Abstract
The anti-inflammatory molecule annexin A1 (ANXA1) determines the ultimate fate of retinal ganglion cell (RGC) in glaucoma. Cytoplasmic and extracellular ANXA1 facilitate resolution of inflammation. However, the nuclear translocation of ANXA1 induces RGC apoptosis in a murine glaucoma model, and the maintenance of ANXA1 secreted in the extracellular environments remains unclear. In this study, we found that intravitreal injection of the recombinant adenovirus vector (Ad)-ATP-binding cassette transporter A1 (ABCA1; carrying full-length ABCA1) improved RGC survival in the ischemia reperfusion (IR) mice model. Upregulation of ABCA1 maintained ANXA1 cytoplasmic location and reduced ANXA1 nuclear translocation, which is due to the decreased binding of ANXA1 with importin β. Moreover, we found that amino acids 903 to 1,344 of ABCA1 interacted with ANXA1 and decreased its nuclear localization. Importantly, intravitreal injection of adenovirus-associated viral (AAV) vector AAV8-ABCA1 (carrying 903 to 1,344 fragments of ABCA1) maintained ANXA1 cytoplasmic location and improved RGC survival in the IR mice model. Thus, overexpression of ABCA1 protects against RGC apoptosis by partially blocking ANXA1 nuclear translocation. This study puts forth a potential gene treatment strategy to prevent RGC apoptosis in glaucoma.
Keywords: retina, retinal ganglion cells, RGCs, ischemia reperfusion, annexin A1, ABCA1
Graphical Abstract
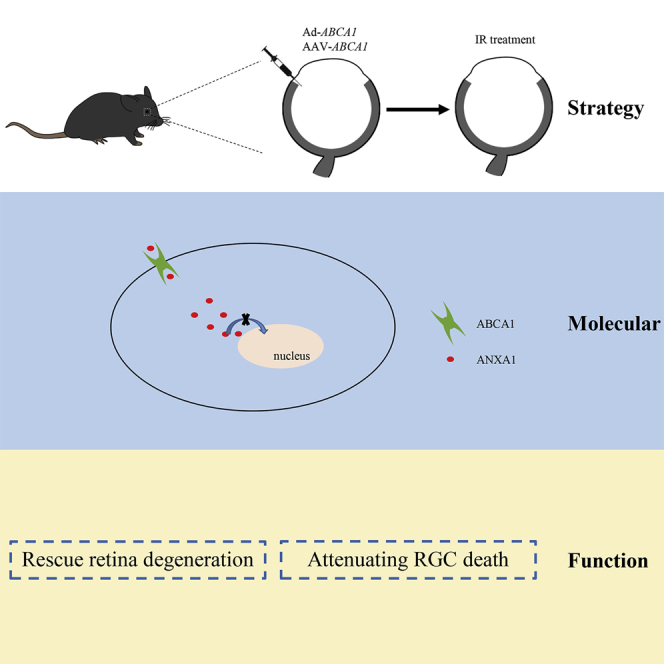
ABCA1 has been identified as a glaucoma risk gene for years. In this study, Zhao and colleagues reported that Ad and AAV, carried with ABCA1 treatment, attenuate retina degeneration in a murine glaucoma model. ABCA1 improved RGC survival via maintaining ANXA1 membrane localization.
Introduction
Glaucoma, one of the severe causes of irreversible blindness worldwide, is characterized by the loss of retinal ganglion cells (RGCs).1 Acute angle-closure glaucoma is an ocular emergency caused by a rapid increase in intraocular pressure (IOP).2 One of the animal models that could mimic acute angle-closure glaucoma is the retinal ischemia reperfusion (IR) injury model.3,4 Both ischemia and reperfusion processes contribute to RGC loss. In the IR model, rapid and transient elevation of IOP induces retinal ischemia and produces reactive oxygen species.5,6
Annexin A1 (ANXA1) is an anti-inflammatory molecule involved in inflammatory diseases.7,8 The subcellular localization of ANXA1 determines cellular fate.9 Following externalization, cytoplasmic ANXA1 interacts with its receptor N-formyl peptide receptor 2 (FPR2), which then activates downstream anti-inflammatory signaling to inhibit phospholipase A2.10,11 However, the nuclear translocation of ANXA1 is associated with cancer progression via regulation of transcription factors and miRNAs.12,13 In neuronal cells, the nuclear translocation of ANXA1 is associated with the induction of cellular apoptosis by regulating transcription factors, such as p53 and p65.14,15 The maintenance of ANXA1 cytoplasmic localization is a critical factor in the RGC fate determination.
ATP-binding cassette (ABC) transporter A1 (ABCA1), a 254-kDa protein, was found associated with ANXA1 externalization.16,17 The expression of ABCA1 was decreased in the IR mice model, and this protein was transcriptionally regulated by the liver X receptor (LXR). Our previous study demonstrated that the LXR agonist upregulated ABCA1 expression, promoted ANXA1 membrane translocation, and reduced RGC apoptosis.18 In addition, the LXR agonist could protect against retinal damage in N-methyl-D-aspartate (NMDA) injury and an autoimmune uveitis animal model.19 However, the mechanism of ABCA1-ANXA1 interactions and ABCA1-mediated ANXA1 subcellular localization is still unclear.
