Abstract
Background
Tumor mutational burden (TMB) and density of tumor-infiltrating lymphocytes (TIL) have been postulated as predictive biomarkers for immunotherapy. Therefore, we investigated the concordance of TMB and TIL of primary/extracranial renal cell carcinoma (RCC) specimens and matched brain metastases (BM).
Patients and methods
Twenty specimens from 10 patients were retrieved from the Vienna Brain Metastasis Registry (6/10 primary tumor, 4/10 lung metastasis, 10/10 matched BM). TMB was assessed using the TruSight Oncology 500 gene panel with libraries sequenced on a NextSeq instrument. TIL subsets (CD3+, CD8+, CD45RO+, FOXP3+, PD-L1+) were investigated using immunohistochemistry (Ventana Benchmark Ultra system) and automated tissue analysis (Definiens software).
Results
No significant difference in TMB, CD3+, CD8+, CD45RO+, FOXP3+ or PD-L1+ expression was observed between extracranial and matched intracranial specimens (P > 0.05). Higher CD8+ TIL (P = 0.053) and CD45RO+ TIL (P = 0.030) densities in the primary tumor compared with the intracranial samples were observed in specimens collected after exposure to systemic treatment. Neither extracranial sample origin (lung metastasis versus primary RCC) nor extracranial disease status at BM diagnosis (progressive versus stable disease) were significantly associated with TMB or TIL densities in extracranial and intracranial samples (P > 0.05). No significant correlation was found between the median differences of TMB or TIL densities from extracranial to intracranial samples and BM-free survival.
Conclusion
The comparable immunological microenvironment of extra- and intracranial tumor samples in our study underscores the immunological activation also in BM from RCC, and therefore, supports the development of immune modulatory treatments also in patients with brain metastatic RCC.
Key words: tumor mutational burden in brain metastases, tumor-infiltrating lymphocytes in brain metastases, brain metastases of renal cell carcinoma, tumor microenvironment in renal cell carcinoma
Highlights
-
•
Immunological microenvironment characteristics were comparable in extra- and intracranial RCC samples.
-
•
Numerically higher CD45RO+ and CD8+ TIL densities in extracranial compared to intracranial samples after systemic therapy.
-
•
The inflammatory microenvironment is a promising therapeutic target in RCC BM.
Introduction
Brain metastases (BM) are a severe complication impairing quality of life and survival prognosis of patients suffering from advanced solid cancer; 8%-15% of renal cell carcinoma (RCC) patients develop BM.1 Treatment with immune checkpoint inhibitors has gained increasing importance in clear cell advanced RCC as combined therapies of nivolumab plus ipilimumab and avelumab plus axitinib and pembrolizumab plus axitinib have shown superior outcome compared with standard tyrosine kinase inhibitors as first-line treatment in untreated intermediate and poor-risk RCC patients.2 Axitinib plus pembrolizumab and axitinib plus avelumab are even considered as standard of care for advanced RCC irrespective of the International Metastatic RCC Database Consortium risk status.3,4 However, brain metastatic RCC patients have been excluded from these registration trials resulting in limited knowledge on the antitumor activity of immunotherapy in RCC BM.
The CheckMate 920 phase IIIb/IV investigated the combination of nivolumab and ipilimumab in patients with advanced RCC in high unmet medical need. Here, cohort 3 also allowed the inclusion of RCC patients with asymptomatic BM.5 In 28 BM patients, the overall response rate was 28% and the median progression-free survival was 9 months. Therefore, the overall response rate as well as the progression-free survival in RCC BM patients were inferior to the one reported for RCC patients without BM in the CheckMate 214 trial, with an overall response rate of 42% and progression-free survival of 11.6 months.2 A further phase II trial evaluated the efficacy and safety of nivolumab in brain metastatic RCC patients after at least one prior anti-angiogenic treatment; it included untreated (cohort A) and pretreated (cohort B) BM patients. Interestingly, nivolumab monotherapy was associated with impaired survival prognosis in cohort A compared with cohort B (overall survival at 12 months: 59% versus 67%, respectively), suggesting that focal therapy before immune checkpoint inhibitor therapy needs to be considered in a specific subgroup of RCC BM patients.6 Based on these so far limited clinical efficacies, we aimed to compare characteristics of the inflammatory microenvironment of extracranial RCC with matched BM of the same patient in order to identify potential immune escape mechanisms.
