Highlights
-
•
Memory for temporal context, vital for episodic memory, shows prolonged development.
-
•
Cognitive processes and neural mechanisms driving age-related improvements unclear.
-
•
Event-related potentials (ERP) used with 7−9-year-olds, 10−12-year-olds, and adults.
-
•
We found age-related improvements, ERP effects, and brain-behavior relations.
-
•
Implications for temporal memory and episodic memory development discussed.
Keywords: Episodic memory development, Temporal memory, Temporal context, Distance-based processes, Event-related potentials
Abstract
Time is a critical feature of episodic memory—memory for events from a specific time and place (Tulving, 1972). Previous research indicates that temporal memory (memory for ‘when’) is slower to develop than memory for other details (e.g., ‘what’ and ‘where’), with improvements observed across middle and late childhood. The factors that drive these changes are not yet clear. We used an event-related potential (ERP) recognition memory paradigm to investigate the underlying processes of memory for temporal context in middle to late childhood (7−9-year-olds; 10−12-year-olds) and young adulthood. Behaviorally, we observed age-related improvements in the ability to place events in temporal context. ERP analyses showed old/new effects for children and adults. We also found brain-behavior relations for 1) episodic memory (ERP mean amplitude difference between source hits and correctly identified new trials was correlated to behavioral accuracy), and 2) temporal memory (ERP mean amplitude difference between source hits and source error trials was correlated to accuracy of temporal memory judgments). This work furthers our understanding of the cognitive processes and neural signatures supporting temporal memory development in middle to late childhood, and has implications for episodic memory development more broadly.
1. Introduction
The events of our lives are organized by when they occurred (Friedman, 1993), and fall into certain lifetime periods (e.g., when I was in college; Conway and Pleydell-Pearce, 2000). Tulving’s (1972) definition of episodic memory—memory for events from a particular time and place—includes temporal organization as a critical feature. We can remember the temporal order of events (relating events to each other in time; e.g., X came before Y) and the broader temporal context (placing an event on a conventional or arbitrary time scale; e.g., event occurred in January; event occurred in List 1). Although temporal memory is an integral part of episodic memory, relatively little is known about temporal memory development, especially memory for temporal context, and even less is known about the cognitive and neural processes supporting temporal memory in childhood.
Memory for temporal order emerges in infancy (Bauer, 2007), and shows continued development throughout childhood (Canada et al., 2020; Friedman, 1991; Pathman et al., 2013a; Pathman et al., 2013b; Pathman and Ghetti, 2014; Picard et al., 2012). Memory for temporal context also shows age-related improvements, however fewer studies exist. From early to middle childhood, children’s ability to place events on arbitrary and conventional time scales (e.g., season) improves with age (Friedman, 1991; Friedman and Lyon, 2005; Pathman et al., 2013b), and studies with older children and adults show age-related improvements into adolescence (Czernochowski et al., 2009; Pathman and Ghetti, 2014). For example, researchers found age-related improvements in accuracy between 7- and 10-year-olds, and 10-year-olds and young adults, when participants judged the arbitrary temporal context of past events (participants selected the object that had appeared in the same study trial as a cue object, among distractors from other study trials; Pathman and Ghetti, 2014). Temporal memory, including memory for temporal context, shows a protracted development. To understand the cause of age-related changes in memory for temporal context, researchers can examine underlying cognitive and neural processes.
Two main processes are implicated in temporal memory: reconstruction and distance-based processes (Friedman, 1993, 2014). Reconstruction processes are slow, effortful processes that involve remembering event details, combined with semantic time knowledge, to infer when past events occurred (e.g., “My trip to Montreal occurred in the winter, because I remember that it was snowing”). Distance-based processes rely on impressions of memory strength to infer when events took place (i.e., more vivid memories occurred more recently). Children’s ability to engage in reconstruction processes improves across childhood (Friedman, 1991; Friedman and Lyon, 2005; Pathman et al., 2013b), however, less is known about children’s use of distance-based processes. Before age 12 children cannot describe the advantages of using distance-based processes, but it is possible that children use these processes without being aware of them (see Friedman, 2007, for discussion). Indeed, a study that manipulated elapsed time between events found that 8−10-year-olds and adults rely on distance-based processes to judge temporal order of two past events (Pathman et al., 2013a). Further work is needed to understand children’s use of distance-based processes to judge temporal context.
Event-related potentials (ERPs) are often used to study the neural substrates of episodic memory (see Voss and Paller, 2017, for review), due to the high temporal resolution, which allows isolation of neural responses to specific stimuli before overt behavioral responses occur (Friedman and Johnson, 2000). Temporal memory is a type of source memory—memory for perceptual and contextual information associated with an event (Johnson et al., 1993). In source memory ERP paradigms, participants study items associated with contextual information (e.g., colored background), and during retrieval participants identify ‘old’ items from encoding and their context (i.e., background color) and ‘new’ items that were not included in the encoding phase (Cycowicz et al., 2003). Differences in ERP waveforms for correctly recognized ‘old’ and ‘new’ stimuli are referred to as old/new or episodic memory (EM) effects (Cycowicz, 2019; Friedman and Johnson, 2000).
As reviewed by Riggins, Rollins, and Graham (2013), EM effects can include ERP differences between all old (i.e., source-correct, source-incorrect) and new trials and between source-correct and correct rejections. ERPs for source-correct/source-incorrect trials are often more positive compared to new trials (see Friedman and Johnson, 2000, and Voss and Paller, 2017, for reviews), but there is also evidence that ERPs for source-correct/source-incorrect trials can be more negative compared to new trials (Cycowicz et al., 2003; Czernochowski et al., 2009). Additionally, old/new effects that are observed for children often differ in topography and timing compared to adult effects (Cycowicz et al., 2003; Czernochowski et al., 2005, Czernochowski et al., 2009).
