Summary
Social learning, which is a mechanism that allows an individual to acquire skills from other individuals, occurs in a social context. Therefore, factors that influence social context, like social structure, will impact social learning opportunities. This review explores how features of social structure affect social learning opportunities in primates, either through their relationship with social tolerance or through the number of social learning models. Features that are investigated in this review and that we hypothesize affect social learning opportunities are parental investment, dominance hierarchy, nepotism, social bonds, dispersal, group size, fission-fusion dynamics, and sex ratio. For most of these features we find evidence, but support varies. Of all primate species, only humans show all the requirements of an optimal social structure to promote social learning. Future research into social learning and culture should not overlook the social context in which it takes place.
Subject areas: Natural Sciences, Cognitive Neuroscience, Sociology
Graphical Abstract
Natural Sciences; Cognitive Neuroscience; Sociology
Introduction
Social learning is an important mechanism used widely by individuals of multiple taxa, ranging from social insects to primates, to acquire skills already exhibited by others (for a review see: Allen, 2019). By using social learning, defined as “learning that is influenced by observation of, or interaction with, another animal or its product” (Heyes, 1994), individuals can circumvent the costs associated with asocial learning (Griffin, 2004; van Schaik and Burkart, 2011). Social learning is used over a wide range of behaviors, such as locating food (Frisch, 1946), mate selection (White, 2004), migration routes (Mueller et al., 2013), and tool-use (Whiten et al., 1999). In addition, it is an essential mechanism for the transmission of innovations and of culture, and it allows individuals to increase the number of skills they can possess in a lifetime (Aplin, 2016; van Schaik and Burkart, 2011; Schuppli and van Schaik, 2019). At the same time, social learning also increases asocial learning skills due to high overlap of cognitive mechanisms (Heyes, 1994; van Schaik and Burkart, 2011).
Interest in social learning has recently intensified, and research has focused on strategies that individuals use to select when and from whom to socially learn (Kendal et al., 2018), the cognitive mechanisms behind social learning, and the adaptive utility of social learning as a driving force behind the diffusion of innovations (Aplin, 2016). However, less attention has been devoted to the social context promoting social learning.
Individuals who use social learning learn by observing or interacting with another individual. The cognitive mechanisms behind social learning range from simple stimulus enhancement to emulation (Whiten, 2000). Through social learning, the knowledge of one individual is altered by another individual, thus social learning is in essence an interaction between a minimum of two individuals. Social transmission of knowledge can occur in different pathways: the most common one is vertical social transmission (i.e., parent to offspring), but it can also be oblique (i.e., adult to unrelated juvenile) or horizontal (i.e., between peers of the same generation). Social learning does not occur in every interaction between individuals; nevertheless, every interaction can be an opportunity for social learning. The probability of social learning actually occurring depends upon both the quantity and quality of the social learning opportunities and the complexity of the behavior to be learnt. An increase in the quantity of social learning opportunities will lead to more social learning in general. This can be achieved by simply increasing the number of potential social learning models in a group (van Schaik and Pradhan, 2003). The number of social learning models is hereby defined as the number of conspecifics with which an individual interacts within a group. The quality of a social learning opportunity can be defined by the social tolerance between the individuals involved (Coussi-Korbel and Fragaszy, 1995; van Schaik, 2003). Social tolerance is hereby defined as the average time and proximity of each interaction between two individuals and, therefore, the quality of their interactions. High levels of social tolerance correlate with high-quality opportunities for social learning. Both the prolonged time and close proximity of these interactions provide higher chances of social learning (Coussi-Korbel and Fragaszy, 1995; van Schaik, Deaner and Merrill, 1999; Rapaport and Brown, 2008; Perry, 2009). In addition, more complex behaviors will require more or longer observations to learn and may be linked to the quality of the social learning opportunities. However, the context promoting social learning of more complex behavior will not be systematically addressed, as we find it difficult to determine how complex a learned behavior is. It is only mentioned when explicitly addressed in a study. Therefore, in this review we will focus on the opportunities to socially learn as determined by the number of social learning models and social tolerance, to gain a greater understanding of the social contexts promoting social learning. Both the number of social learning models and the social tolerance depend on the social structure an individual is part of.