Adenovirus vectors (Ads) and adeno-associated virus (AAV) vectors are the most commonly used vectors for gene therapy.20 However, AAV vectors are limited by their small packaging capacity (considered to be <5 kb). The human ABCA1 is 2,261 amino acids in length, which corresponds to more than 6.7 kb. In this study, we used Ad to carry full-length human ABCA1 (Ad-ABCA1) and AAV to carry an ABCA1 fragment to investigate the effect and mechanism of ABCA1 overexpression on ANXA1 subcellular localization.
Results
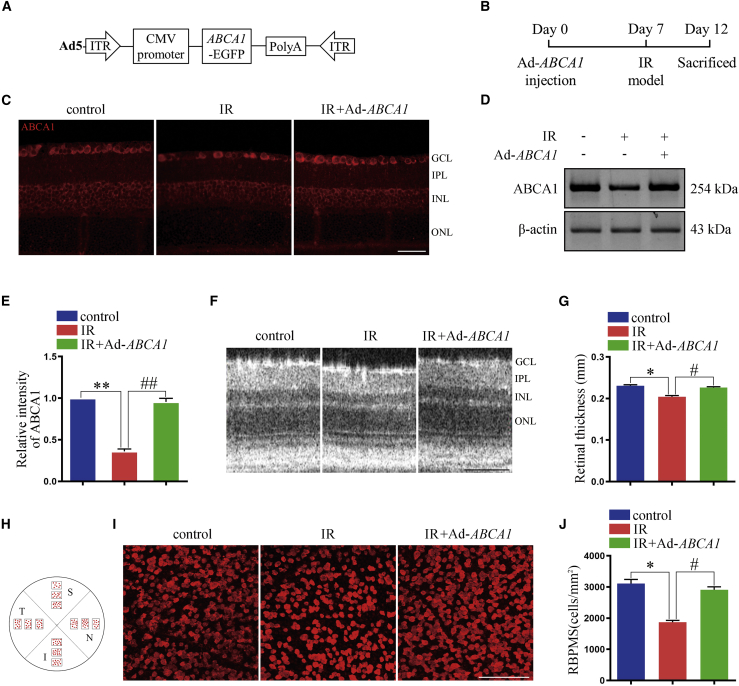
Ad-ABCA1 treatment reduced RGC loss in IR mice model
Intravitreal injection of the LXR agonist could upregulate ABCA1 expression and reduce RGC loss in the IR mice model.18 To confirm the effect of ABCA1 overexpression on RGC apoptosis, Ad-ABCA1 and immunofluorescence (IF) staining were used to examine the expression of ABCA1 in the retina. We found that compared with the IR group, the expression of ABCA1 was increased in the Ad-ABCA1 group (Figure 1C). The expression of ABCA1 in retina extract was increased in the Ad-ABCA1 group (Figures 1D and 1E). qPCR results also confirmed that Ad-ABCA1 could increase ABCA1 mRNA expression (Figure S2B). In Figures S3A and S3B, we have presented the GFP images from an Ad-ABCA1-infected retina. We used spectral-domain optical coherence tomography (SD-OCT) to examine the morphological changes in the retina in vivo. As shown in Figures 1F and 1G, after IR, the thickness of the retina decreased, whereas after Ad-ABCA1 treatment, the retina exhibited sufficient thickness. Most importantly, retinal whole-mount immunostaining with RNA-binding protein with multiple splicing (RBPMS) showed that the RGC number in the IR group decreased, and Ad-ABCA1 treatment could reverse this reduction (Figures 1H−1J).
Figure 1.
The effect of Ad-ABCA1 overexpression on retina degeneration after IR in vivo
(A) Graphic illustration of the structure of Ad-ABCA1. (B) Experimental scheme of Ad-ABCA1 administration and the mouse IR model. (C) Representative immunofluorescence images for ABCA1 expression in retina slices. Scale bar, 50 μm. (D) Western blot analysis showing the expression of ABCA1 in retina after Ad-ABCA1 administration. These results are obtained from three independent experiments. (E) Statistical analysis of (D). ∗∗p < 0.01, compared with control retina; ##p < 0.01 compared with the IR group (n = 3 retinas per group). (F) Representative SD-OCT images for the thickness of the retina. Scale bar, 100 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; and ONL, outer nuclear layer. (G) Statistical analysis of (F). ∗p < 0.05 compared with control retina; #p < 0.05 compared with the IR group (n = 8 retinas per group). (H) Graphic illustration of the RBPMS analysis. S, superior; N, nasal; I, inferior; and T, temporal. (I) Representative immunofluorescence results showing the number of retinal ganglion cell (RGC), n = 8 retinas per group. Scale bar, 100 μm. (J) Statistical analysis of (I). ∗p < 0.05, compared with control retina; #p < 0.05 compared with the IR group. All of the data are expressed as mean ± SEM, one-way ANOVA followed by a Tukey’s post hoc test.
