], [13]. Interestingly, although radiobiological dose-response modelling of CNS re-irradiation exists based on clinical published data [14], [15], no true dose accumulation and biologically effective dose re-calculation has been performed to derive safe dose recommendations for re-irradiation. In this retrospective analysis we therefore evaluated dosimetric data of patients that received re-irradiation of the brain by generating accumulated equivalent uniform doses with rigid-registration of all intracranial treatments and investigated clinical outcome of normal tissue toxicity.
2. Methods and material
2.1. Patient characteristics
Between February 2013 and September 2016, 76 patients were treated with re-irradiation for primary or metastatic brain tumors at the Department of Radiation Oncology of the University Hospital of Zurich. A re-irradiation was defined as two administered doses overlapping significantly within at least the 50% isodose. The analysis was approved by the local ethics committee of Zurich (BASEC-Nr: 2017-01027). 34 patients were treated for primary brain tumors, 42 patients had brain metastases. Prior to treatment all patients were discussed in a multidisciplinary tumor board. Further patient and treatment characteristics are shown in Table 1.
Table 1.
Patient and treatment characteristics.
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 43 | 56.6 |
| Female | 33 | 43.4 |
| Age at first Re-RT | ||
| Median (range) | 55 years (18–83 years) | |
| ECOG at first Re-RT | ||
| 0–1 | 48 | 63.2 |
| 2–3 | 7 | 9.2 |
| 4–5 | 0 | 0 |
| Unknown | 21 | 27.6 |
| Tumor Entitiy | ||
| Primary brain tumor | 34 | 44.7 |
| Brain Metastases | 42 | 55.3 |
| Primary Braintumor | ||
| Glioblastoma (GBM) | 16 | 47.1 |
| Oligodendroglioma | 2 | 5.9 |
| Astrocytoma | 5 | 14.7 |
| Ependymoma | 2 | 5.9 |
| Meningeoma | 5 | 14.7 |
| Others | 4 | 11.8 |
| Primary Tumor (Brain metastases) | ||
| Small cell Lung Cancer (SCLC) | 4 | 9.5 |
| Non-small cell Lung Cancer (NSCLC) | 22 | 52.4 |
| Melanoma | 5 | 11.9 |
| Breast | 2 | 4.8 |
| Renal cell carcinoma | 2 | 4.8 |
| Others | 7 | 16.7 |
| Initial WHO Grade (primary brain tumor) | ||
| 1 | 1 | 2.9 |
| 2 | 4 | 11.8 |
| 3 | 11 | 32.3 |
| 4 | 17 | 50 |
| Unknown | 1 | 2.9 |
| Initial UICC Stage (Brain metastases) | ||
| 1 | 0 | 0 |
| 2 | 5 | 11.9 |
| 3 | 8 | 19 |
| 4 | 25 | 59.5 |
| Unknown | 4 | 9.5 |
| Additional systemic therapy at first re-RT | ||
| Yes | 31 | 40.8 |
| No | 45 | 59.2 |
| Additional surgery at first re-RT | ||
| Yes | 10 | 13.2 |
| No | 66 | 86.8 |
| Number of repeated RT courses | ||
| 2 | 76 | 100 |
| 3 | 23 | 30.3 |
| 4 | 8 | 10.5 |
| 5 | 3 | 3.9 |
| Time interval between RT Courses | ||
| 1st–2nd course: median (range) | 56 months (3–1901 months) | |
| 2nd–3rd course: median (range) | 30 months (2–112 months) | |
| 3rd–4th course: median (range) | 24 months (11–29 months) | |
| 4th–5th course: median (range) | 12 months (9–19 months) | |
| Characteristics for the planning target volume | ||||||||||
| course | 1st (n = 76) | 2nd (n = 76) | 3rd (n = 23) | 4th (n = 8) | 5th (n = 3) | |||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| Volume (cc) | 78.15 | 0.2–436.4 | 14.2 | 0.1–2006.8 | 7.7 | 0.2–1653.2 | 4 | 0.4–14.7 | 1.2 | 1.1–1.3 |
| dose/fraction mean (Gy) | 2.5 | 1.8–20 | 3.5 | 2–20 | 5 | 2.5–20 | 5 | 3–20 | 3 | 2.5–20 |
| EQD2 mean (Gy) | 48.3 | 23.3–60 | 37.5 | 20–55.9 | 37.5 | 23.33–100 | 40 | 32.5–50 | 32.5 | 31.25–50 |
2.2. Radiation treatment planning and delivery for first and re-irradiation
Planning computer tomography (CT) was acquired in treatment position and setup with contrast i.v. injection if possible with a slice thickness of 0.6–1 mm. For Patients receiving a non-stereotactic radiation a thermoplastic mask, for patients receiving stereotactic radiotherapy (SRT) or stereotactic radiosurgery (SRS) a dedicated stereotactic mask system (Brainlab ®, CIVCO®) was used. A planning magnetic resonance imaging (MRI) with a dedicated volumetric contrast enhanced T1 sequence was acquired. For primary brain tumors an additional FET-PET in treatment position was acquired as well.