We limit this review in several ways. First, there are many different features of social structure (Mitani et al., 2012), so here we review only the features that most likely influence social learning opportunities, due to their effect on the number of social learning models, on social tolerance, or a combination of both. On the basis of our ideas and findings, we propose a conceptual framework of how social structure can influence opportunities for social learning. Second, identifying what features of social structure promote or limit opportunities for social learning sets the stage for future research that may explore their evolutionary link. The need for social learning may select for specific social settings, yet, alternatively, social settings resulting from other evolutionary pressures may favor social learning. In this article, the potential for these different evolutionary connections is only shortly addressed in the discussion. Third, although social learning is widespread taxonomically, we limit this review to the order of primates, because there is substantial information available on both their social structure and social learning. Furthermore, primates show impressive variation in their social structure (Mitani et al., 2012). Last, many diurnal and group-living primates possess very similar capacities, like good vision and manual dexterity, making them comparable to each other.
We review several features of social structure and argue which of these may affect social learning opportunities through either their effect on the number of social learning models or their effect on social tolerance. First, we propose that parental investment will have a positive influence on social learning opportunities through the high levels of social tolerance between parent(s) and offspring. Second, we hypothesize that dominance hierarchy and nepotism will have a negative influence on social learning opportunities through low levels of social tolerance, whereas social bonds show an opposite effect. Third, we argue that dispersal will have a positive influence on social learning opportunities through an increase in the number of social learning models. Fourth, we propose that group size will have a conflicting influence on social learning opportunities by having a contrasting effect on the number of social learning models and social tolerance, whereby larger groups have more social learning models but lower social tolerance. Fifth, we hypothesize that fission-fusion dynamics will have a positive influence on social learning opportunities through increased levels of social tolerance. Last, we argue that different facets of group composition, such as sex ratio, will influence social learning opportunities through varying levels of social tolerance. Because this review aims to investigate the influence of social structure on social learning opportunities and not on social learning itself, we do not consider the mechanisms behind social learning. Furthermore, we will mainly focus on socially learned behavior in the physical realm, because the results of social learning in the physical realm, as opposed to the social realm, are rather unambiguous and have been well studied. Examples of behaviors in the physical realm are tool-use, foraging, or food extracting behaviors. When clear examples of socially learned social behaviors are available, we do mention them. Finally, we indicate interesting directions for future research focusing on the social context of social learning. To conclude, we will summarize these components of social structure and hypothesize what forms an ideal social structure to promote social learning opportunities.
Learning from primary caretakers
Mothers and other caretakers
Social learning is most likely to take place when the model is tolerant and available for a long period of time, and primary caretakers (e.g., mothers) fit these requirements. Indeed, primate infants adhere to this, as they have slow life histories in which offspring spend a long time in close proximity to their mother (Whiten and van de Waal, 2018). One extreme case is that of Sumatran orangutans (Pongo abelii), which can spend up to 10 years with their mother (Wich et al., 2004). But even in less extreme cases, primate infants still spend a relatively long period, often several years, close to their mother (Lonsdorf et al., 2014). In addition to this long association time, mother and offspring typically show high levels of social tolerance (Japanese macaques, Macaca fuscata: Belisle and Chapais, 2001; gorillas, Gorilla gorilla: Maestripieri, Ross and Megna, 2002; chimpanzees, Pan troglodytes: Lonsdorf et al., 2014; review: Whiten and van de Waal, 2018). Therefore, it is likely that mother-infant dyads show high levels of social learning, also called vertical social transmission (Bebko and Russon, 2015). This will result in mother and infant showing high overlap in their learned skills.