Patients and methods
Patients
A total of 20 matched extracranial and intracranial specimens from 10 patients with brain metastatic clear cell RCC were retrieved from the Vienna Brain Metastasis Registry (6/10 primary renal cell tumor, 4/10 lung metastasis, 10/10 matched BM). Tissue samples containing viable solid cancer tissue of histologically confirmed RCC were included in the present analysis. None of the included patients were treated with an immune checkpoint inhibitor, neither before nor after diagnosis of BM. All the included patients received a radical resection of BM. Clinical characteristics including primary tumor disease status, applied therapies and brain-metastatic-free survival were retrospectively evaluated by chart review.
This study was approved by the ethics committee of the Medical University of Vienna (vote 078/2004).
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks were cut into serial 3 μm slices with a microtome for further immunohistochemical processing. All immunohistochemical analyses were carried out using the Ventana Benchmark Ultra system (Ventana, Tucson, AZ). Immunohistochemistry for T-cell subtypes was carried out as previously published.7,8 PD-L1 expression was assessed by immunohistochemistry using a monoclonal mouse anti-human PD-L1 antibody (1 : 50, Clone 22C3, Dako). Human placenta for PD-L1 and human non-malignant lymph nodes for tumor-infiltrating lymphocytes (TIL) were used as control tissue.
Evaluation of immunohistochemistry
Automated image analyses of TIL density were carried out for CD8+, CD3+, FOXP3+ and CD45RO+ using the Definiens software (Definiens AG, Munich, Germany). Therefore, all slides were digitalized using an automatic slide scanner (Hamamatsu, Herrsching am Ammersee, Germany).
The PD-L1 signal was semi-quantitatively scored based on the percentage of tumor cells that presented with a specific membranous signal as previously described (Tumor Proportion Score).7 Samples with ≥1% distinct membranous anti-PD-L1 labeling of all tumor cells were classified as PD-L1 positive.
Evaluation of tumor mutation burden
Formalin-fixed paraffin-embedded tissue samples were used to analyze the tumor mutational burden (TMB). TMB was assessed using the TruSight Oncology 500 DNA gene panel according to the manufacturer's recommendations. After preparation and quality control of individual libraries, eight libraries were pooled and sequenced on a NextSeq 500 instrument (Illumina) using 100 bp paired-end chemistry.
Statistics
Differences of T-cell subtypes and TMB between extracranial and intracranial samples were calculated by subtracting the results of the extracranial samples from the results of the matched intracranial sample of the same patient. Therefore, a positive difference indicated a higher value in extracranial samples and a negative difference a higher value in intracranial samples. The median difference was used as a cut-off to explore survival correlation.
Data normality was tested using the Shapiro–Wilk test. Differences between two groups were calculated by unpaired, two-sided Mann–Whitney U tests and differences between more than two groups were calculated by Kruskal–Wallis tests.
In order to obtain information on the association of the inflammatory microenvironment in BM and the brain-metastatic-free survival, we used Cox regression models to receive hazard ratios for the differences from extracranial to intracranial TIL subsets and TMB. Brain-metastatic-free survival was defined as time from diagnosis of primary tumor to radiological diagnosis of BM.
A two-sided P value of <0.05 was considered to indicate statistical significance. Due to the hypothesis-generating approach of the current paper, no correction for multiple testing was applied.9
Results
Patients characteristics
Ten BM specimens and 10 matched extracranial RCC tumor specimens from 10 individual patients (male 8/10, 80%; female 2/10, 20%) were available for further analysis. One (10%) patient presented with synchronous diagnosis of BM and primary tumor, while the median BM-free survival of 9/10 (90%) patients with subsequent diagnosed BM was 27 months (1-122 months). Further patients and clinical characteristics are listed in Table 1.
Table 1.