Gross tumor volume (GTV) was contoured as the visible tumor in the planning MRI/CT supplemented by information from i.v. contrast or further imaging including FET PET.
For non-stereotactic treatment techniques in the first series for primary brain tumors, an additional clinical target volume (CTV) was generated around the GTV. An additional 3 mm margin was added to derive the planning target volume (PTV). In case of whole brain radiotherapy for brain metastases as one of the delivered series the brain was contoured as the CTV and an additional 3 mm PTV margin was added.
For stereotactic treatment techniques a 1 mm (definite RT) or 2 mm (postoperative RT) was added to the GTV to generate the PTV in the case of re-irradiation of brain metastases. For re-irradiation of primary brain tumors a dedicated PTV margin of 3 mm was applied.
All plans were calculated by a radiation therapy technologist using our institutional target prescription standards and the respective constraints for the organs at risk and were finally reviewed by a board certified physicist and a board certified radiation oncologist. For treatment planning, Eclipse software (Varian Medical Systems, Version 10–15) was used.
2.3. EQD2 sum plans and statistical analysis
Equivalent uniform dose in 2-Gy fraction (EQD2) sum plans were calculated for all courses of brain radiotherapy using MIM (MIM Software Inc. Version 6.7.9). The CT scans, dose distribution and the structure sets of all relevant courses were exported from ARIA to MIM. Then the EQD2 doses of each course were estimated in MIM. After inserting the number of fractions, the structures were matched with their different α/β-values and the equivalent uniform dose. To calculate the EQD2 the following formula was used:
where n is the number of fractions and d the fraction dose. Different EQD2 parameters were calculated based on different dose metrics (e.g. Dmean, D1cc etc.).
The α/β-values chosen were 10 Gy for GTV, 2 Gy for the myelon, brainstem and the optical nerve and 3 Gy for the body contour. Thereafter, EQD2 was calculated for the different structures in MIM and then transferred back to ARIA/Eclipse for final dose accumulation and further detailed dose-volume analysis.
The EQD2 plans were matched in Eclipse treatment planning system using rigid automatic bone match (translation and rotations) image registration. All organs at risk (OARs) were contoured on the most recent CT scan.
Dose parameters were then extracted for OARs (brain as defined as “whole brain minus GTV”, brainstem, chiasm, right and left optic nerves) and target volumes.
2.4. Endpoints and toxicity definitions
Regarding acute treatment related toxicity all patients were monitored daily during treatment. Follow-up 6 weeks after completion of RT and every 3–4 months thereafter included physical examination and CT and/or MRI until tumor progression.
Toxicity was scored according to the National Cancer Institute CTCAE v5.0 criteria. Toxicity was defined as either acute (<12 weeks after RT) or late (>12 weeks after RT) toxicity.
Local failure of a lesion was defined as either reappearance after complete remission or re-growth after initial partial response in follow-up CT or MRI scans.