As expected, many examples of infants learning from their mothers (review: Whiten and van de Waal, 2018) are found of which we will mention a few here. Mantled howler monkeys (Alouatta palliata) learn to avoid toxic mature leaves by co-feeding with their mother (Whitehead, 1986). Furthermore, both Bornean orangutans (Pongo pygmaeus) and vervet monkeys (Chlorocebus pygerythrus) show food preferences similar to their mother (Jaeggi et al., 2010; van de Waal, Borgeaud and Whiten, 2013). Similarly, chimpanzees learn the use of tools from their mother (Boesch, 1991; Lonsdorf, 2006). The long association time and the high levels of social tolerance may be particularly important for learning complex behavior, especially when this behavior cannot be learned instantaneously, but requires repeated observations at close proximity. Indeed, the complex behavior of nut cracking, where chimpanzee and capuchin infants attend to their mother's behavior, can take several years to master (Inoue-Nakamura and Matsuzawa, 1997; tufted capuchin monkey, Sapajus apella: Resende et al., 2008). These are just a few examples of infants learning from their mother.
When the social tolerance between caretakers and offspring is high, having multiple caretakers greatly increases the social learning opportunities (Enquist et al., 2010). In most primate species, rearing is mostly done by mothers (Mitani et al., 2012). However, in callitrichids, fathers predominantly carry the offspring, reducing the role of the mother (Fernandez-Duque et al., 2012), and other genetically related individuals help taking care of the infant, forming a cooperative breeding system (Mitani and Watts, 1997; Burkart and van Schaik, 2016). In addition to carrying infants, callitrichid allocaretakers also provide infants with food and allow scrounging (Feistner and Price, 1991; Price and Feistner, 1993; Ruiz-Miranda et al., 1999). Because of the higher number of social learning models that show high levels of social tolerance, species that live in cooperative breeding systems are expected to show high levels of social learning. Indeed, studies show that infants learn about novel food from their provisioning allocaretakers (Rapaport, 1999; Rapaport and Brown, 2008; Voelkl, Schrauf and Huber, 2006; for a review see: Snowdon, 2001).
Overall, mothers form an ideal social learning model because of the long association time and high levels of social tolerance. Accordingly, offspring learn many and also very complex behaviors from their mother. In addition, social systems with allocaretakers show oblique social transmission and provide additional important models to learn from.
Creating learning opportunities
The evolutionary importance of infant social learning from their primary caregivers may also set the stage for primary caretakers and/or offspring to actively create social learning opportunities. Although social learning can be achieved by just being near a social learning model, actively creating a social learning opportunity increases the chance of social learning to occur. Active creation of a social learning opportunity is defined as an individual changing its behavior in such a way that it enhances the possibility of learning. The active creation of a social learning opportunity can be initiated by both primary caretakers and infants.
Many examples of caretakers actively creating social learning opportunities can be found (Rapaport and Brown, 2008). For example, caretakers can create social learning opportunities by transferring food to their offspring, where they supply novel food more often than familiar food (Rapaport, 1999; Jaeggi, van Noordwijk and van Schaik, 2008). Chimpanzee mothers facilitate nut-cracking learning opportunities for their infants by supplying them with the right tools at the right location (Boesch, 1991). An even more active approach on the caretakers' side is teaching or tutoring (Thornton and Raihani, 2010). Teaching is hereby defined as an individual modifying its behavior only in the presence of a naive observer, without receiving any immediate benefit, and as a result, the naive observer will acquire knowledge or a skill quicker than it would otherwise (Caro and Hauser, 1992; Thornton and Raihani, 2010). Although rare, occurrences of teaching have been seen in chimpanzees (Boesch, 1991; Musgrave et al., 2016) and golden-lion tamarins (Leontopithecus rosalia: Rapaport and Ruiz-Miranda, 2002).