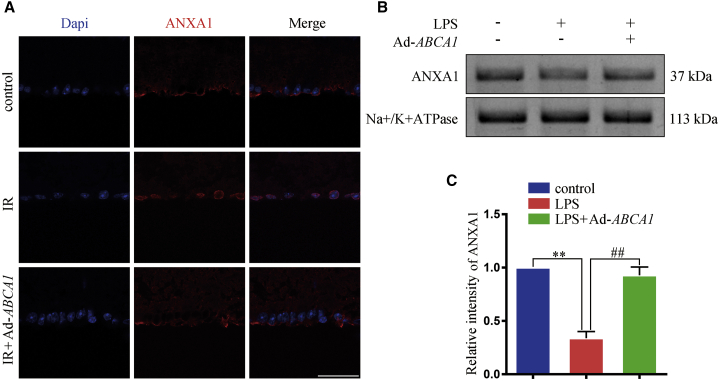
Ad-ABCA1 treatment maintained ANXA1 membrane translocation after IR
Previous studies found ABCA1 to be associated with ANXA1 externalization.16,17 To investigate the effect of ABCA1 overexpression on ANXA1 subcellular localization, immunostaining was performed on retina slices to examine the expression of ANXA1. As shown in Figure 2A, IR induced ANXA1 nuclear localization, visualized using 4′,6-diamidino-2-phenylindole (DAPI), whereas treating with Ad-ABCA1, decreased ANXA1 nuclear translocation. We also examined ANXA1 expression in the cellular membrane extract. In Figures 2B and 2C, we found that lipopolysaccharide (LPS) treatment decreased ANXA1 expression, whereas Ad-ABCA1 maintained ANXA1 expression in membrane extract in HEK293 cells.
Figure 2.
The effect of Ad-ABCA1 overexpression on ANXA1 nuclear localization in vivo and in vitro
(A) Representative immunofluorescence images of ANXA1 expression on the retinal GCL, n = 4 retinas per group. Scale bar, 50 μm. (B) Western blot analysis showing the expression of ANXA1 in cellular membrane extract in HEK293 cells. (C) Statistical analysis of (B); the data of three independent experiments expressed as the mean ± SD. ∗∗p < 0.01, compared with control; ##p < 0.01 compared with the LPS group, one-way ANOVA with Bonferroni post hoc test.
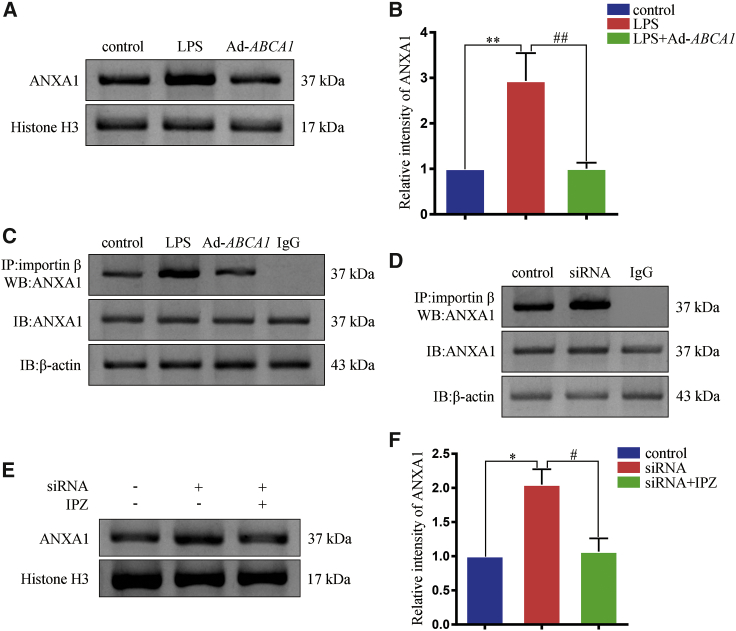
ABCA1 decreased ANXA1 nuclear translocation by competing with importin β
Previous studies found that ANXA1 nuclear translocation was associated with importin β;15,21,22 however, whether the decrease in ANXA1 nuclear translocation associated with ABCA1 overexpression was due to competition with importin β remained unclear. Here, we used Ad-ABCA1 and ABCA1 small interfering RNA (siRNA) to upregulate (Figure S2A) and downregulate (Figures S2D−S2F) ABCA1 expression, respectively. We found that in HEK239 cells, LPS induced ANXA1 nuclear translocation, which could be partially blocked by Ad-ABCA1 (Figures 3A and 3B). We further found that Ad-ABCA1 could decrease binding of ANXA1 with importin β (Figure 3C). We also found that decreased ABCA1 expression could increase binding of ANXA1 with importin β (Figure 3D). In addition, we used the importin β1-specific inhibitor importazole (IPZ) to block the importin β function. We found that ABCA1 siRNA increased ANXA1 nuclear localization, whereas IPZ could partially block its effect (Figures 3E and 3F).
Figure 3.
The mechanism of ABCA1 overexpression on ANXA1 nuclear localization
(A) Western blot analysis showing the expression of ANXA1 in the cellular nuclear extract in HEK293 cells. (B) Statistical analysis of (A). ∗∗p < 0.01, compared with control; ##p < 0.01 compared with the LPS group. (C) A western blotting analysis indicates that LPS induces the interaction between importin β and ANXA1, and this effect is inhibited by Ad-ABCA1 treatment. IP, immunoprecipitation; IB, immunoblotting; and WB, western blot. (D) A western blotting analysis indicates that ABCA1 siRNA induces the interaction between importin β and ANXA1. (E) Western blot analysis showing the expression of ANXA1 in the cellular nuclear extract in HEK293 cells. IPZ, importazole. (F) Statistical analysis of (E). ∗p < 0.05, compared with controls; #p < 0.05 compared with the ABCA1 siRNA group. All of the data of the three independent experiments are expressed as the mean ± SD, one-way ANOVA with Bonferroni post hoc test.