Clinical characteristics of the total patient cohort (N = 10)
| Clinical characteristics | N = 10 | % |
|---|---|---|
| Primary tumor (RCC) | 6 | 60 |
| Lung metastasis from RCC | 4 | 40 |
| IMDC risk score | ||
| Favorable | 5 | 50 |
| Intermediate | 4 | 40 |
| Poor | 1 | 10 |
| Systemic treatment before BM diagnosis | ||
| None | 3 | 30 |
| Performed | 7 | 70 |
| Interferon-based treatment regimes | 6 | 60 |
| Capecitabine-based treatment regimes | 2 | 20 |
| Systemic treatment lines before BM diagnosis | ||
| One line of systemic treatment | 6 | 60 |
| Two lines of systemic treatment | 1 | 10 |
| Median BM-free survival (range) | 27 months (1-122) | |
| BM-free survival | ||
| <2 years | 3 | 15.0 |
| 2-4 years | 5 | 25.0 |
| >4 years | 2 | 10.0 |
| Status of RCC at BM diagnosis | ||
| Stable disease | 5 | 25.0 |
| Progressive disease | 2 | 10.0 |
| Complete response | 1 | 5.0 |
| Partial response | 1 | 5.0 |
BM, brain metastasis; IMDC, International Metastatic RCC Database Consortium; RCC, renal cell carcinoma.
TIL and TMB in extracranial and matched intracranial RCC specimens
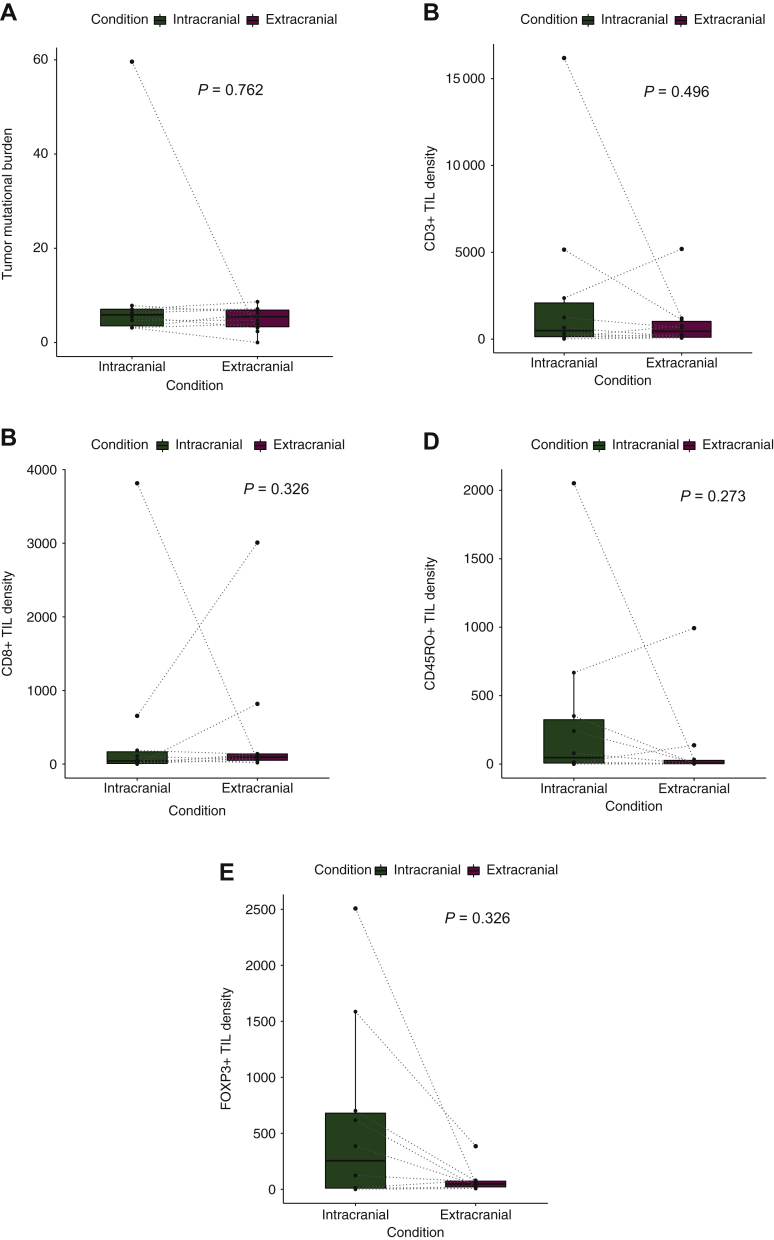
Median TMB was 5.4 mut/Mb (0-8.6 mut/Mb) in the extracranial and 5.9 mut/Mb (3.1-59.6 mut/Mb) in the intracranial samples. No significant difference in TMB was observed from extracranial to matched intracranial specimen (Mann–Whitney U test; P = 0.762; Figure 1A; Table 2).
Figure 1.