Infants can also create social learning opportunities. Infants can do this by peering, which is defined as: “Directly looking at the action of another individual […] at a close enough range that enables the peering individual to observe the details of the action” (Schuppli et al., 2016, Figure 1). Infant peering has been reported in multiple species (Maestripieri, Ross and Megna, 2002; Fragaszy and Visalberghi, 2004; Schiel and Huber, 2006; Schuppli et al., 2016). Infants often peer more at novel food or novel situations than familiar situations, thereby creating new social learning opportunities (Voelkl, Schrauf and Huber, 2006; Jaeggi et al., 2010). Another way in which infants can create social learning opportunities is by begging. Especially when begging is done for novel or difficult-to-process food items, the potential information gain is large (Brown et al., 2004). This pattern has been seen in multiple primate species (orangutans: Jaeggi, van Noordwijk and van Schaik, 2008; chimpanzees: Nishida and Turner, 1996; golden lion tamarins: Ruiz-Miranda et al., 1999).
Figure 1.
An infant vervet monkey (Xinji, 2y, male) actively creating a social learning opportunity for novel food by peering at his mother (Xian, 8 years, female) eating a stick insect (Phasmatodea)
Photograph was taken by Lukas Schad at the Inkawu Vervet Project, South Africa.
In conclusion, both caretakers and infants actively create social learning opportunities.
Variation in social tolerance
The nature of the average interaction between individuals, and thereby tolerance, is determined by the relationship of these two individuals. These relationships are considered from three different perspectives: dominance, nepotism, and social bond. These perspectives are based on, respectively, agonistic interactions, relatedness, and affiliative interactions; however, additional social patterns may also affect tolerance.
Dominance hierarchy
The hierarchical style of the dominance hierarchy in a group affects both social tolerance and learning opportunities. The formation of a dominance hierarchy in a group is a way that primates avoid intragroup competition (Bernstein et al., 1974; de Waal, 1986; Sapolsky, 1983). Social tolerance has been linked to the steepness of the dominance hierarchy (Thierry, 2007). Primates show great variation in the hierarchical style of their dominance hierarchy, ranging from extremely steep in despotic societies to substantially less steep in egalitarian societies (Thierry, 2000; Mitani et al., 2012). In despotic societies there is on average more intense aggression, little counter-aggression, and lower average proximity between individuals compared with egalitarian societies (Sterck, Watts and van Schaik, 1997; Matsumura, 1998; Thierry, 2000). More despotic societies also show lower levels of social tolerance between individuals (Thierry, 2000, 2007). Lower levels of social tolerance will lead to less social learning opportunities (Coussi-Korbel and Fragaszy, 1995; van Schaik, 2003).
As far as we are aware, no study has yet investigated the impact of different hierarchical styles on social learning. However, studies have revealed that more egalitarian societies perform better at cooperation tasks than despotic species, which may be a proxy for social learning (Hare et al., 2007; Joly et al., 2017). Another proxy for social learning is tool-use. Most primate species that show the use and manufacturing of tools are relatively egalitarian (Tonkean macaque, Macaca tonkeana: Anderson, 1985; tufted capuchin monkey: Ottoni, de Resende and Izar, 2005; orangutan: van Schaik et al., 2003; lion-tailed macaque, Macaca silenus: Westergaard, 1988; chimpanzee: Whiten et al., 1999; for a review see Bentley-Condit and Smith, 2010). However, tool-use also exists in a subspecies of the despotic long-tailed macaques (Macaca fascicularis aurea: Malaivijitnond et al., 2007).
Although strong despotism in dominance hierarchies decreases the levels of social tolerance, this effect is not equally strong for all individuals. In despotic societies, aggression is asymmetrical, meaning that lower-ranked individuals receive more aggression (Sterck, Watts and van Schaik, 1997; Thierry, 2000). Moreover, lower-ranked individuals are more often displaced and are forced more toward the periphery of the group (van de Waal, Borgeaud and Whiten, 2013; Carter, Ticó and Cowlishaw, 2016; Tan et al., 2018). Consequently, lower-ranked individuals also have fewer social learning opportunities compared with higher-ranked individuals.