The aims of the current study were to use a laboratory-based task to examine the development of memory for temporal context from middle to late childhood (i.e., 7- to 9-year-olds and 10- to 12-year-olds) and into adulthood, and to investigate behavioral accuracy and associated neural activity using ERP. The use of laboratory-based studies is ideal for studying mechanisms and processes involved in memory for temporal context because items are placed on arbitrary time scales, which does not allow for reliance on knowledge of conventional time or scripts and routines to make temporal judgments. We adapted a traditional recognition memory paradigm to include a temporal component by separating the presentation of two object lists (i.e., List 1, delay, List 2), similar to previous studies (Curran and Friedman, 2003, Curran and Friedman, 2004; Trott et al., 1999). ERPs were recorded during retrieval, where participants identified objects from the previous lists and novel objects. The task design encouraged the use of distance-based processes, as opposed to reconstruction, because study context remained the same between lists and thus participants had to rely on memory strength to differentiate lists (Curran and Friedman, 2003, Curran and Friedman, 2004). We predicted age-related improvements in behavioral accuracy, and differences in ERP waveforms between source hits (correctly identified list) and correct rejections (correctly identified new items). We expected to see differences in the timing and/or topography of the ERP effects for children compared to adults. We also expected to observe meaningful individual differences that underscore brain-behavior relations.
2. Materials and methods
2.1. Participants
One hundred and three children and adults participated in this study. Child participants were recruited from a mid-sized city in the South Eastern United States, and adult participants were either university students or community volunteers. Fourteen participants were excluded from data analyses for reasons explained in Supplemental Methods. The final sample consisted of twenty-nine 7- to 9-year-olds (Mage=8.37, SD = 0.84; 17 females and 12 males), twenty-nine 10- to 12-year-olds (Mage=11.55, SD = 0.91; 14 females and 15 males), and 31 young adults (Mage=21.80, SD = 3.21; 16 females and 15 males). All participants provided informed consent, which included written parental consent, children’s verbal or written assent, and written consent for adults. Each participant took part in one session that was approximately 2 h in length, including breaks. Families of child participants received monetary compensation ($10), and adults either received course credit or monetary compensation ($10).
2.2. Stimuli
Visual stimuli for the temporal memory task consisted of 150 photographs of everyday objects selected from the Bank of Standardized Stimuli Version 1.0 (BOSS; Brodeur et al., 2010). Each image was placed on a gray background, with dimensions of 1920 × 1080 pixels and a resolution of 72 dpi. The 150 images were randomly assigned to one of three lists (50 images per list); each list was counterbalanced across participants such that it served as List 1, List 2, or New (see below).
2.3. Procedure
After consenting procedures, participants began the temporal memory task, composed of an encoding and a retrieval phase. During encoding, participants saw two lists of 50 objects each on a computer screen (i.e., List 1, List 2) separated by a 10-minute break. For each list, a fixation (+) appeared on the screen for 500 ms prior to each image, and then each image was presented for 2 s. Participants indicated if they would see each object at school by pressing either the left button for “Yes,” or the right button for “No” on a response box. After encoding, participants were fitted for an electrode cap, and application of the cap took about the same amount of time for the children and adults (M younger children = 21.38 min, M older children = 19.13 min, M adults=23.95 min). During retrieval, participants saw old objects (i.e., objects from Lists 1 and 2) and new objects (not from encoding) for 2 s each. A fixation (+) preceded each object, and the timing varied from 1,500−2,500 ms. Participants identified each object as being from List 1, List 2, or New using the response box, similar to Curran and Friedman (2003, 2004). After the temporal memory task, participants completed the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence: Second Edition (WASI-II; Wechsler, 2011). At the end of the session, participants completed additional tasks outside the scope of this paper.
2.4. Electroencephalogram (EEG) recording and data processing
We recorded EEG with Ag/AgCl active electrodes using the Brain Vision actiCHamp amplifier and PyCorder computer software. The electrophysiological data was processed using Brain Vision Analyzer 2.0 software. Data were filtered at a low cutoff of 0.1 Hz and a high cutoff of 40 Hz. We re-referenced the data to linked mastoids (Czernochowski, et al., 2005; Sprondel et al., 2011). Ocular correction was applied using the Analyzer ICA (Independent Component Analysis; Makeig et al., 1996) method, and then manual inspection confirmed segments identified as blinks. All 150 trials were selected in a 2000 ms epoch that was representative of the 2000 ms presentation time during the retrieval task, with a 200 ms baseline correction applied to all trials. The criteria for artifact rejection was +/- 150μV, and we employed the Brain Vision Analyzer 2.0 semiautomatic procedure, which screens for muscle-related artifacts. We then used manual inspection to confirm the removal of artifacts and specific electrodes if needed.
ERPs were averaged for each of the following conditions based on behavioral responses: source hits (old items; correctly identified as either List 1 or 2), source errors (incorrectly identified list) and correct rejections (new items; correctly identified as new). The trial numbers for source hits per group were: 7- to 9-year-olds (M = 39.59, SD = 7.94, Range: 21–54), 10- to 12-year-olds (M = 49.38, SD = 8.58, Range: 30–65), and adults (M = 59.23, SD = 7.32, Range: 42–72). The trial numbers for source errors were: 7- to 9-year-olds (M = 33.86, SD = 6.69, Range: 16–46), 10- to 12-year-olds (M = 31.90, SD = 6.93, Range: 19–47), and Adults (M = 32.45, SD = 6.07, Range: 21–46). The trial numbers for correct rejections were: 7- to 9-year-olds (M = 41.14, SD = 5.41, Range: 26–49), 10- to 12-year-olds (M = 45.03, SD = 3.63, Range: 36–50), and adults (M = 47.32, SD = 2.81, Range: 39–50). For each age group and trial type, the number of trials was well above the a priori trial cutoff (15 trials). Trial numbers were similar to previous work on ERPs of source memory with similar age groups (Czernochowski et al., 2009; Sprondel et al., 2011). Additionally, we used mean amplitude as the dependent measure which is not biased by condition differences in trial numbers (Luck, 2014).