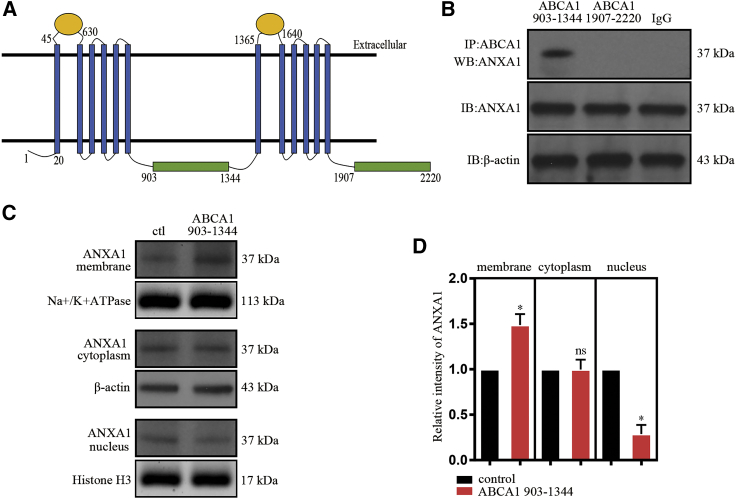
ABCA1 interacted with ANXA1
The structure of ABCA1 has been recently reported.23 This protein mainly contains two transmembrane domains, two extracellular domain, and two main intracellular domains (Figure 4A). Each intracellular domain contains one intracellular helix, one nucleotide-binding domain, and one regulatory domain. Here, we constructed two plasmids containing ABCA1 intracellular domains (amino acids 903 to 1,344 and amino acids 1,907 to 2,220). Coimmunoprecipitation results showed that the amino acids 903 to 1,344 fragment of ABCA1 interacted with ANXA1 (Figure 4B). In addition, we transfected HEK293 cells with a plasmid containing the ABCA1 903–1,344 fragment and found that the expression of ANXA1 was increased in the membrane extract and decreased in the nuclear extract (Figures 4C and 4D). These results indicate that the amino acids 903–1,344 fragment of ABCA1 contains the domain that binds with ANXA1 and maintains ANXA1 membrane localization.
Figure 4.
The interaction between ABCA1 and ANXA1
(A) Schematic diagram of full-length ABCA1. (B) A western blotting analysis is showing the interaction between the 903−1,344 fragment and the 1,907−2,220 fragment of ABCA1 and ANXA1. (C) Western blot analysis showing the expression of ANXA1 in membrane, cytoplasm, and nucleus extract after ABCA1 903−1,344 fragment overexpression. (D) Statistical analysis of (C); the data of three independent experiments expressed as the mean ± SD. ∗p < 0.05, unpaired Student’s t test versus control.
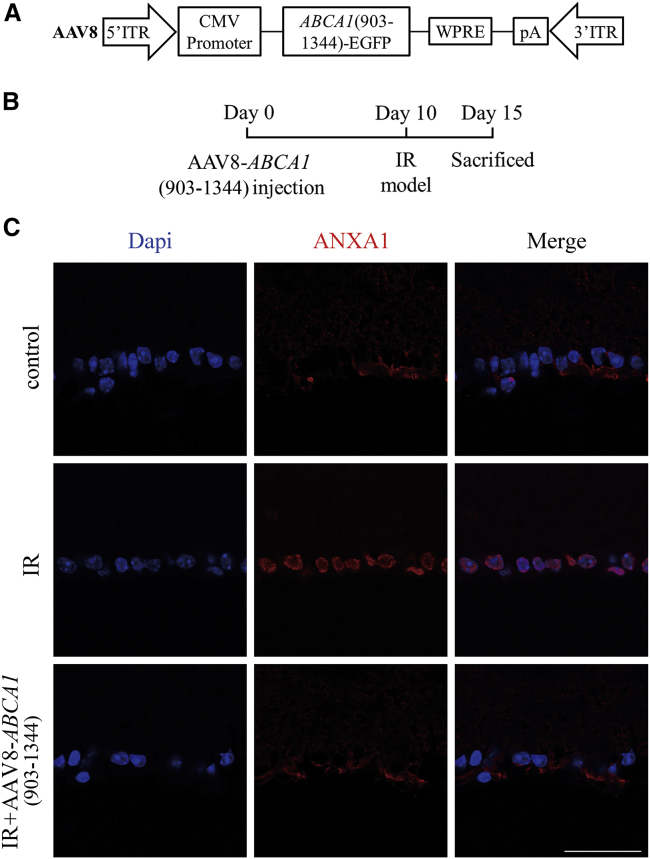
AAV8-ABCA1 (903–1,344) pretreatment decreased ANXA1 nuclear translocation after IR
To assess the impact of ABCA1 amino acids 903–1,344 on ANXA1 nuclear localization in vivo, we used AAV8-ABCA1 (903–1,344) and examined the expression of ANXA1 using retinal slices. The qPCR results revealed that AAV8-ABCA1 (903–1,344) could upregulate ABCA1 (fragment 903–1,344) mRNA expression (Figure S2C). The AAV8-ABCA1 (903–1,344) pretreatment group showed less nuclear staining upon general observation (Figure 5C).