Overall survival (OS) was calculated from first re-irradiation until death or last follow-up, progression free survival (PFS) from RT until tumor relapse or last follow-up and local control from end of RT until last imaging follow-up.
2.5. Statistical analysis
For radiation treatment parameters, descriptive statistics, e.g. median, maximum and minimum values were calculated. Occurrence of toxicity of any grade (composite endpoint G1–G4 toxicity) was correlated to whole brain dose parameters using univariable logistic regression. OS and PFS were calculated according to the Kaplan-Meier method. For statistical analysis, SPSS version 25 and R version 3.5.0 were used.
3. Results
3.1. Radiation treatment
76 patients received at least 2 courses of intracranial RT. Twenty-three of those patients had a third, 8 a fourth and 3 a fifth repeated course of RT. In total 99 in-field re-irradiations (re-RT) were administered, and 11 patients received a second in-field re-RT course. To access as much clinical data as possible we pooled patients with primary brain tumors and brain metastases, as shown in Table 1.
The median single fraction dose for the first RT of primary brain tumors was 2 Gy (range 1.8–2 Gy), the median cumulative dose was 60 Gy (range 24–60 Gy). For the first re-RT the median doses were 3 Gy (range: 2–18 Gy) and 30 Gy (range 18–48 Gy), respectively. In patients with brain metastases the median single fraction dose for the first RT was 5 Gy (range 2–20 Gy) and the median cumulative dose was 30 Gy (range 20–60 Gy). The doses for the first re-RT were 5 Gy (range 2–20 Gy) and 30 Gy (range 15–55 Gy), respectively. A detailed overview of all administered radiation doses in the re-irradiation situation is summarized in Table 1.
Additional systemic therapy or surgery was given in up to 85% of patients. During the first three treatment courses in each case between 40 and 46% of patients received an additional systemic therapy. Notably, the percentage of patients receiving additional surgery decreased with every repeated treatment course.
The median time interval between the first and second RT course was 56 weeks (range 3–1901 weeks), between the second and the third course 30 weeks (range 2–112 weeks), between the third and the fourth course 24 weeks (range 11–29 weeks) and between the fourth and the fifth course 12 weeks (range 9–19 weeks).
3.2. EQD2 statistics
Accumulated equivalent uniform doses were generated to analyze the dose statistics. The median prescription dose (EQD2) was 96.5 Gy10 for all RT courses combined and 48.3 Gy10,(n = 76), 37.5 Gy10 (n = 76), 37.5 Gy10 (n = 23), 40 Gy10 (n = 8) and 32.5 Gy10 (n = 3) for the individual RT courses, respectively.
Further dose and dose-volume parameters for the PTV of each course individually are shown in Table 1.
The median Dmean for the brain was 35 Gy3 (range 0.9–57.7 Gy) with a median D1cc of 99.1 Gy3 (range 40.9–142.2). The median D1cc of the brainstem was 38.4 Gy2 (range 0.1–94.6 Gy) and the median D0.1 cc for the chiasm was 33.2 Gy2 (range 0.04–72.2 Gy). Further dose parameters for intracranial OARs are shown in Table 2.
Table 2.
EQD2 dose statistics for the organs at risk.