3. Results
3.1. Behavioral data
We will refer to 7- to 9-year-olds as ‘younger children’ and 10- to 12-year-olds as ‘older children.’ Table 1 shows all the different response categories.
Table 1.
Temporal Memory Task Response Categories.
| Trial Types | |||
|---|---|---|---|
| List 1 | List 2 | New | |
| Participant Responses | |||
| List 1 | Source Hit | Source Error | False Alarm |
| List 2 | Source Error | Source Hit | False Alarm |
| New | Miss | Miss | Correct Rejection |
We examined age group and List (i.e., List 1 versus List 2) differences for the following response types: source hits (e.g., correctly identifying List 1 items as List 1, List 2 items as List 2), source errors (e.g., incorrectly identifying List 1 items as List 2, List 2 items as List 1), and false alarms (i.e., incorrectly identifying a new item as either List 1 or List 2). Source hits proportions reflect how many total source hits participants had out of a total of 100 old items (i.e., 50 items in List 1, 50 items in List 2). Similarly, source errors proportions reflect how many total source errors participants had out of a total of 100 old items. We examined the responses by List to see if there were any differences in memory for more distant compared to more recent events. Additionally, we looked at the effect of age group on correct rejections. Source errors and false alarms analyses are presented in Supplemental Results.
3.1.1. Source hits
To investigate age differences in memory for temporal context, we first ran a 2 (List: List 1, List 2) x 3 (Age group: 7- to 9-year-olds, 10- to 12-year-olds, adults) ANOVA on source hits. There was a main effect of age group, F(2, 86) =20.15, p < 0.001, ηp2 = 0.319. Follow-up comparisons showed significant differences between all three groups: adults (M = 0.62, SD = 0.06) had more overall source hits than both younger children (M = 0.51, SD = 0.06; p < 0.001) and older children (M =0.58, SD = 0.08; p = 0.037), and older children had more overall source hits than younger children (p<0.001). The pattern here indicates that memory for temporal context improves from middle to late childhood. There was also a main effect of list, F(1, 86) = 5.55, p = 0.02, ηp2 =0 .061, indicating that across age groups there were more List 2 hits (M = 0.61, SD = 0.16) than List 1 hits (M = 0.54, SD = 0.18). There was no list by age group interaction, F(2, 86) = 2.24, p = 0.11, ηp2=0.050.
3.1.2. Correct rejections
There were no age differences in correct rejections, F(2, 86) = 1.24, p = 0.29, ηp2 =0 .028. Across age correct rejections were very high: younger children (M = 0.96, SD =0 .04), older children (M = 0.97, SD =0 .03), and adults (M=0.97, SD =0 .04).
3.2. ERP data
We included 9 electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4) in the following analyses based on previous work examining old/new effects in children and adults with source memory tasks (Czernochowski et al., 2005; Haese and Czernochowski, 2016; Riggins and Rollins, 2015; Riggins et al., 2013; Rollins and Riggins, 2013; Sprondel et al., 2011) and previous temporal source studies with these 9 electrodes in adults (Trott et al., 1997, 1999; see also Curran and Friedman, 2004). We conducted ANOVAs with the factors Coronal plane (frontal, central, parietal), Sagittal plane (left, midline, right), and Age group (7- to 9-year-olds, 10- to 12-year-olds, adults) for each of the following condition comparisons: (a) source hits versus correct rejections, (b) source errors versus correct rejections, and (c) source hits versus source errors. Analysis (a) is presented in the main manuscript; (b) and (c) are in Supplemental Results. The dependent measure for all analyses was mean amplitude. Only significant effects with Condition will be reported and discussed. Greenhouse-Geisser corrected p-values and uncorrected degrees of freedom are reported for instances where sphericity was violated. Interactions with the Condition were followed up with additional ANOVAs and t-tests, as appropriate. All ERP results tables report direction of effects. We focus on Condition differences in either direction (e.g., source hits mean amplitude more positive or more negative than correct rejections mean amplitude), given that previous studies have found both types of differences (Cycowicz et al., 2003; Czernochowski et al., 2009).
The ANOVA analyses reported in the main manuscript (source hits and correct rejections) is consistent with Curran and Friedman’s (2003, 2004) investigation of ERPs and adults’ memory for temporal context because they only analyzed correct responses (e.g., correct list items, correctly identified new items). As a reminder, our retrieval task, like theirs, did not require participants to make an initial old/new judgment followed by a source judgment (e.g., List 1, List 2). Rather, participants identified items as being from either List 1, List 2, or New. Given that participants’ judgments of temporal context were combined with judging items as ‘old,’ the comparison between source hits and correct rejections is not just a comparison of correct responses to ‘old’ and ‘new’ stimuli. A source hit indicates that participants both recognized an item as old and correctly recalled its temporal context.