Correlation of intracranial with extracranial tumor mutational burden (A), densities of CD3+ TIL (B), CD8+ TIL (C), CD45RO+ TIL (D) and FOXP3+ TIL (E).
TIL, tumor-infiltrating lymphocyte.
Table 2.
Characteristics of the total patient cohort according to intra- and extracranial specimens
| Specimen characteristics | ||
|---|---|---|
| Tumor mutational burden | Mut/Mb | P value |
| Median TMB intracranial (range) | 5.9 (3.1-59.6) | 0.762 |
| Median TMB extracranial (range) | 5.4 (0-8.6) | |
| In RCC specimen | 5.4 (2.4-7.1) | |
| In lung metastasis specimen | 5.5 (0.0-8.6) | |
| Tumor-infiltrating lymphocytes | Cells/mm2 | P value |
|---|---|---|
| Median density of CD8+ TIL (range) intracranial | 43 (1-3815) | 0.326 |
| Median density of CD8+ TIL (range) extracranial | 95 (20-3009) | |
| In RCC specimen | 119 (46-3009) | |
| In lung metastasis specimen | 52 (20-817) | |
| Median density of CD3+ TIL (range) intracranial | 495 (18-16186) | 0.496 |
| Median density of CD3+ TIL (range) extracranial | 454 (70-5192) | |
| In RCC specimen | 453 (71-5192) | |
| In lung metastasis specimen | 616 (70-1206) | |
| Median density of CD45RO+ TIL (range) intracranial | 47 (1-2052) | 0.273 |
| Median density of CD45RO+ TIL (range) extracranial | 6 (2-993) | |
| In RCC specimen | 4 (2-993) | |
| In lung metastasis specimen | 20 (4-138) | |
| Median density of FOXP3+ TIL (range) intracranial | 255 (1-2507) | 0.326 |
| Median density of FOXP3+ TIL (range) extracranial | 47 (7-384) | |
| In RCC specimen | 23 (7-54) | |
| In lung metastasis specimen | 77 (68-384) |
| PD-L1 expression | % | P value |
|---|---|---|
| Median density of PD-L1 expression (range) intracranial | 0 (0-20) | 0.503 |
| Median density of PD-L1 expression (range) extracranial | 0 (0-1) | |
| In RCC specimen | 0 (0-0) | |
| In lung metastasis specimen | 0 (0-1) |
RCC, renal cell carcinoma; TIL, tumor-infiltrating lymphocytes; TMB, tumor mutational burden.
Median density of CD3+, CD8+, CD45RO+, FOXP3+ and PD-L1+ in extracranial and matched intracranial specimens is given in Table 2. Median density of CD3+ (P = 0.496), CD8+ (P = 0.326), CD45RO+ (P = 0.273), FOXP3+ (P = 0.326) and PD-L1 (P = 0.503) did not differ significantly between extracranial and matched intracranial specimens (Mann–Whitney U test; Table 2; Figure 1B-E).
Differences in TMB and TIL density according to clinical parameters
Systemic treatment before BM diagnosis
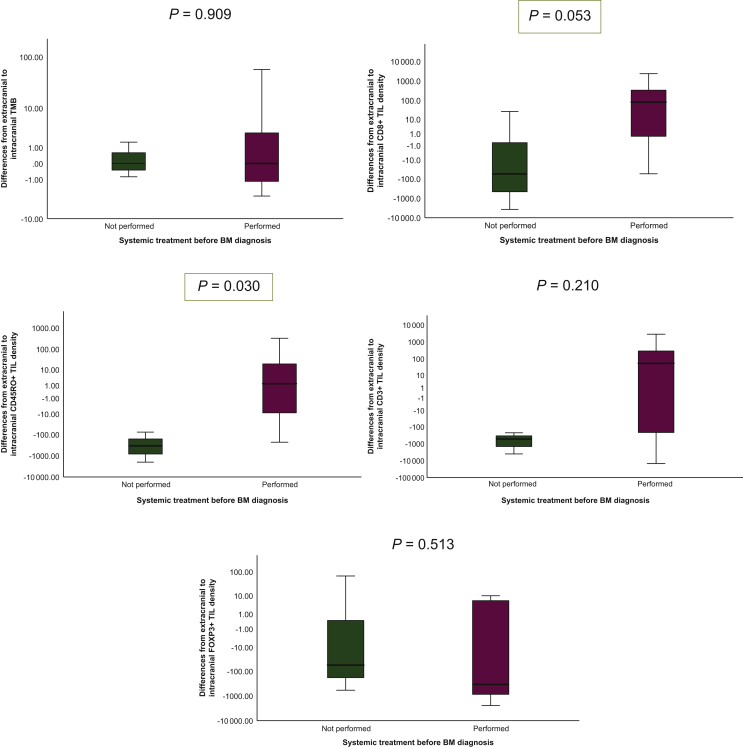
Systemic anticancer treatment was applied between diagnosis of the primary tumor and BM in 7/10 (70%) patients (5/10 patients received interferon-based treatment; 2/10 received capecitabine-based therapy). Systemic treatment before BM diagnosis had no statistically significant impact on the median TMB difference between extracranial and intracranial site (P = 0.909; Mann–Whitney U test; Table 3; Figure 2A). Higher CD8+ TILs (P = 0.053) and CD45RO TILs (P = 0.030) densities were observed in extracranial samples compared with matched intracranial specimens after previous systemic treatment (Mann–Whitney U test; Table 3; Figure 2B and C). No differences in CD3+ and FOXP3+ TIL densities or expression of PD-L1 according to applied systemic treatment was observed (P > 0.05; Mann–Whitney U test; Table 3; Figure 2E).