| Median | Maximum | Minimum | |||
|---|---|---|---|---|---|
| Brain |
0.1 cc > 80 Gy3 EQD2 (n = 69) |
D(0.1 cc) in Gy3 EQD2 | 107.40 | 161.50 | 80.03 |
| D(mean) in Gy3 EQD2 | 34.40 | 50.80 | 0.90 | ||
| 0.1 cc > 90 Gy3 EQD2 (n = 62) | D(0.1 cc) in Gy3 EQD2 | 108.37 | 161.50 | 90.26 | |
| D(mean) in Gy3 EQD2 | 34.16 | 50.80 | 0.90 | ||
| 0.1 cc > 100 Gy3 EQD2 (n = 46) | D(0.1 cc) in Gy3 EQD2 | 114.00 | 161.50 | 100.1 | |
| D(mean) in Gy3 EQD2 | 33.30 | 50.80 | 0.90 | ||
| Brainstem | 0.1 cc > 70 Gy3 EQD2 (n = 12) | D(0.1 cc) in Gy3 EQD2 | 86.07 | 100.68 | 76.33 |
| D(mean) in Gy3 EQD2 | 37.81 | 72.14 | 9.35 | ||
| 0.1 cc > 80 Gy3 EQD2 (n = 9) | D(0.1 cc) in Gy3 EQD2 | 90.36 | 100.68 | 80.57 | |
| D(mean) in Gy3 EQD2 | 41.60 | 72.14 | 15.91 | ||
| Chiasm | 0.01 cc > 50 Gy3 EQD2 (n = 11) | D(0.01 cc) in Gy3 EQD2 | 57.71 | 76.01 | 50.52 |
| D(mean) in Gy3 EQD2 | 47.97 | 70.61 | 32.53 | ||
| 0.01 cc > 70 Gy3 EQD2 (n = 2) | D(0.01 cc) in Gy3 EQD2 | 75.21 | 76.01 | 74.41 | |
| D(mean) in Gy3 EQD2 | 53.17 | 70.61 | 35.72 | ||
| Optical Nerve right | 0.01 cc > 50 Gy3 EQD2 (n = 5) | D(0.01 cc) in Gy3 EQD2 | 56.67 | 59.11 | 51.32 |
| D(mean) in Gy3 EQD2 | 45.96 | 50.20 | 39.02 | ||
| Optical Nerve left | 0.01 cc > 50 Gy3 EQD2 (n = 6) | D(0.01 cc) in Gy3 EQD2 | 57.08 | 58.02 | 51.80 |
| D(mean) in Gy3 EQD2 | 34.25 | 43.48 | 26.68 | ||
3.3. Clinical outcome and toxicity
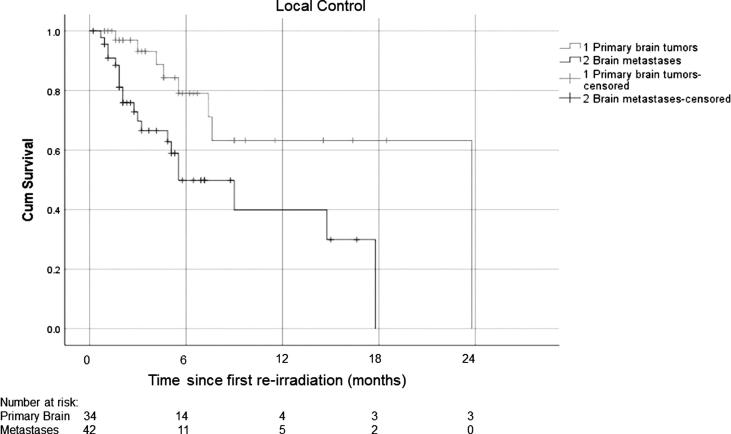
At the time of analysis, 40 out of 76 patients were still alive. The median OS for patients with brain metastases was 78 weeks (range: 4–186 weeks; Fig. 1) and the median time to local failure after Re-RT was 24 weeks (range 1–77 weeks; Fig. 2). Patients with brain tumors had a median OS of 54 weeks (range: 7–166 weeks; Fig. 1) and the median time to local failure after re-RT was not reached.
Fig. 1.
Survival of patients after first reirradiation (n = 76; 1: primary brain tumors, 2: brain metastasis.
Fig. 2.
Local control after re-irradiation, (n = 76; 1: primary brain tumors, 2: brain metastasis).