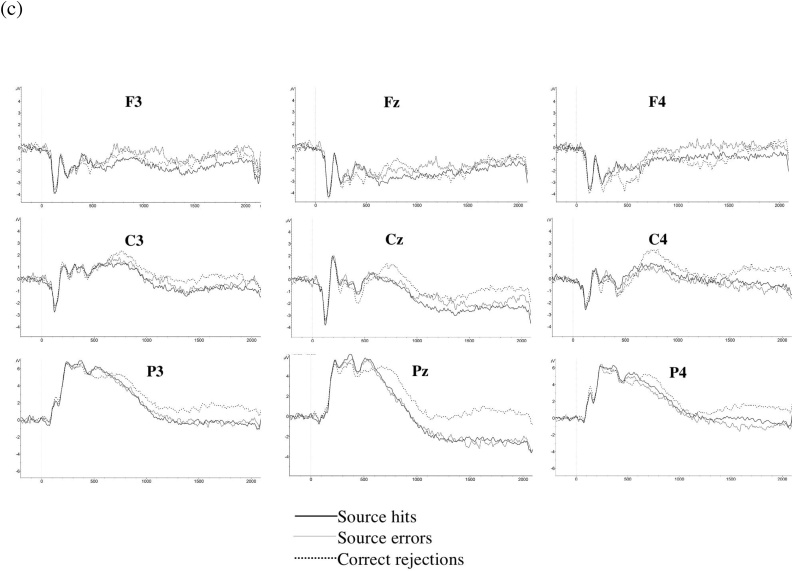
ERP analyses focused on post-stimulus time windows beginning at 300−500 ms. This window was selected since it is the earliest window used by Curran and Friedman (2004). Given that we expected effects in later time windows in children compared to adults, and might expect effects in different time windows due to methodological differences between the present work and past studies, we report an objective, exploratory approach (not based on visual inspection of grand averages). We started with the 300−500 ms time window and examined subsequent time windows in 200 ms duration intervals up to 1900 ms. Note that Curran and Friedman report results from early time windows (300−500 & 500−700 ms) and late time windows (800–1800ms) in separate papers (2003, 2004). Time windows also generally overlap with other studies examining temporal memory and ERPs with adults and children (Czernochowski, et al., 2009; Trott, et al., 1997; 1999). We do not correct for multiple comparisons in the omnibus ANOVAs since the analysis approach is exploratory, given the novelty of this work, not confirmatory. This follows statistics guidelines (Bender and Lange, 2001). However, we provide all of the significance levels so that readers can focus on particular results at their own discretion. For example, they may choose to use Bonferroni correction and only focus on effects for which the p-value is less than 0.006 (0.05 divided by 8 time windows equals 0.00625; noted in tables as effects with 3 asterisks). However, we also note that previous papers have suggested that Bonferroni is too stringent (e.g., Perneger, 1998). See Fig. 1 for grand averages for each age group.
Fig. 1.
Grand averages for (a) younger children, (b) older children, and (c) adults.
3.2.1. Source hits versus correct rejections
Results for the Condition x Age Group x Coronal x Sagittal ANOVA are reported in Table 2 for all time windows. In the earliest time window (300−500 ms), mean amplitude for source hits was more positive than correct rejections for both older children and adults (consistent with effects for adults in Curran and Friedman, 2004). Both older children and adults showed these effects in sites that included frontal electrodes and Pz; additionally, older children showed effects in P3, whereas adults showed additional effects in midline and right hemisphere sites. For the 500−700 ms window, only older children showed differences between source hits and correct rejections. There were no differences between source hits and correct rejections for younger children in these two earliest windows that directly map to time windows used by Curran and Friedman (2004).
Table 2.
ERP Results for Condition (Source Hits, CR) x Age Group x Coronal x Sagittal ANOVA.
| Time Window | Omnibus ANOVA Condition Effects | Appropriate Follow-up Tests (Where condition differences found) |
||
|---|---|---|---|---|
| Younger Children | Older Children | Adults | ||
| 300−500 | 4-way: F(8,340) = 2.33, ηp2 = .05** C x G x Co * C x Co ** |
ns | Across Frontal: F(1,28) = 4.91, ηp2 = .15* P3: t(28) = 2.71, d = .50** Pz: t(28) = 4.18, d = .78*** |
Midline (Fz,Cz,Pz): t(29) = 3.01, d = .55*** Right (F4,C4,P4): t(30) = 2.33, d = .42** |
| 500−700 | C x G x Co: F(4,340) = 4.94, ηp2 = .10*** C x S x Co ** C x Co *** C x S * |
ns | Frontal: t(28) = -3.06, d = .57*** Parietal: t(28) = 3.50, d = .65*** |
ns |
| 700−900 | 4-way: F(8, 340) = 2.00, ηp2 = .05* C x G x S ** C x S x Co ** C x S *** C *** |
Right (F4, C4, P4) t(28) = -2.76, d = .51** |
Fz: t(28) = -2.81, d = .52** F4: t(28) = -2.25, d = .42* C4: t(28) = -2.35, d = .44** |
Across all electrodes: F(1, 29) = 17.16, ηp2 = .37*** (C x S x Co ANOVA revealed main effect of C) |
| 900−1100 | 4-way: F(8, 340) = 2.08, ηp2 = .05* C x S x Co *** C x S *** C** |
Fz: t(28)=-3.11, d = .58*** F4: t(28) = -2.39, d = .44** |
Fz: t(28) = -2.12, d = .39* |
Across Central: F(1, 29) = 6.02, ηp2 = .17** P3: t(30) = -2.12, d = .38* Pz: t(30) = -3.44, d = .62*** |
| 1100−1300 | 4-way: F(8, 340) = 3.15, ηp2 = .07*** C x S x Co *** C x S *** C x Co * C * |
Cz: t(28) = -2.14, d = .40* P3: t(28) = -2.51, d = .47** Pz: t(28) = -3.04, d = .56*** |
ns | Central: F(1, 29) = 7.67, ηp2 = .21** P3: t(30) = -3.14, d = .56*** Pz: t(30) = -4.84, d = .87*** |
| 1300−1500 | 4-way: F(8, 340) = 2.89, ηp2 = .06*** C x S x Co ** C x S ** C x Co *** |
P3: t(28) = -2.26, d = .42* Pz: t(28) = -2.38, d = .44** |
Parietal: t(28) = -2.10, d = .39* |
Central: F(1, 29) = 4.70, ηp2 = .14* P3: t(30) = -3.73, d = .67*** Pz: t(30) = -5.80, d = 1.04*** P4: t(30) = -2.46, d = .44** |
| 1500−1700 | C x S x Co: F(4, 340) = 3.72, ηp2 = .04** C x Co *** C x S ** |
Across ages effect: Parietal: t(88) = -2.89, d = .31*** |
||
| 1700−1900 | C x S x Co: F(4, 340) = 3.81, ηp2 = .04** C x Co *** |
Across ages effect: Parietal: t(88) = -2.89, d = .31*** |
||
Note. *p < .05, **p < .03, ***p < .006. C=Condition; G=Age Group; S=Sagittal; Co=Coronal; 4-way=4-way interaction. For the Omnibus ANOVA column, we present full statistics (e.g., df, test statistic, effect size) for the largest interaction effect that was significant; for remaining Condition effects we only report significance level for brevity. For the last 3 columns, we report only the final follow-up test findings for brevity; lower level tests were only pursued if higher level main effects or interactions were significant. If there is an interaction effect but the lower level tests are not significant, we report the abbreviation ns. Direction of effects are reported with positive or negative statistic (i.e., if t-statistic is positive source hits > correct rejections; if t-statistic is negative source hits < correct rejections).