Figure 5.
The effect of AAV8-ABCA1 (903−1,344) overexpression on ANXA1 nuclear localization after IR in vivo
(A) Graphic illustration of the structure of AAV8-ABCA1 (903−1,344). (B) Experimental scheme of AAV8-ABCA1 (903−1,344) administration and the mouse IR model. (C) Representative immunofluorescence images of ANXA1 expression on retina GCL, n = 4 retinas per group. Scale bar, 50 μm.
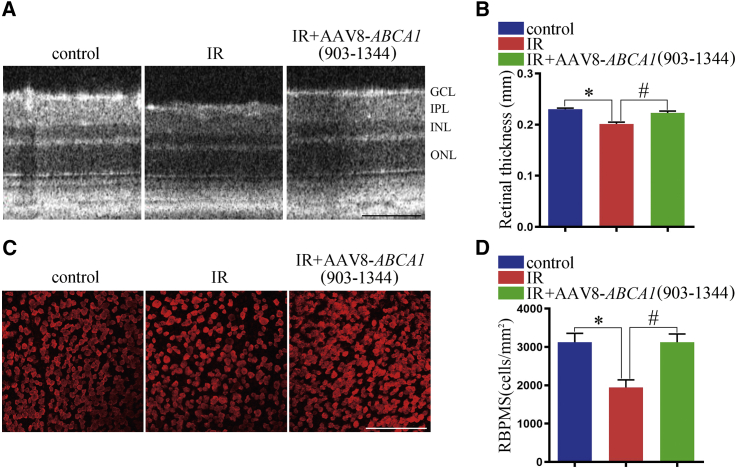
AAV8-ABCA1 (903–1,344) pretreatment reduced RGC loss after IR
To confirm the treatment effect of AAV8-ABCA1 (903–1,344) on RGC apoptosis after IR, we performed SD-OCT to examine the morphological changes in the retina in vivo. We found that AAV8-ABCA1 (903–1,344) pretreatment could reduce retina degeneration after IR (Figures 6A and 6B). Furthermore, we found that AAV8-ABCA1 (903–1,344) pretreatment could reverse RGC loss after IR (Figures 6C and 6D). However, AAV8-ABCA1 (1,907–2,220) injection could not reverse retina degeneration or RGC apoptosis (Figures S1A and S1B). We also examined the effect of AAV8-ABCA1 (903–1,344) post-treatment on retina degeneration and RGC apoptosis. We found that AAV8-ABCA1 (903–1,344) post-treatment could not improve retina degeneration or RGC apoptosis after IR (Figures S1D−S1F). Similarly, an AAV empty vector could not reverse retina degeneration after IR (Figure S1C).
Figure 6.
The effect of AAV8-ABCA1 (903−1,344) overexpression on retina degeneration after IR in vivo
(A) Representative SD-OCT images for the thickness of the retina (n = 8 retinas per group). Scale bar, 100 μm. (B) Statistical analysis of (A). ∗p < 0.05 compared with control retina; #p < 0.05 compared with the IR group. (C) Representative immunofluorescence results showing the number of RGCs, n = 8 retinas per group. Scale bar, 100 μm. (D) Statistical analysis of (C). ∗p < 0.05, compared with control retina; #p < 0.05 compared with the IR group. All of the data are expressed as mean ± SEM, one-way ANOVA followed by Tukey’s post hoc test.
Discussion
This study demonstrated that ABCA1 overexpression decreased ANXA1 nuclear translocation in vivo and in vitro and mitigated retina degeneration and RGC loss after IR. These results suggest that ABCA1 or/and ANXA1 could be considered as a gene therapy target for glaucoma disease.
ANXA1 subcellular localization determines cellular fate. In inflammatory diseases and the immune response system, treatment with exogenous ANXA1 mimetic peptides can decrease the expression of proinflammatory mediators, attenuating the infiltration of inflammatory cells.24 By interacting with FPR2, ANXA1 can also limit viral replication in the influenza A virus infection model.25 Extracellular ANXA1 promotes stem cell differentiation26 and reduces cancer cell growth and aggressiveness.27 However, ANXA1 nuclear translocation induces cellular apoptosis by interacting with p53 and p65. In this study, we found that ABCA1 overexpression could promote RGC survival in an IR mouse model. We speculated that overexpression of full-length ABCA and its amino acids 903–1,344 fragment promoted the RGC survival rate by reducing ANXA1 nuclear localization.
Previous studies have reported that ABCA1 could interact with ANXA1 and induce externalization of ANXA1. However, the underlying mechanism remains unclear. S100A11 is another protein reported to be an ANXA1 nuclear translocation regulator. S100A11 overexpression can downregulate ANXA1 binding with importin β, which partially interferes with ANXA1 nuclear translocation.28 Similarly, in this study, we found that ABCA1 overexpression could reduce ANXA1 binding with importin β and decrease ANXA1 nuclear localization. Moreover, we found that the amino acids 903–1,344 fragment of ABCA1 interacted with ANXA1 and could be considered a key domain for the ABCA1-ANXA1 interaction. We speculated that ABCA1 reduced ANXA1 nuclear translocation by decreasing ANXA1 binding with importin β, which indicated that ABCA1 was competitively binding with ANXA1 and reducing ANXA1 nuclear translocation. However, whether importin β can proactively and specifically transport ANXA1 into the nucleus and reduce ABCA1–ANXA1 binding remains unclear.