74% of patients suffered from low grade (G1–G2) acute toxicity, usually in the form of headache (18.4%) and fatigue (19.7%) or edema (21.1%). There were only two patients suffering from G3–G4 toxicity due to newly developing seizures. One patient died during a course of re-irradiation. There was no significant correlation between any dose variable of the whole brain and any particular type of acute toxicity in logistic regression. Only when we associated the general occurrence of acute toxicity (headache or fatigue or edema or any other type) of any grade with dose variables, there was a significant positive association with D1ccm (odds ratio = 1.033, p = 0.0223).
Twenty-one percent of patients suffered from low grade (G1–G2) late toxicity, usually in the form of headache, fatigue or radiological findings of suspected radionecrosis. There were no G3–G5 late toxicities. Due to the small number of events a logistic regression analysis was not performed.
The acute (<12 weeks) and late (>12 weeks) toxicities are shown in Table 3.
Table 3.
Acute toxicity (<12 weeks) and late toxicity (>12 weeks) after radiotherapy.
| Acute toxicity (<12 weeks) n = 76 |
Late toxicity (>12 weeks) n = 58 |
|||||
|---|---|---|---|---|---|---|
| G1–G2 | G3–G4 | G5 | G1–G2 | G3–G4 | G5 | |
| Seizure | 4 (5.3%) | 2 (2.6%) | 0 | 1 (1.7%) | 0 | 0 |
| Radionecrosis | 4 (5.3%) | 0 | 0 | 5 (8.6%) | 0 | 1 (1.7%) |
| Edema | 16 (21.1%) | 0 | 0 | 8 (13.8%) | 0 | 0 |
| Bleeding | 1 (1.3%) | 0 | 0 | 0 | 0 | 0 |
| Headache | 14 (18.4%) | 0 | 0 | 3 (5.2%) | 0 | 0 |
| Fatigue | 15 (19.7%) | 0 | 0 | 4 (6.9%) | 0 | 0 |
| Others | 2 (2.6%) | 0 | 1 (1.3%) | 1 (1.7%) | 0 | 0 |
| Sum | 56 | 2 | 1 | 22 | 0 | 0 |
4. Discussion
The aim of this study was to analyze the toxicity and efficacy of repeated courses of cranial radiotherapy to primary or secondary recurrent brain lesions. Therefore, we analyzed all patients re-irradiated with regards to clinical outcome and toxicity and generated cumulative EQD2 sum plans for all patients receiving multiple intracranial irradiation courses.
As there are no guidelines regarding the dose schemes for re-irradiation, available published data on this subject is very heterogeneous, especially with regards to dose fractionation and cumulative doses applied [7], [9], [16], [17], [18], [19], [20]. This also applies to the current analysis: depending on total dose in the first RT, modality of the radiation, location, distance to organs at risk and re-irradiation volume individual dose schemes were used. To facilitate analysis and comparison of the dosimetrical data of the patients, accumulated equivalent uniform doses were generated in this study.
In this analysis, the median D0.1 cc of the brain was 105.27 Gy (range 48.3–161.5 Gy). Despite such high cumulative doses only 3 patients (2.3%) had a >G3 acute or late toxicity. We observed 74% with a low-grade toxicity, mainly consisting of edema, headache and fatigue. This is consistent with published data [9], [16], [17], [18], [19]. In addition, only one G3 or greater late toxicity was observed: one patient with a suspected fatal radionecrosis received a cumulative EQD2Gy of only 80.2 Gy at D(0,1cc) of the brain and was later found to harbor both recurrence and signs of radiation necrosis. If considering all organs at risk, maximum doses exceeded 65 Gy also for brainstem (D0.1 cc = 100.68 Gy) and the chiasm (D0.01 cc = 76.01 Gy), although the cumulative doses were much lower compared to the observed brain doses.
In agreement with our findings, published data on re-irradiation reveals that it seems quite well tolerated with acceptable treatment related toxicity [7], [8], [9], [10], [16], [17], [18], [19], [20], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Still, no analysis reports on cumulative radiation doses based on patient individual dose accumulation and respective EQD2Gy dose calculation. Therefore, clear recommendation of safe and tolerable cumulative doses to OARs in the situation of CNS re-RT cannot be derived.