Beginning at the 700−900 ms window, there were differences in mean amplitude for source hits compared to correct rejections for all age groups, but the topography of the effects differed based on age group. Differences were found in right hemisphere sites (i.e., F4, C4, P4) for younger children, and in two frontal sites (F4, F3) and one central site for older children. For adults, differential neural processing for source hits compared to correct rejections was observed across all 9 electrode sites. From 900−1100 ms, source hits differed from correct rejections across age groups, with differences in both child groups found in frontal sites and those for adults found in central and parietal sites. To summarize, differences between source hits and correct rejections were present for all age groups from 700−900 ms and 900−1100 ms. These are the first condition differences observed for younger children, which is consistent with previous studies showing that ERP effects occur later for children compared to adults (Cycowicz, et al., 2003; Czernochowski et al., 2009). Further, effects seemed to be more topographically widespread for adults. Effects for remaining time windows can be seen in Table 2; we see continued differential processing of source hits compared to correct rejections, including in the last two time windows (1500–1700ms and 1700–1900ms) in which effects were found in parietal electrodes across age groups.
3.3. Relations between brain and behavior
To examine relations between neural activity and behavioral performance, we ran correlation analyses between the magnitude of ERP effects (indices reflecting neural processes associated with episodic memory and temporal memory; see below) in early time windows and behavioral accuracy (proportions of source hits and source errors). Using an approach used by Riggins et al. (2013), we averaged across electrode sites to produce values for planned ERP regions of interest (Frontal: F3, Fz, F4; Parietal: P3, Pz, P4), which decreased the number of correlation analyses needed. Thus, for each participant, we calculated an average amplitude for the conditions listed below, for each region. Then using a procedure similar to Riggins et al., we created differences scores to account for individual differences in amplitude. We focused on our two earliest time windows (300−500 and 500−700 ms; windows used by Curran and Friedman, 2004). The difference scores were: (a) EM ERP magnitude [mean amplitude for Source Hits – mean amplitude for Correct Rejections] and (b) temporal ERP magnitude [mean amplitude for Source Hits – mean amplitude for Source Errors]. These difference scores were a proxy for individual differences in the neural correlates of episodic (i.e., EM ERP magnitude) and temporal memory (i.e., temporal ERP magnitude). We ran partial correlations, controlling for age (in months) and WASI performance, between ERP magnitudes and behavior (proportion of source hits, source errors). This correlational analysis parallels the group-level (ANOVA) analyses already reported (e.g., source hits vs. correct rejections; source hits vs. source errors). We report additional regressions, which complement the findings below, in Supplemental Results.
In the 300−500 ms time window, EM ERP magnitude in the parietal region was positively related to source hits, r = 0.224, p = 0.038, and negatively related to source errors, r=-0.289, p = 0.007. Temporal ERP magnitude in the parietal region was also positively related to source hits, r = .296, p = 0.006, and negatively related to source errors, r=-0.274, p =0.011. In the 500−700ms time window, EM ERP magnitude in the parietal region was positively related to source hits, r = .216, p = 0.046, and negatively related to source errors, r=-0.232, p = 0.032. In the 500−700ms the relation between Temporal ERP magnitude in the parietal region was not related to source hits (p = 0.12) or errors (p = 0.18). There were no significant correlations in the frontal region. Thus, individual differences in the magnitude of early EM and temporal ERP effects in parietal regions were related to behavioral temporal context memory accuracy.
To check that the partial correlations were unique to participants’ memory for temporal context (and not solely old/new recognition), we ran the same analyses with participants’ item memory (source hits + source errors) as the behavioral measure. These partial correlations were not significant (ps>0.20), strengthening the evidence for a relation between individual differences in participants’ neural activity and memory for temporal context.
4. Discussion
The goal of the present study was to examine the behavioral and neural correlates of memory for temporal context in 7- to 9-year-olds, 10- to 12-year-olds, and young adults. Participants encoded individual objects, presented in one of two lists separated by a break. During retrieval, while we recorded ERPs, participants judged the temporal context (i.e., list) of each object. To our knowledge, this is the first study to use ERPs to investigate temporal context memory with two groups of children (middle and late childhood) and adults. We found that accuracy of temporal context memory improved with age, and we observed ERP EM effects that began in early time windows for older children and adults (i.e., amplitude differences between source hits and correct rejections) and later time windows for younger children. Adults showed robust and widespread differences between source hits and correct rejections; differences between source errors and correct rejections were only apparent in later time windows. Older children showed neural activity differences between source errors and correct rejections even in early time windows. Importantly, we also observed brain-behavior relations between the magnitude of early ERP effects in parietal regions and behavioral performance.