Indeed, in the despotic Japanese macaque, only higher-ranked females managed to manufacture tools in a feeding experiment (Tokida et al., 1994). In the famous example of potato-washing, also in Japanese macaques, adolescent males were the last to pick up the behavior as they resided more at the periphery of the group (Kawai, 1965) and had fewer learning opportunities. In chacma baboons (Papio ursinus), lower-ranked individuals, which are on the periphery of the group, are not able to socially learn from others compared with the more central higher-ranked individuals (Carter, Ticó and Cowlishaw, 2016).
In conclusion, societies with more pronounced and despotic dominance hierarchies are expected to show less social learning because of their reduced social tolerance. However, there is some evidence indicating that tool-use, an important outcome of social learning, although prominent in egalitarian societies, also occurs in despotic societies. In addition, dominance hierarchies create an asymmetry in both social tolerance and opportunities to learn from models decreasing with rank, with lower-ranked individuals at the bottom. Accordingly, lower-ranked individuals learn fewer behaviors socially.
Nepotism
Along with the hierarchical style of the dominance hierarchy, there are other features that cause a variation in the levels of social tolerance. Nepotism, which is inter-related with despotic societies (Thierry, 1990; Sterck, Watts and van Schaik, 1997; Matsumura, 1998), is one of them. In nepotistic societies, individuals from the same matriline are more closely ranked compared with individuals of different matrilines. Nepotistic societies often show lower levels of social tolerance between matrilines than within matrilines (Belisle and Chapais, 2001; Smith, 2014). Nepotism can promote vertical social transmission, whereas it inhibits horizontal (between peers) and oblique (between unrelated individuals) social transmission, except for horizontal social transmission between siblings. Therefore, social learning opportunities are numerous within matrilines, but less common between matrilines.
Indeed, in Japanese macaques wheat-washing is prevalent in some matrilines, but almost absent in other matrilines from the same group (Kawai, 1965). This effect may be more pronounced in complex behaviors that require high levels of social tolerance. Indeed, the development of tool-use in long-tailed macaques (Tan et al., 2018) and the cleaning method of food in vervet monkeys (van de Waal et al., 2012) is more similar within than between matrilines. This effect is even visible in a social behavior of chimpanzees, in which the grooming style converges within matrilines, but not at the level of the group (Wrangham et al., 2016), suggesting social learning of social behavior. Altogether, nepotism promotes social learning within matrilines, but inhibits social learning between matrilines. Accordingly, socially learned traditions can be limited to only one or a few matrilines.
Social bonds
Although the hierarchical style of the dominance hierarchy and nepotism explain a large part of the variation in social tolerance, they do not explain all the variation. They both fail to identify affiliative behaviors between non-kin, which can be differently distributed (Lehmann and Ross, 2011). Unrelated individuals that show many affiliative interactions are considered to have a strong bond (Silk, 2002; Massen, Sterck and De Vos, 2010). Bonded individuals also show close proximity and high social tolerance (Silk, 2002). Moreover, individuals with many bonds have access to increased numbers of social learning models. Therefore, more social learning opportunities will occur between individuals with strong bonds and that have many bonds, promoting both horizontal and oblique social transmission.
Indeed, in common squirrel monkeys (Saimiri sciureus), individuals with a higher number of bonds had a higher chance of socially learning a new behavior during an experiment than those with few bonds (Claidière et al., 2013). Similarly, long-tailed macaques are more likely to learn tool-use from models they groom relatively often (Tan et al., 2018). However, tufted capuchin monkeys show no correlation between bond strength and social learning bias (Coelho et al., 2015), yet this may be due to the overall high levels of social tolerance in capuchin monkeys (Fernandez-Duque et al., 2012).