The structure of human ABCA1 was recently identified.23 We speculated that ABCA1 binding with ANXA1 occurred on its intracellular domain. Coimmunoprecipitation results also identified that the intracellular domain of ABCA1 (amino acids 903–1,344 fragment) could bind with ANXA1. The ABCA1 fragment (amino acids 1,907–2,220) could not bind with ANXA1 or reverse retina degeneration. We assumed that this is due to the presence of the unconjugated form of ANXA1. A limitation of this study was that the amino acids 903–1,344 fragment of ABCA1 contained three functional domains, including one nucleotide-binding domain, one regulatory domain, and one intracellular helices domain. Further studies should identify which domain of this fragment could specifically bind with ANXA1. Further, more studies are needed to identify other structures in ABCA1 that could potentially interact with ANXA1, such as two transmembrane domains and two extracellular domains. Another limitation of this study is that the adenovirus injection might have activated the immune system, including the Toll-like receptor (TLR)-dependent and TLR-independent pathways. More studies are warranted to silence retinal microglia and thereafter investigate the function of Ad-ABCA1 on retina degeneration.
Our findings identify a novel treatment strategy for RGC loss in the IR mice model. Overexpression of the full-length and amino acids 903–1,344 fragment of ABCA1 decreased ANXA1 nuclear localization. The reduced RGC loss associated with ABCA1 overexpression was brought about due to interference of the ANXA1-importin β interaction and maintenance of ANXA1 membrane localization. These findings reveal ABCA1 and ANXA1 as novel targets for glaucoma gene therapies.
Materials and methods
Animals
Male C57BL/6J mice (3 months old, 20–25 g) were obtained from the Experiment Animal Center of Tongji Medical College. All of the mice were fed with a standard rodent diet ad libitum and kept on a 12-h light/12-h dark cycle. The procedures concerning animals were performed according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research and under protocols approved by the Institutional Animal Care and Use Committees at Huazhong University of Science and Technology (HUST; 2015-K-023).
Reagents and antibodies
IPZ was obtained was from Calbiochem (Darmstadt, Germany; 401105, 8 μM). Human ABCA1 siRNA was obtained from Thermo Fisher Scientific (Shanghai, China; assay ID 107728, catalog #AM16708).
The following antibodies were used in this study: anti-ABCA1 (ab-18180, Research Resource Identifier [RRID]: AB_444302; Abcam; 1:500 for western blot, 1:200 for IF), anti-ANXA1 (ab-214486; Abcam; 1:2,000 for western blot, 1:1,000 for IF), anti-RBPMS (ABN1362; Millipore; 1:500), anti-β-actin (sc-47778, RRID: AB_2714189; Santa Cruz; 1:1,000), anti-importin β (#51186, RRID: AB_2799386; Cell Signaling Technology; 1:1,000), anti-beta 1 sodium potassium ATPase (ab-2873, RRID: AB_303375; Abcam; 1:250), and anti-histone H3 (#4499, RRID: AB_10544537; Cell Signaling Technology; 1:1,000).
Intravitreal injections
The Ad-ABCA1 gene (1 × 1011 viral genomes/mL) and AAV-encompassing amino acids 903–1,344 fragment of ABCA1 (AAV8-ABCA1 903−1,344; 1 × 1012 viral genomes/mL) were obtained from BrainVTA (Wuhan, China). The experimental eyes were injected with Ad-ABCA1 (1.5 μL) and AAV8-ABCA1 903–1,344 (1.5 μL) into the vitreous cavity through a 35G needle with a 10-μL Hamilton micro syringe (Hamilton, Reno, NV, USA) before the onset of reperfusion at 7 days and 10 days, respectively. Tobramycin was applied to prevent bacterial infection.
IR mouse model
Mice were dark adapted overnight and anesthetized with an intraperitoneal injection of ketamine (15 mg/g body weight) and xylazine (7 mg/g). Their right eyes were dilated with 5% tropicamide, followed by topical anesthesia using proparacaine. We inserted a 30G needle into the anterior chamber (right eyes); the needle was connected to a saline reservoir. We elevated the saline reservoir to keep the IOP at 75 mmHg for 45 min. Retinal ischemia was confirmed by whitening of the iris. Then, we withdrew the needle and treated the mice with tobramycin to avoid bacterial infection. We kept the left eyes as control. All mice were sacrificed 120 h after this surgery.
Retinal thickness measurement in vivo
Retina thickness was measured by SD-OCT using the Spectralis HRA+OCT system (Heidelberg Engineering). Mice were anesthetized by intraperitoneal injection of ketamine (15 mg/g body weight) and xylazine (7 mg/g). Pupils were dilated with drops of Tropicamide Ophthalmic Solution USP 1% (Bausch & Lomb). An artificial tear was applied bilaterally to prevent corneal dehydration during the procedure. Total retinal thickness was defined as the width from the inner-limiting membrane to the retinal pigment epithelium (RPE)/choroid layer on the cross-sectional images and measured using custom-automated image segmentation routines in MATLAB.