The behavioral findings show significant age-related improvements between 7−9-year-olds and 10−12-year-olds, and between children and young adults. The observed age-related improvements parallel findings from previous studies examining memory for temporal context of personal and lab-based events (Friedman, 1991; Friedman and Lyon, 2005; Pathman et al., 2013b), including studies showing that children in late childhood are less accurate than young adults (Jack et al., 2016; Pathman and Ghetti, 2014) and shows that this type of memory is slow to develop. Patterns of behavioral performance suggest that all age groups were relying on memory strength to make their judgements. Across age groups, there was an effect of List, where there were more source hits for List 2 compared to List 1 items. Given that List 2 items were seen more recently, it is possible that participants had more vivid or clear memory for those items compared to those from List 1. Indeed, Friedman (1993) discussed how better accuracy for more recent compared to more distant events serves as evidence for the use of distance-based processes. Further, when errors occurred, all age groups were more likely to incorrectly attribute the old item to the more recent list. Since both lists were relatively recent and thus would feel relatively vivid, compared to events that occurred days or weeks ago, reliance on distance-based processes would lead to more errors in which List 1 items are judged as coming from List 2 than vice versa, which is the pattern found across age groups. Overall, behavioral accuracy is in line with our participants (including children as young as 7−9-years-old) using distance-based processes, and is consistent with other work showing that 8- to-10-year-olds and adults use distance-based processes in similar ways (Pathman et al., 2013a).
The use of ERP in the present study, a methodology used to examine the neural correlates of source memory in children and adults (Cycowicz et al., 2003; Czernochowski et al., 2005), provides novel insights into the development of memory for temporal context. The presence of early and late ERP old/new effects for adults and older children, as well as the later effects for younger children, are consistent with effects observed in studies of source memory and ERPs with similar age groups (Cycowicz et al., 2003; Czernochowski et al., 2005; Sprondel et al., 2011).
In adults, we found that mean amplitude for source hits was more positive than correct rejections during the earliest time window (300−500 ms), which is consistent with findings from Curran and Friedman (2004; ‘day test’) in which adults saw two lists (i.e., List 1, List 2) in the same room, separated by a day and then participated in a 3-choice discrimination task (i.e., List 1, List 2, New). They found that correct List 1/List 2 trials were associated with more positive amplitudes than for correct new trials. Ratings of strategies used was in line with the use of distance-based processes to perform this task. Curran and Friedman (2003) examined ERP differences between correct responses (correctly identified list items vs. correctly identified new items) in frontal sites during the 300−500 ms window and they examined ERP differences in parietal regions in the 500−700 ms window, and found expected differences. We found effects in midline and the right hemisphere for adults (which included frontal and parietal electrodes) in the 300−500 time window, however, we might expect topographical differences given methodological differences between studies. We did not observe differences in adult ERPs for source hits and correct rejections from 500−700 ms, in contrast to findings from Curran and Friedman (2004). However, we did find evidence of widespread differences between the conditions before this window and after this particular window in our study. For later time windows, we are cautious to draw direct comparisons between our results and Curran and Friedman (2003) since the aims of that study were different and they did not examine late (800−1800 ms) ERP effects using List 1 trials. However, they found EM effects in frontal electrode clusters (they did not examine central or parietal electrodes). We also found EM effects in late time windows associated with correct source compared to new items, especially in parietal electrodes.
Researchers have linked ERP EM effects to familiarity and recollection processes (see Friedman and Johnson, 2000; Rugg and Curran, 2007, for reviews), which come from the dual-process model of recognition memory (see Yonelians, 2002, for review). Familiarity processes are described as rapid and semiautomatic, which rely on a feeling of knowing or judgment of memory strength (Yonelinas, 2002). Whereas recollection processes involve the controlled retrieval of contextual details associated with an item or stimulus. Curran and Friedman (2003) suggested that distance-based processes and reconstruction processes that underly temporal memory are similar to familiarity and recollection processes discussed in the recognition memory literature. Reminiscent of distance-based processes, familiarity processes involve assessing whether an item is old or new based on quantitative memory strength (Yonelinas, 1994; Yonelinas et al., 2010), not requiring the retrieval of associated contextual details. Like reconstruction processes, recollection processes are controlled and involve retrieval of details associated with an event. Curran and Friedman discussed differences found in ERPs for source hits and correct rejections from 300−500 ms as evidence of an early, frontal old/new effect. In terms of temporal memory processes, however, Curran and Friedman state although they predicted to find ERP differences between certain conditions that would show evidence of distance-based processing, “no such differences were unambiguously observed” (p. 117). The present study was not designed to assess the distinction between familiarity and recollection, although previous behavioral research indicates that familiarity develops more quickly than recollection (Ghetti and Angelini, 2008), and more research is needed to explain how distance-based and reconstruction processes might parallel familiarity and recollection processes. Future work could examine if distance-based and reconstruction processes are affected in the same ways as familiarity and recollection. For example, research shows that familiarity processes are unaffected by divided attention, while recollection processes are affected (Espinosa-García et al., 2017). Thus, future work in which temporal memory is assessed with a paradigm that incorporates a divided attention manipulation could help to illuminate the relation between temporal memory processes (e.g., distance-based processes) with familiarity and recollection.