Mayer et al. published an overview based on clinical data on the re-irradiation tolerance of the human brain by comparing several papers on re-RT of gliomas. They analyzed the late adverse effects based on the doses, not the volumes and described a cumulative equivalent dose in 2-Gy fractions of >100 Gy increasing the risk of radionecrosis [14]. Ho et al. analyzed multiple case series. Based on median overall survival and neurological toxicity considering the equivalent uniform doses and the median PTV they recommended that the cumulative total dose in 2-Gy fractions should not exceed 100 Gy [5]. However, this analysis is to our knowledge the first to address a dosimetric analysis by generating accumulated biological effective doses of all radiotherapy treatments performed and reporting the cumulative EQD2 to the target volumes and the respective OARs.
Re-irradiation is increasingly performed but still lacks firm guidelines regarding dose to target volumes and OARs. Scoccianti et al. tried to derive recommendations based on a literature review, concluding that low cumulative equivalent doses depending on the target volume (<65 Gy when <12.5 cc and <36 Gy when >35 cc) would keep the risk of severe side effects lower than 3.5% [21]. Still, this recommendation is solely related to the brain as an OAR and not to other OARs such as brainstem and the optic system. Moreover, as most published data on the efficacy and safety of repeated radiotherapy of the brain is retrospective in nature, caution is warranted with the reported rate of early and late toxicity [14], [5], [6], [7], [8], [9], [10], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. As an exception, Shaw et al. gathered prospective data on single dose radiosurgery treatment in recurrent primary brain tumors or brain metastases which had been irradiated before. Depending on the diameter of the recurrence the dose of the re-irradiation was calculated. High grade toxicity was more common in patients with larger tumors [11].
In this analysis we could find no dose-response relationship between doses to the brain and the different recorded acute toxicity despite the wide range of applied EQD2. The only significant association was observed when all acute toxicities were jointly analyzed. Then a significant correlation with brain D1ccm was observed. Still, this finding should be interpreted with caution due to the large number of tested correlations. For late toxicity, no modelling was performed due to the small number of events. Overall, the rate of side effects in this analysis, regarding doses for organs at risk and the number of re-irradiations, seemed to be acceptable.
Thus, EQD2 up to 100 Gy to the brain and brainstem are feasible and safe, while brain doses up to 120 Gy EQD2appear reasonably possible within the range of target volumes in this series. Beyond 120 Gy EQD2 no firm recommendation is possible due to the very limited number of patients receiving such doses to the brain and brainstem. Due to the cautious application of re-iRT, cumulative doses to the chiasm did not exceed 75 Gy EQD2. No conclusion can be drawn with regards to the optic nerves, as cumulative EQD2 did not exceed doses acceptable in a first line irradiation setting.
Patients with primary brain lesions displayed a median OS from the time of the first Re-RT of 54 weeks (range: 1–77 weeks) which is comparable to other published reports with a range of 8–11 months [7], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. However, most of the studies only included glioblastomas, whereas in our study population WHO grade II and III brain tumors were analyzed as well. Combs et al. differentiated between the histological grading and reported a median OS after Re-RT in patients with glioblastomas of 8 months, in grade III tumors of 16 months and in grade II tumors of 22 months [30]. As the number of patients in our study was smaller, we did not distinguish between the different grades for calculating the median OS.
Patients with brain metastases had a median OS of 78 weeks (range: 4–186 weeks) which is more favorable to other published data for metastatic lesions with a range of 2.8–10.8 months [9], [23], [24], [36], [37], [16], [17], [18], [19]. Although this comparison has to be viewed with caution, it still emphasizes that with careful patient selection very encouraging OS can be achieved.