IF
We washed 5 μm paraffin-embedded retinal sections twice with phosphate-buffered saline (PBS; at 37°C). Afterward, the sections were incubated with 5% bovine serum albumin (BSA) for 1 h and were then treated with mouse monoclonal ABCA1 antibody and rabbit monoclonal ANXA1 antibody in 5% BSA at 4°C overnight. Retinal sections were then washed and treated with secondary antibody (1:200; Invitrogen) in 5% BSA at 37°C for 1 h. A Zeiss confocal microscope was used to acquire fluorescence images (Zeiss LSM 700; Germany).
Coimmunoprecipitation
We used a buffer containing 400 mM KCl, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 5 mM ethylenediaminetetraacetic acid, 0.4% NP-40, protease inhibitors, and 5% glycerol to obtain cell lysates by sonication. We incubated the cell lysates with an anti-importin β antibody overnight at 4°C. Then, we used protein A/G PLUS-Agarose beads (sc-2003; Santa Cruz) to incubate the reaction mixture for 2 h at 4°C. The precipitates were then washed three times with a washing buffer and eluted from the beads by boiling with 1 × sodium dodecyl sulfate (SDS) for 5 min at 95°C. The protein samples were detected by SDS-polyacrylamide gel electrophoresis.
Protein extraction and preparation
We prepared the protein samples using the Membrane and Cytosol Protein Extraction Kit (P0033; Beyotime, China). In brief, we washed the HEK293 cells with ice-cold PBS and harvested the cells using a cell scraper. The cells were centrifuged at 3,000 × g for 5 min. We then resuspended the cell pellets in a membrane and cytoplasmic extraction buffer that contained protease inhibitors and phenylmethanesulfonyl fluoride. The cell pellets were placed on ice with the extraction buffer for 30 min, and the lysates were then centrifuged at 12,000 × g at 4°C for 10 min. The membrane-bound and cytoplasmic protein fractions were harvested for expression analysis, which was performed later.
We used ImageJ (Fiji) to detect the western blotting bands and performed a band density analysis.
Statistical analysis
We used GraphPad Prism software (version 5.0; GraphPad Software) to perform the statistical analysis. Data were analyzed using Student’s t test and a one-way analysis of variance (ANOVA) to determine statistically significant differences. A value of p <0.05 was considered statistically significant.
Acknowledgments
The current studies were supported by funding from the National Natural Science Foundation of China grant no. 31800868 and Tongji Hospital (HUST) Foundation for Excellent Young Scientist grant no. 2020YQ18 (to Y.Z.); and National Natural Science Foundation of China grant no. 31900657, the China Postdoctoral Science Foundation grant nos. 2018M643375 and 2020T130251, and the Postdoctoral Fund of the First Affiliated Hospital, Jinan University grant no. 801323 (to J.L.).
Author contributions
Y.Z. conceived and designed the project and wrote the manuscript. J.L. performed the experiments. S.W. and Z.Z. helped to revise the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2021.01.012.
Supplemental information
References
- 1.Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emanuel M.E., Parrish R.K., 2nd, Gedde S.J. Evidence-based management of primary angle closure glaucoma. Curr. Opin. Ophthalmol. 2014;25:89–92. doi: 10.1097/ICU.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 3.Chi W., Li F., Chen H., Wang Y., Zhu Y., Yang X., Zhu J., Wu F., Ouyang H., Ge J. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA. 2014;111:11181–11186. doi: 10.1073/pnas.1402819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J., Zhao Q., Li M., Duan Q., Zhao Y., Zhang H. The effects of endothelium-specific CYP2J2 overexpression on the attenuation of retinal ganglion cell apoptosis in a glaucoma rat model. FASEB J. 2019;33:11194–11209. doi: 10.1096/fj.201900756R. [DOI] [PubMed] [Google Scholar]
- 5.Minhas G., Sharma J., Khan N. Cellular Stress Response and Immune Signaling in Retinal Ischemia-Reperfusion Injury. Front. Immunol. 2016;7:444. doi: 10.3389/fimmu.2016.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne N.N., Casson R.J., Wood J.P., Chidlow G., Graham M., Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Purvis G.S.D., Solito E., Thiemermann C. Annexin-A1: Therapeutic Potential in Microvascular Disease. Front. Immunol. 2019;10:938. doi: 10.3389/fimmu.2019.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobbetti T., Cooray S.N. Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature. Biol. Chem. 2016;397:981–993. doi: 10.1515/hsz-2016-0200. [DOI] [PubMed] [Google Scholar]
- 9.Boudhraa Z., Bouchon B., Viallard C., D’Incan M., Degoul F. Annexin A1 localization and its relevance to cancer. Clin. Sci. (Lond.) 2016;130:205–220. doi: 10.1042/CS20150415. [DOI] [PubMed] [Google Scholar]