Early in the recording epoch we observed differential neural processing for source hits compared to correct rejections, for older children and adults, but not younger children, which provides neural evidence that could account for developmental differences we see in behavioral temporal memory accuracy. This robust evidence of early differentiation of source hits compared to correct rejections in adults is in contrast to the analysis of source errors compared to correct rejections, in which we found fewer time windows overall, and no time windows early in the recording epoch, in which adults showed differential neural processing. Although source errors are technically ‘old’ trials, in our study the task was always to retrieve temporal source (and source errors are failures to do so). This is different from previous temporal memory studies that involved an old/new recognition decision first, followed by other decisions that included a judgment of whether the old item was presented in List 1 or 2 (Trott et al., 1997). Curran and Friedman (2004) did not report analyses with incorrect source trials and so we cannot directly link our source errors analyses to this previous work. However, we suggest that the robust ERP effects for source hits compared to correct rejections in early time windows for adults (in combination with less robust ERP effects for source errors compared to correct rejections) reflect early neural processing related to successful temporal context decisions. Our analysis of individual differences in early neural processing further extends this claim.
Notably, we found that individual differences in the magnitude of ERP effects in parietal sites in the earliest time window were related to behavioral accuracy. Riggins et al. (2013) observed similar brain-behavior relations in early childhood when looking at source memory, but this is the first evidence of such brain-behavior relations between ERPs and memory for temporal context, to our knowledge. Specifically, we found brain-behavior relations based on individual differences in neural correlates of overall episodic memory but also individual differences in neural correlates of more specific temporal context memory. Further, our regression analyses (in Supplemental Results) revealed that individual differences in the magnitude of our temporal ERP effect early in the recording epoch contributed unique variance to behavioral temporal accuracy (correctly attributing an item to the correct temporal context, i.e., list), but this temporal ERP effect did not predict behavioral recognition accuracy (judging an old item as old). Thus, it is possible that similar early retrieval processes were used by adults and children who performed better on the temporal memory task. In an eye-tracking study of temporal memory, Pathman and Ghetti (2014) observed relations (controlling for age) between the magnitude of eye-movement effects (i.e., longer looking time at correct stimuli compared to incorrect stimuli in early time windows; eye movement effects that may be dependent on the hippocampus: Hannula and Ranganath, 2009) and behavioral temporal memory accuracy. Pathman and Ghetti’s study is relevant to the present work because they found eye tracking effects for adults, but not for the 7-year-old group (at least at the group level); 10-year-olds showed effects but at later time windows, which is reminiscent of the present findings in which we found delayed old/new ERP effects for our youngest age group. Further, Pathman and Ghetti showed that even in the absence of group level effects (e.g., presence of eye movement effect), individual differences in those effects predicted overt temporal memory accuracy. This parallels our findings in which we did not find that ERPs differed for source hits compared to source errors at the group level, but individual differences in early temporal ERP magnitude (mean amplitude for source hits minus source errors) predicted overt accuracy. Pathman and Ghetti (2014) attributed the different patterns of eye movement effects based on age to the continued development of the hippocampus during middle and late childhood. Future work in which temporal memory is examined with neuroimaging is required to test how structural or functional changes in the hippocampus and prefrontal cortex contribute to improvements in temporal memory across childhood and into adulthood. Future work in which eye movements and ERP are combined to examine temporal memory in childhood could also be fruitful.
4.1. Conclusions
We examined the development of memory for temporal context in middle and late childhood and adulthood using a lab-based recognition memory paradigm and recording of ERPs during retrieval. This is the first study that allows examination of distance-based memory processes, and neural signatures of temporal memory in different groups of children and young adults. We found both behavioral and ERP effects, which indicate the continued improvement of temporal memory beyond childhood and differences in memory processes across age. Our findings add to the relatively small literature on temporal memory development, and can lead to multiple new directions of future research.
CRediT authorship contribution statement
Kathleen M. Bettencourt: Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review & editing. Laurel H. Everett: Funding acquisition, Investigation, Methodology. Yixin Chen: Investigation, Methodology. Thanujeni Pathman: Conceptualization, Funding acquisition, Methodology, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the families and undergraduate students who participated. Some of the funding for this work came from an Undergraduate Research and Creativity Award from UNCG awarded to LE and TP. We thank members of the MDLaB for help with data collection and scoring. We acknowledge the support of a Discovery Grant awarded to TP from the Natural Sciences and Engineering Research Council of Canada (NSERC), [funding reference number RGPIN-2018-05916].
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100932.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bauer P.J. Lawrence Erlbaum; Mahwah, NJ: 2007. Remembering the Times of Our Lives: Memory in Infancy and Beyond. [Google Scholar]
- Bender R., Lange S. Adjusting for multiple testing – when and how? J. Clin. Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Brodeur M.B., Dionne-Dostie E., Montreuil T., Lepage M. The bank of standardized stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010773. 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada K.L., Pathman T., Riggins T. Longitudinal development of memory for temporal order in early to middle childhood. J. Genet. Psychol. 2020;181(4):237–254. doi: 10.1080/00221325.2020.1741504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway M.A., Pleydell-Pearce C.W. The construction of autobiographical memories in the self-memory system. Psychol. Rev. 2000;107(2):261–288. doi: 10.1037/0033-295X.107.2.261. [DOI] [PubMed] [Google Scholar]