Table 3.
Association between clinical characteristics with median difference from extra- to intracranial TMB and TIL density
| Clinical characteristics | Median difference from extra- to intracranial specimens (range) |
|||||
|---|---|---|---|---|---|---|
| TMB | CD8+ | CD3+ | CD45RO | FOXP3+ | PD-L1+ | |
| Systemic treatment before BM diagnosis | P = 0.909 | P = 0.053 | P = 0.210 | P = 0.030 | P = 0.425 | P = 0.513 |
| None | −0.005 mut/Mb (−0.8 to 1.5) | −57 cells/mm2 (−3764 to 26) | −537 cells/mm2 (−4044 to −229) | −345 cells/mm2 (−2018 to −77) | −56 cells/mm2 (−591 to 68) | 0.0 % (0 to 0) |
| Performed | −0.001 mut/Mb (−57.3 to −3.1) | 81 cells/mm2 (−55 to 2354) | 53 cells/mm2 (−14980 to 2827) | 2 cells/mm2 (−232 to 325) | −346 cells/mm2 (−2454 to 10) | 0.0 % (0 to −20) |
| Extracranial disease status at BM diagnosis | P = 0.439 | P = 0.699 | P = 0.245 | P = 0.699 | P = 0.245 | P = 0.527 |
| Stable disease | −0.008 mut/Mb (−0.85 to 57.3) | 81 cells/mm2 (−57 to 2354) | 53 cells/mm2 (−596 to 2827) | −12 cells/mm2 (−345 to 325) | 7 cells/mm2 (−2454 to 68) | 0.0 % (0 to 0) |
| Progressive disease | −0.5 mut/Mb (−1.6 tp1.5) | 422 cells/mm2 (26 to 817) | −7604 cells/mm2 (−14979 to −229) | −27 cells/mm2 (−77 to 131) | −896 cells/mm2 (−1201 to −591) | 0.0 % (0 to 0) |
| Extracranial tumor specimens | P = 0.831 | P = 0.201 | P = 0.055 | P = 0.670 | P = 0.831 | P = 0.414 |
| RCC specimen | 0.7 mut/Mb (−3.1 to 57.3) | 79 cells/mm2 (−55 to 2354) | 87 cells/mm2 (−597 to 2827) | −9 cells/mm2 (−233 to 325) | −170 cells/mm2 (−2454 to 10) | 0.0 % (−20 to 0) |
| Lung metastasis | −0.4 mut/Mb (−1.6 to 3.1) | −46 cells/mm2 (−3767 to 817) | −2291 cells/mm2 (−14979 to −80) | −171 cells/mm2 (−2018 to 131) | −341 cells/mm2 (−1201 to 68) | 0.0 % (0 to 0) |
| BM-free survival | P = 0.503 | P = 0.270 | P = 0.369 | P = 0.330 | P = 0.638 | P = 0.738 |
| HR = 0.99; 95% CI 0.95-1.02 | HR = 1.02; 95% CI 0.9-1.01 | HR = 1.0; 95% CI 1.0-1.01 | HR = 0.9; 95% CI 0.9-1.01 | HR = 1.0; 95% CI 0.9-1.01 | HR = 1.01; 95% CI 0.9-1.02 | |
BM, brain metastases; CI, confidence interval; HR, hazard ration; RCC, renal cell carcinoma; TIL, tumor-infiltrating lymphocyte; TMB, tumor mutational burden.