Altogether, individuals with strong bonds and with a high number of bonds are expected to share more social learning opportunities, because of increased levels of social tolerance. However, although this effect may be limited to despotic societies, systematic empirical data linking dominance hierarchy, nepotism, and bonds are still lacking.
Additional variation in social tolerance
Although dominance, nepotism, and social bonds are recognized to structure social interactions, other features may also affect social interactions, such as additional patterns identified by social network analyses (SNA) and leadership. SNA is an important tool for recognizing social structure in relationships different from dominance, nepotism, or bonds (Kasper and Voelkl, 2009; Lehmann and Ross, 2011). Moreover, it can identify individual (e.g., centrality), dyadic (e.g., vertex strength), and group-level patterns (e.g., density) (Kasper and Voelkl, 2009). In addition, SNA has already successfully been used to show that some of these additional patterns can reflect social learning (Claidière et al., 2013).
In addition, leadership may form an additional phenomenon that structures social learning. Leadership has been assigned to individuals with specific competences (Chapais 2015) that indicate lines of action (Vermande and Sterck 2020) and, in addition to dominance and affiliation, may structure social behavior (Vermande and Sterck 2020). In humans, leaders are readily copied (Henrich and Gil-White, 2001). Thus, behavior of leaders might be preferentially copied. However, research on primate leadership is in its infancy, and an empirical connection to social learning remains to be established.
Dispersal
Immigrating individuals
An individual has more social learning opportunities when it encounters a larger number of social learning models in its life, and dispersing does just that. In many primate species one or both sexes disperse from their natal group to another group (Strier, 1994; Mitani et al., 2012). Dispersal can occur to avoid incest, infanticide, feeding competition, or mating competition (Moore, 1984; Sterck, Watts and van Schaik, 1997; Sterck and Korstjens, 2000). The goal of dispersing individuals is to immigrate into another group. Although this new group can show an overlap in behavior with the previous group, there are also good chances that this group will have different socially learned traditions compared with the origin (natal or non-natal) group (van Schaik and Knott, 2001; Luncz and Boesch, 2014; Tan et al., 2015; van de Waal, 2018). Consequently, members of this new group form new potential social learning models for the immigrant individual. By dispersing, an individual will have access to a larger group of learning models and a larger variety of socially learned behaviors. Because natal individuals disperse without their mother, dispersal inhibits vertical social transmission. However, it does promote horizontal and oblique social transmission by increasing the pool of potential social learning models available for the immigrant.
Indeed, immigrating individuals are able to adopt new behaviors present in the group, showing behavioral flexibility (Whiten and van de Waal, 2018). Immigrating vervet monkeys adopt the food preference in a group, even when it is the opposite of their own preference (van de Waal, Borgeaud and Whiten, 2013). In chimpanzees, transferring females adapt to local social customs (Nakamura and Uehara, 2004), and, in addition, complex behaviors, like tool-use, are also adopted by immigrating females (Luncz and Boesch, 2014). However, whether dispersing individuals have a broader learned repertoire than resident individuals has not yet been studied.
In conclusion, by dispersing, an individual increases the number of social learning models it encounters in its life, potentially increasing the variety of learned behaviors. Accordingly, immigrating individuals show that they can adopt novel behavior from these new social learning opportunities.
Resident individuals
Dispersal not only enhances the learning opportunities for the dispersing individual but also increases the social learning opportunities for resident individuals. After all, new immigrants may have different traditions compared with residents of their new group. Therefore, immigrants can form a new social learning model for resident individuals through both oblique and horizontal social transmissions and can spread new behaviors between groups (Nunn et al., 2009).
Although rarely seen, two potential examples of this have been reported for chimpanzees. A group of chimpanzees seems to have learned how to crack a newly introduced species of nut from one female who immigrated from a group in which that particular nut species was cracked habitually (Biro et al., 2003). In another group of chimpanzees, a new female that immigrated into the group may have introduced a tool-assisted ant-fishing technique into a group where this behavior had been absent for several decades (O’Malley et al., 2012).