- Curran T., Friedman W.J. Differentiating location- and distance-based processes in memory for time: an ERP study. Psychon. Bull. Rev. 2003;10(3):711–717. doi: 10.3758/bf03196536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Friedman W.J. ERP old/new effects at different retention intervals in recency discrimination tasks. Cogn. Brain Res. 2004;18(2):107–120. doi: 10.1016/j.cogbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M. Orienting memory to unexpected and/or unfamiliar visual events in children and adults. Dev. Cogn. Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Duff M. Pictures and their colors: what do children remember? J. Cogn. Neurosci. 2003;15(5):759–768. doi: 10.1162/jocn.2003.15.5.759. [DOI] [PubMed] [Google Scholar]
- Czernochowski D., Mecklinger A., Johansson M., Brinkmann M. Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adults. Cogn. Affect. Behav. Neurosci. 2005;5(4):417–433. doi: 10.3758/cabn.5.4.417. [DOI] [PubMed] [Google Scholar]
- Czernochowski D., Mecklinger A., Johansson M. Age-related changes in the control of episodic retrieval: an ERP study of recognition memory in children and adults. Dev. Sci. 2009;12(6):1026–1040. doi: 10.1111/j.1467-7687.2009.00841.x. doi: 10.111/j.1467-7687.2009.00841.x. [DOI] [PubMed] [Google Scholar]
- Espinosa-García M., Vaquero J.M.M., Milliken B., Tudela P. Recollection and familiarity for words and faces: A study comparing remember-know judgments and the process dissociation procedure. Memory. 2017;25(1):19–34. doi: 10.1080/09658211.2015.1120310. [DOI] [PubMed] [Google Scholar]
- Friedman W.J. The development of children’s memory for the time of past events. Child Dev. 1991;62(1):139–155. doi: 10.1111/j.1467-8624.1991.tb01520.x. [DOI] [Google Scholar]
- Friedman W.J. Memory for the time of past events. Psychol. Bull. 1993;113(1):44–66. doi: 10.1037/0033-2909.113.1.44. [DOI] [Google Scholar]
- Friedman W.J. The development of temporal metamemory. Child Dev. 2007;78(5):1472–1491. doi: 10.1111/j.1467-8624.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- Friedman W.J. The development of memory for the times of past events. In: Bauer P.J., Fivush R., editors. The Wiley Handbook on the Development of Children’s Memory. Wiley-Blackwell; West Sussex, UK: 2014. pp. 394–407. [Google Scholar]
- Friedman D., Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc. Res. Tech. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51. [DOI] [PubMed] [Google Scholar]
- Friedman W.J., Lyon T.D. Development of temporal-reconstructive abilities. Child Dev. 2005;76(6):1202–1216. doi: 10.1111/j.1467-8624.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 2008;79(2):339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Haese A., Czernochowski D. Task characteristics are critical for the use of familiarity: an ERP study on episodic memory development in middle childhood. Cogn. Dev. 2016;40:82–100. doi: 10.1016/j.cogdev.2016.08.008. [DOI] [Google Scholar]
- Hannula D.E., Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63(5):592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack F., Friedman W., Reese E., Zajac R. Age-related differences in memory for time, temporal reconstruction, and the availability and use of temporal landmarks. Cogn. Dev. 2016;37:53–66. doi: 10.1016/j.cogdev.2015.12.003. [DOI] [Google Scholar]
- Luck S. MIT Press; Cambridge, MA: 2014. An Introduction to the Event-related Potential Technique. [Google Scholar]
- Makeig S., Bell A.J., Jung T., Sejnowski T.J. Independent component analysis of electroencephalographic data. In: Touretzky D., Mozer M., Hasselmo M., editors. VOL 8. MIT Press; Cambridge, MA: 1996. pp. 145–151. (Advances in Neural Information Processing Systems). [Google Scholar]
- Pathman T., Ghetti S. The eyes know time: a novel paradigm to reveal the development of temporal memory. Child Dev. 2014;85(2):792–807. doi: 10.1111/cdev.12152. [DOI] [PubMed] [Google Scholar]
- Pathman T., Doydum A., Bauer P.J. Bringing order to life events: memory for the temporal order of autobiographical events over an extended period in school-aged children and adults. J. Exp. Psychol. 2013;115(2):309–325. doi: 10.1016/j.ecp.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Pathman T., Larkina M., Burch M.M., Bauer P.J. Young children’s memory for the times of personal past events. J. Cognit. Dev. 2013;14(1):120–140. doi: 10.1080/15248372.2011.641185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger T.V. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard L., Cousin S., Guillery‐Girard B., Eustache F., Piolino P. How do the different components of episodic memory develop? Role of executive functions and short‐term feature‐binding abilities. Child Dev. 2012;83:1037–1050. doi: 10.1111/j.1467-8624.2012.01736. [DOI] [PubMed] [Google Scholar]
- Riggins T., Rollins L. Developmental differences in memory during early childhood: insights from event-related potentials. Child Dev. 2015;86(3):889–902. doi: 10.1111/cdev.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Rollins L., Graham M. Electrophysiological investigation of source memory in early childhood. Dev. Neuropsychol. 2013;38(3):180–196. doi: 10.1080/87565641.2012.762001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins L., Riggins T. Developmental changes in memory encoding: insights from event-related potentials. Dev. Sci. 2013;16(4):599–609. doi: 10.1111/desc.12072. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Curran T. Event-related potentials and recognition memory. Trends Cogn. Sci. (Regul. Ed.) 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Sprondel V., Kipp K.H., Mecklinger A. Developmental changes in item and source memory: evidence from an ERP recognition memory study with children, adolescents, and adults. Child Dev. 2011;82(6):1638–1653. doi: 10.1111/j.1467-8624.2011.01642.x. [DOI] [PubMed] [Google Scholar]
- Trott C.T., Friedman D., Ritter W., Fabiani M., Snodgrass J.G. Episodic priming and memory for temporal source: event-related potentials reveal age-related differences in prefrontal functioning. Psychol. Aging. 1999;14(3):390–413. doi: 10.1037/0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E., Donaldson W., editors. Organization of Memory. Academic Press; New York: 1972. pp. 381–403. [Google Scholar]
- Voss J.L., Paller K.A. Neural substrates of remembering: event-related potential studies. In: Byrne J.H., editor. Learning and Memory: A Comprehensive Reference. Academic Press; 2017. pp. 81–98. [DOI] [Google Scholar]
- Yonelinas A.P. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J. Exp. Psychol. Learn. Mem. Cogn. 1994;20(6):1341–1354. doi: 10.1037/0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46(3):441–517. doi: 10.1006/jmla.2002.2864. [DOI] [Google Scholar]
- Yonelinas A.P., Aly M., Wang W., Koen J.D. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.