Figure 2.
Association between systemic treatment before brain metastases diagnosis and median differences between extracranial to intracranial tumor mutational burden (A), CD3+ TIL (B), CD8+ TIL (C), CD45RO+ TIL (D) and FOXP3+ TIL (E).
BM, brain metastases; TIL, tumor-infiltrating lymphocyte; TMB, tumor mutational burden.
In accordance with applied systemic treatment before BM diagnosis, no significant differences in TIL densities or TMB values between interferon-based and capecitabine-based treatment regimens were observed (P > 0.05; Mann–Whitney U test). However, numerically higher densities of TIL were observed in samples collected after capecitabine-based compared with interferon-based therapy (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100057).
Extracranial disease status at BM diagnosis
Extracranial disease status was stable in 5/10 (50%) patients and progressive in 2/10 (20%) at time of BM diagnosis. TMB difference from primary tumor to BM did not differ according to primary tumor status at BM diagnosis (P = 0.439; Mann–Whitney U test; Table 3; Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2021.100057). No statistically significant association between extracranial disease status and densities of TIL or PD-L1 expression was observed in the present cohort (P > 0.05; Mann–Whitney U test; Table 3, Supplementary Figure S1B-E, available at https://doi.org/10.1016/j.esmoop.2021.100057).
Extracranial samples—primary tumor versus lung metastasis
Analysis included 6/10 (60%) extracranial samples from the primary kidney tumor and 4/10 (40%) extracranial samples from a resected lung metastasis. No statistical significance was found in median TMB difference between extracranial and intracranial samples according to extracranial specimen origin (lung metastasis versus primary tumor; P = 0.831; Mann–Whitney U test; Table 3, Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100057). Furthermore, the median differences from extracranial to intracranial samples according to infiltration of CD3+, CD8+, CD45RO+, FOXP3+ TIL as well as expression of PD-L1+ were also not significantly associated with extracranial tumor origin (P > 0.05; Mann–Whitney U test; Table 3; Supplementary Figure S2B-E, available at https://doi.org/10.1016/j.esmoop.2021.100057).
Differences in TMB and TIL density according to BM-free survival
No significant correlation was found between the median difference of TMB from extracranial to intracranial samples and BM-free survival [hazard ratio (HR) = 0.99; 95% confidence interval (CI) 0.95-1.02; P = 0.503; Cox regression model; Table 3]. Furthermore, no significant association between the median differences of infiltration of CD3+ TIL (HR = 1.0; 95% CI 1.0-1.01; P = 0.369), CD8+ TIL (HR = 1.02; 95% CI 0.9-1.01; P = 0.270), CD45RO+ TIL (HR = 0.9; 95% CI 0.9-1.01; P = 0.330) and FOXP3+ TIL (HR = 1.0; 95% CI 0.9-1.01; P = 0.638) were observed (Cox regression model; Table 3).
The median difference of the expression of PD-L1+ between extracranial and intracranial site was also not statistically significantly associated with BM-free survival (HR = 1.04; 95% CI 0.9-1.2; P = 0.475; Cox regression model; Table 3).
Discussion
Immunological characteristics potentially change throughout the treatment course of RCC. Therefore, we aimed to analyze biomarkers associated with response to immune checkpoint inhibitors in a unique cohort of matched extracranial and intracranial RCC specimens. TMB, density of TILs and expression of PD-L1 showed numerically higher results in RCC BM specimens compared with matched extracranial specimens in the present cohort. Although interpretation of the present results has to be taken with caution due to the small sample size, our findings provide a unique insight in the composition of the inflammatory microenvironment of matched extra- and intracranial RCC specimens and support the further development of immune modulatory strategies for RCC BM.