5. Conclusion
To quantitatively analyze the dose values for target volumes and organs at risk of re-irradiations of the brain, accumulated biological effective doses were generated after rigid image registration of all treatment courses. Despite high cumulative EQD2Gy to the brain and brainstem, only a very low rate of high-grade treatment related toxicities and an encouraging median OS were observed. Cumulative doses up to 100 Gy EQD2Gy to the brainstem and 120 Gy EQD2Gy to the brain appear both safe and efficient in patients with recurrent primary or secondary brain tumors. Larger cohort analyses are necessary to validate the finding that the D1cc brain is correlated with any grade of acute toxicity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Stelzer K.J. Epidemiology and prognosis of brain metastases. Surg Neurol Int. 2013;2(4):192–202. doi: 10.4103/2152-7806.111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallner K.E., Galicich J.H., Krol G., Arbit E., Malkin M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16(6):1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.De Robles P., Fiest K.M., Frolkis A.D., Pringsheim T., Atta C. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-Oncology. 2015;17(6):776–783. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linskey M.E., Andrews D.W., Asher A.L., Burri S.H., Kondziolka D. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho A.L.K., Jena R. Re-irradiation in the brain: primary gliomas. Clin Oncol. 2018;30:124–136. doi: 10.1016/j.clon.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Dörr W., Gabryś D. The principles and practice of re-irradiation in clinical oncology: an overview. Clin Oncol. 2018;30:67–72. doi: 10.1016/j.clon.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Lee J., Cho J., Chang J.H., Suh C.O. Re-irradiation for recurrent gliomas: treatment outcomes and prognostic factors. Yonsei Med J. 2016;57(4):824–830. doi: 10.3349/ymj.2016.57.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazici G., Cengiz M., Ozyigit G., Eren G., Yildiz F. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol. 2014;120:117–123. doi: 10.1007/s11060-014-1524-0. [DOI] [PubMed] [Google Scholar]
- 9.Scharp M., Hauswald H., Bischof M., Debus J., Combs S.E. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: retrospective analysis. Radiat Oncol. 2014;9:4. doi: 10.1186/1748-717X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greto D., Livi L., Bonomo P., Masi L., Detti B. Cyberknife stereotactic radiosurgery for the re-irradiation of brain lesions: a single-centre experience. Radiol Med. 2014;119:721–726. doi: 10.1007/s11547-014-0383-2. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E., Scott C., Souhami L., Dinapoli R., Kline R. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 12.Combs S.E., Kessel K.A., Hesse J., Straube C., Zimmer C. Moving second courses of radiotherapy forward: early re-irradiation after surgical resection for recurrent gliomas improves efficacy with excellent tolerability. Neurosurgery. 2018;83:1241–1248. doi: 10.1093/neuros/nyx629. [DOI] [PubMed] [Google Scholar]
- 13.Straube C., Scherb H., Gempt J., Kirschke J., Zimmer C. Adjuvant stereotactic fractionated radiotherapy to the resection cavity in recurrent glioblastoma – the GlioCave study (NOA 17 – ARO 2016/3-DTK ROG trial) BMC Cancer. 2018;18:15. doi: 10.1186/s12885-017-3928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer R., Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncology Biol Phys. 2008;70(5):1350–1360. doi: 10.1016/j.ijrobp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Nieder C., Andratschke N.H., Grosu A.L. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016;36:4985–4996. doi: 10.21873/anticanres.11067. [DOI] [PubMed] [Google Scholar]
- 16.Bernhardt D., Bozorgmehr F., Adeberg S., Opfermann N., von Eiff D. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. 2016;101:76–81. doi: 10.1016/j.lungcan.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Akiba T., Kunieda E., Kogawa A., Komatsu T., Tamai Y. Re-irradiation for metastatic brain tumors with whole-brain radiotherapy. Jpn J Clin Oncol. 2012;42(4):264–269. doi: 10.1093/jjco/hys007. [DOI] [PubMed] [Google Scholar]