- 10.Parente L., Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm. Res. 2004;53:125–132. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 11.Girol A.P., Mimura K.K.O., Drewes C.C., Bolonheis S.M., Solito E., Farsky S.H.P., Gil C.D., Oliani S.M. Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro. J. Immunol. 2013;190:5689–5701. doi: 10.4049/jimmunol.1202030. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y., Anbalagan D., Lee L.H., Samy R.P., Shanmugam M.K., Kumar A.P., Sethi G., Lobie P.E., Lim L.H.K. ANXA1 inhibits miRNA-196a in a negative feedback loop through NF-kB and c-Myc to reduce breast cancer proliferation. Oncotarget. 2016;7:27007–27020. doi: 10.18632/oncotarget.8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anbalagan D., Yap G., Yuan Y., Pandey V.K., Lau W.H., Arora S., Bist P., Wong J.S.B., Sethi G., Nissom P.M. Annexin-A1 regulates microRNA-26b∗ and microRNA-562 to directly target NF-κB and angiogenesis in breast cancer cells. PLoS ONE. 2014;9:e114507. doi: 10.1371/journal.pone.0114507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Li X., Gong J., Li L., Chen L., Zheng L., Chen Z., Shi J., Zhang H. Annexin A1 nuclear translocation induces retinal ganglion cell apoptosis after ischemia-reperfusion injury through the p65/IL-1beta pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1350–1358. doi: 10.1016/j.bbadis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Zhao Y., Xia Q., Zheng L., Liu L., Zhao B., Shi J. Nuclear translocation of annexin 1 following oxygen-glucose deprivation-reperfusion induces apoptosis by regulating Bid expression via p53 binding. Cell Death Dis. 2016;7:e2356. doi: 10.1038/cddis.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman L.P., Epton M.J., Buckingham J.C., Morris J.F., Christian H.C. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology. 2003;144:1062–1073. doi: 10.1210/en.2002-220650. [DOI] [PubMed] [Google Scholar]
- 17.Omer S., Meredith D., Morris J.F., Christian H.C. Evidence for the role of adenosine 5′-triphosphate-binding cassette (ABC)-A1 in the externalization of annexin 1 from pituitary folliculostellate cells and ABCA1-transfected cell models. Endocrinology. 2006;147:3219–3227. doi: 10.1210/en.2006-0099. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Xu L., Chen W., Li X., Xia Q., Zheng L., Duan Q., Zhang H., Zhao Y. Reduced Annexin A1 Secretion by ABCA1 Causes Retinal Inflammation and Ganglion Cell Apoptosis in a Murine Glaucoma Model. Front. Cell. Neurosci. 2018;12:347. doi: 10.3389/fncel.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S., Yang H., Chen Z., Zheng C., Lei C., Lei B. Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Invest. Ophthalmol. Vis. Sci. 2015;56:1168–1180. doi: 10.1167/iovs.14-15612. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.H., Keiser M.S., Davidson B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018;8:e58. doi: 10.1002/cpmo.58. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y., Wang J., Jiang H., Yu Z., Li X., Shi J. Following OGD/R, annexin 1 nuclear translocation and subsequent induction of apoptosis in neurons are assisted by myosin IIA in a TRPM7 kinase-dependent manner. Mol. Neurobiol. 2015;51:729–742. doi: 10.1007/s12035-014-8781-y. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Zheng L., Xia Q., Liu L., Mao M., Zhou H., Zhao Y., Shi J. A novel cell-penetrating peptide protects against neuron apoptosis after cerebral ischemia by inhibiting the nuclear translocation of annexin A1. Cell Death Differ. 2019;26:260–275. doi: 10.1038/s41418-018-0116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian H., Zhao X., Cao P., Lei J., Yan N., Gong X. Structure of the Human Lipid Exporter ABCA1. Cell. 2017;169:1228–1239.e10. doi: 10.1016/j.cell.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., An D., Xu J., Shao B., Li X., Shi J. Ac2-26 Induces IKKβ Degradation Through Chaperone-Mediated Autophagy Via HSPB1 in NCM-Treated Microglia. Front. Mol. Neurosci. 2018;11:76. doi: 10.3389/fnmol.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schloer S., Hübel N., Masemann D., Pajonczyk D., Brunotte L., Ehrhardt C., Brandenburg L.O., Ludwig S., Gerke V., Rescher U. The annexin A1/FPR2 signaling axis expands alveolar macrophages, limits viral replication, and attenuates pathogenesis in the murine influenza A virus infection model. FASEB J. 2019;33:12188–12199. doi: 10.1096/fj.201901265R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa C.M.V., Fock R.A., Hastreiter A.A., Reutelingsperger C., Perretti M., Paredes-Gamero E.J., Farsky S.H.P. Extracellular annexin-A1 promotes myeloid/granulocytic differentiation of hematopoietic stem/progenitor cells via the Ca2+/MAPK signalling transduction pathway. Cell Death Discov. 2019;5:135. doi: 10.1038/s41420-019-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecchi L., Alves Pereira Zóia M., Goss Santos T., de Oliveira Beserra A., Colaço Ramos C.M., França Matias Colombo B., Paiva Maia Y.C., Piana de Andrade V., Teixeira Soares Mota S., Gonçalves de Araújo T. Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1368–1382. doi: 10.1016/j.bbamcr.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Xia Q., Li X., Zhou H., Zheng L., Shi J. S100A11 protects against neuronal cell apoptosis induced by cerebral ischemia via inhibiting the nuclear translocation of annexin A1. Cell Death Dis. 2018;9:657. doi: 10.1038/s41419-018-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.