In addition to PD-L1 expression, high TMB has been postulated as a hallmark of response to immunotherapy in many solid tumors such as non-small-cell lung cancer (NSCLC), melanoma or head and neck cancer.10, 11, 12, 13 In line with our results, TMB has been shown to vary significantly between primary and metastatic sites in the same patient, with higher rates of TMB recorded in metastases than in primary tumor.14 A study of NSCLC specimens presented an increase of TMB in metastatic specimens with the highest in BM samples with 13 Mut/Mb compared with 6 Mut/Mb in primary lung adenocarcinoma. In addition, further differences were identified in the NSCLC metastatic sites with brain and adrenal metastases showing the highest TMB, and bone and liver metastases, the lowest TMB across all metastatic sites.15 However—in contrast with our study—the specimens were not matched for the single patient. Certainly, only limited data on the feasibility of these markers to predict BM response exist as BM patients were frequently excluded from initial large registration trials investigating potential predictive biomarkers. Indeed, BM often present with neurological symptoms and the symptomatic burden as well as the need for steroid treatment to control the perifocal edema are clinically challenging.
Diffusion restriction of systemic agents into the central nervous system was considered a potential obstacle for clinically meaningful intracranial efficacy of immune checkpoint inhibitors, which are unable to cross the blood–brain barrier due to their large molecular weight. However, as shown in several studies, in BM patients the blood–brain barrier is leaky and rather substituted by a blood–tumor barrier with much wider fenestration leading to a higher efflux of fluid.16,17 Furthermore, activated T cells are able to cross even an intact blood–brain barrier.18 Modern registration trials of immune checkpoint inhibitors, including ones for RCC, have therefore allowed the inclusion of asymptomatic BM patients in defined but rather small sub-cohorts. Indeed, in NSCLC and melanoma BM, similar intra- and extracranial response rates were observed for patients with newly diagnosed, asymptomatic BM.19, 20, 21 The limited patient number does not allow a final conclusion on the value of biomarkers to predict intracranial response of immune checkpoint inhibitors and further studies are needed to clarify the role of biomarkers in BM therapy.
Patient-specific characteristics during the brain metastatic cascade as well as therapies applied in between sample acquisition can impact inflammatory characteristics, underscoring the importance of analyzing matched samples. In line, significantly higher CD8+ TIL and CD45RO+ TIL densities in intracranial compared to extracranial specimens collected after application of systemic treatment were observed in our study cohort. On the one hand, data on the impact of systemic therapies on TIL and TBM changes have to be interpreted with caution, as the used treatment in the present cohort—interferon and capecitabine—are not within the initial treatment recommendations for metastatic RCC anymore. Nevertheless, systemic treatment—especially interferon-based therapy—can interact with local inflammatory processes affecting the microenvironment of tumor cells. As immune checkpoint inhibitors are an important new treatment backbone in RCC, the caused inflammation might—as observed in the present cohort after interferon therapy—alter the composition of the BM inflammatory microenvironment. Further studies, specifically focusing on RCC BM after immune checkpoint inhibitor therapy are warranted to investigate changes.22
On the other hand, changes in the inflammatory tumor microenvironment can also be associated with a branched evolution of tumor cells during the course of cancer disease. According to mutation patterns and expressions of tumor cells, genetic sequencing of primary tumor and extracranial metastases with matched intracranial metastases revealed heterogeneity between the sites indicating that metastases can undergo genetic evolution between the sites.23 However, our results showed no statistically significant differences in the investigated inflammatory characteristics between the sites and according to primary tumor status (stable versus progressive) with a potential bias due to the small sample size. Matched pairs are rare and to our best knowledge the present cohort represents the largest cohort of matched extra- and intracranial RCC specimens focusing on inflammatory characteristics. Further larger studies are certainly needed to confirm the discrepancies of TMB in primary and brain metastatic tissue and provide further insight into the immunological evolution as the basis for future clinical trial development.
In conclusion, we could observe numerically higher values of TMB and TIL in intracranial compared with extracranial specimens of advanced RCC samples. These findings are in line with characteristics of TMB and TIL described in other metastatic cancer diseases and support further investigation of local and systemic inflammatory characteristics as well as site-specific TMB in larger cohorts of patients with BM from RCC.
Acknowledgements
We thank Illumina for providing the TruSight Oncology 500 and sequencing reagents.
Funding
This work was supported by the Clinical Division of Oncology/Department of Medicine I, Medical University of Vienna and costs for this project were covered by the research budget.