- 18.Son C.H., Jimenez R., Niemierko A., Loeffler J.S., Oh K.S. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):167–172. doi: 10.1016/j.ijrobp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z., Sun B., Shen G., Cha L., Meng X. Brain metastasis reirradiation in patients with advanced breast cancer. J Radiat Res. 2016;1 doi: 10.1093/jrr/rrw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard S.P., Krauze A., Chan M.D., Tsien C., Tomé W.A. The evolving role for re-irradiation in the management of recurrent grade 4 glioma. J Neurooncol. 2017;134:523–530. doi: 10.1007/s11060-017-2392-1. [DOI] [PubMed] [Google Scholar]
- 21.Scoccianti S., Francolini G., Carta G.A., Greto D., Detti B. Re-irradiation as salvage treatment in recurrent glioblastoma: a comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018;126:80–91. doi: 10.1016/j.critrevonc.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Møller S., Munck P., Law I., Poulsen H.S., Engelholm S.A. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother Oncol. 2017;125(2):223–227. doi: 10.1016/j.radonc.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Wong W.W., Schild S.E., Sawyer T.E., Shaw E.G. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34(3):585–590. doi: 10.1016/0360-3016(95)02156-6. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Wahab M.M.R., Wolfson A.H., Raub W., Landy H., Feun L. The role of hyperfractionated re-irradiation in metastatic brain disease: a single institutional trial. Am J Clin Oncol. 1997;20(2):158–160. doi: 10.1097/00000421-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Dincoglan F., Beyzadeoglu M., Sager O., Demiral S., Gamsiz H. Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori. 2015;101:179–184. doi: 10.5301/tj.5000236. [DOI] [PubMed] [Google Scholar]
- 26.Laing R.W., Warrington A.P., Graham J., Britton J., Hines F. Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (phase I/II study) Radiother Oncol. 1993;27:22–29. doi: 10.1016/0167-8140(93)90040-f. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd S.F., Laing R.W., Cosgrove V.P., Warrington A.P., Hines F. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37:393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 28.Hudes R.S., Corn B.W., Werner-Wasik M., Andrews D., Rosenstock J. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Rhys. 1999;43:293–298. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 29.Veninga T., Langendijk H.A., Slotman B.J., Rutten E.H., van der Kogel A.J. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol. 2001;59:127–137. doi: 10.1016/s0167-8140(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 30.Combs S.E., Thilmann C., Edler L., Debus J., Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 31.Vordermark D., Kölbl O., Ruprecht K., Vince G.H., Bratengeier K. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55. doi: 10.1186/1471-2407-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henke G., Paulsen F., Steinbach J.P., Ganswindt U., Isijanov H., Kortmann R.-D. Hypofractionated reirradiation for recurrent malignant gliomaHypofraktionierte Rebestrahlung bei rezidivierten malignen Gliomen. Strahlenther Onkol. 2009;185:235–240. doi: 10.1007/s00066-009-1969-9. [DOI] [PubMed] [Google Scholar]
- 33.Ciammella P., Podgornii A., Galeandro M., D’Abbiero N., Pisanello A. Hypofractionated stereotactic radiation therapy for recurret glioblastoma: single institutional experience. Radiat Oncol. 2013;8:222. doi: 10.1186/1748-717X-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKenzie J.T., Guarnaschelli J.N., Vagal A.S., Warnick R.E., Breneman J.C. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol. 2013;113:403–409. doi: 10.1007/s11060-013-1126-2. [DOI] [PubMed] [Google Scholar]
- 35.Combs S.E., Widmer V., Thilmann C., Hof H., Debus J. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM) Cancer. 2005;104(10):2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 36.Cooper J.S., Steinfeld A.D., Lerch I.A. Cerebral metastases: value of reirradiation in selected patients. Radiology. 1990;174:883–885. doi: 10.1148/radiology.174.3.2305074. [DOI] [PubMed] [Google Scholar]
- 37.Sadikov E., Bezjak A., Yi Q.-L., Wells W., Dawson L., Millar B.-A. Value of whole brain re-irradiation for brain metastases – single centre experience. Clin Oncol (R Coll Radiol) 2007;19:532–538. doi: 10.1016/j.clon.2007.06.001. [DOI] [PubMed] [Google Scholar]