Disclosure
ASB has research support from Daiichi Sankyo (≤ €10 000), Roche (> €10 000) and honoraria for lectures, consultation or advisory board participation from Roche, Bristol-Meyers Squibb, Merck, Daiichi Sankyo (all < €5000) as well as travel support from Roche, Amgen and AbbVie. GW received restricted travel grants from NX Development Corp. MS received honoraria for ad boards and lectures from Pfizer, Roche, Ipsen, Exelixis, EUSA, Eisai, Alkermes, BMS, MSD and Merck. MP has received research support from Böehringer-Ingelheim, GlaxoSmithKline, Merck Sharp & Dome and Roche and honoraria for lectures, consultation or advisory board participation from Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, AstraZeneca, AbbVie, Lilly, MEDahead, Daiichi Sankyo, Merck Sharp & Dome. All other authors have declared no conflicts of interest.
Footnotes
Note: The results of this study were presented as a poster discussion at the ESMO congress 2019 in Barcelona.
Supplementary data
References
- 1.Massard C., Zonierek J., Gross-Goupil M., Fizazi K., Szczylik C., Escudier B. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol. 2010;21:1027–1031. doi: 10.1093/annonc/mdp411. [DOI] [PubMed] [Google Scholar]
- 2.Motzer R.J., Tannir N.M., McDermott D.F. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini B.I., Plimack E.R., Stus V. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 4.Motzer R.J., Penkov K., Haanen J. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emamekhoo H., Olsen M., Carthon B.C. Safety and efficacy of nivolumab plus ipilimumab (NIVO+IPI) in patients with advanced renal cell carcinoma (aRCC) with brain metastases: interim analysis of CheckMate 920. J Clin Oncol. 2019;37:4517. [Google Scholar]
- 6.Flippot R., Dalban C., Laguerre B. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J Clin Oncol. 2019;37(23):2008–2016. doi: 10.1200/JCO.18.02218. [DOI] [PubMed] [Google Scholar]
- 7.Berghoff A.S., Fuchs E., Ricken G. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5:e1057388. doi: 10.1080/2162402X.2015.1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berghoff A.S., Kiesel B., Widhalm G. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender R., Lange S. Adjusting for multiple testing – when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 10.Greillier L., Tomasini P., Barlesi F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7:639–646. doi: 10.21037/tlcr.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labriola M., Zhu J., Gupta R. Characterization of tumor mutational burden (TMB), PD-L1, and DNA repair genes to assess correlation with immune checkpoint inhibitors (ICIs) response in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2019;37:e16079. [Google Scholar]
- 12.Snyder A., Makarov V., Merghoub T. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrielatou N., Doumas S., Economopoulou P., Foukas P.G., Psyrri A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat Rev. 2020;84:101977. doi: 10.1016/j.ctrv.2020.101977. [DOI] [PubMed] [Google Scholar]
- 14.Schnidrig D., Turajlic S., Litchfield K. Tumour mutational burden: primary versus metastatic tissue creates systematic bias. Immuno-Oncology Technol. 2019;4:8–14. doi: 10.1016/j.iotech.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein M.K., Pandey M., Xiu J. Tumor mutational burden is site specific in non-small-cell lung cancer and is highest in lung adenocarcinoma brain metastases. JCO Precis Oncol. 2019;3:1–-13. doi: 10.1200/PO.18.00376. [DOI] [PubMed] [Google Scholar]
- 16.Percy D.B., Ribot E.J., Chen Y. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol. 2011;46:718–725. doi: 10.1097/RLI.0b013e318226c427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis Phillips G.D., Nishimura M.C., Lacap J.A. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res Treat. 2017;164:581–591. doi: 10.1007/s10549-017-4279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preusser M., Berghoff A.S., Thallinger C., Zielinski C. CECOG educational illustrations: the blood-brain barrier and its relevance for targeted cancer therapies and immuno-oncology. ESMO Open. 2017;2:e000194. doi: 10.1136/esmoopen-2017-000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crinòa L., Bronte G., Bidoli P. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2019;129:35–40. doi: 10.1016/j.lungcan.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Long G.V., Atkinson V., Lo S. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 21.Steindl A., Berghoff A.S. Brain metastases in metastatic cancer: a review of recent advances in systemic therapies. Expert Rev Anticancer Ther. 2020;30:1–15. doi: 10.1080/14737140.2021.1851200. [DOI] [PubMed] [Google Scholar]
- 22.Provance O.K., Lewis-Wambi J. Deciphering the role of interferon alpha signaling and microenvironment crosstalk in inflammatory breast cancer. Breast Cancer Res. 2019;21:59. doi: 10.1186/s13058-019-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brastianos P.K., Carter S.L., Santagata S. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.