Altogether, dispersal allows resident individuals to encounter new social learning models and thus increase the variety of possible socially learned behaviors. However, evidence is still rare. Although it has already been demonstrated that the immigrating individual adopts traditions of the residents, we suggest that social learning from migrants may best be documented when experimentally tested.
Group size, fission-fusion dynamics, and group composition
Group size
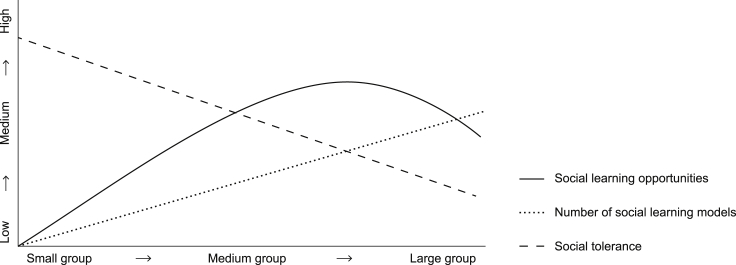
Group size can affect both the number of social learning models and their tolerance. When a larger number of social learning models is encountered, more social learning opportunities will arise, and one way of achieving this is by increasing group size. Therefore, group size should have a positive influence on social learning opportunities. Indeed, in theoretical models group size does have a positive influence on social learning (van Schaik and Pradhan, 2003; Nunn et al., 2009). In contrast, in one meta-analysis of 116 species of primates, no link was found between group size and social learning (Reader and Laland, 2002). This suggests that there are more forces at play not included when only considering group size. Within species, the degree of tolerance may depend on group size.
The level of food competition in a group of a particular size may affect tolerance. Food availability is one of several factors that influence group size in a species (van Schaik, 1983; Janson and Goldsmith, 1995). Increasing the size of a group, when food availability stays the same, will increase within-group feeding competition (Chapman et al., 2012). This increase in within-group competition will decrease social tolerance between individuals (Sterck, Watts and van Schaik, 1997; van Schaik, 2003), and this will have a negative effect on the social learning opportunities (Coussi-Korbel and Fragaszy, 1995). So, due to increased within-group food competition, a larger group size will have a negative influence on social learning. We propose that the interaction between social tolerance and the number of social learning models will lead to an optimal group size that maximizes social learning opportunities (Figure 2). At the optimal group size the benefits of having more social learning models will balance with the disadvantage due to the decreasing social tolerance caused by within-group feeding competition. The slope of the lines and where they meet will depend on different ecological and social factors. The opposing effects of group size may explain the failure of the meta-analysis to find an average effect of group size on social learning (Reader and Laland, 2002). In addition, the optimal group size may differ between species, such that in more tolerant species larger optimal group sizes can be reached.
Figure 2.
Hypothetical figure showing the influence of group size on number of social learning models (dotted line) and social tolerance (dashed line)
This has consequences for the social learning opportunities (solid line). When group size increases, the number of social learning models will increase as well. At the same time, social tolerance will decrease due to within-group food competition. These opposing effects will lead to an optimal group size for the maximum amount of social learning opportunities. The slopes of the lines and the resulting optimal group size will depend on different ecological and social factors.
As far as we are aware, no study has separated these opposing, but tied, effects of group size and tolerance, thus we urge researchers to test it experimentally.
Fission-fusion dynamics
Social learning is expected to increase if individuals are able to circumvent the negative effect of group size on social tolerance, and fission-fusion dynamics is one way of achieving this. Social groups have fission-fusion dynamics when they consist of one large pool of individuals that form smaller parties that have no fixed membership (Nishida, 1968; Aureli et al., 2008; Watts, 2012). By splitting up into temporary parties, individuals can reduce within-group competition (Chapman, Chapman and Wrangham, 1995; Boesch and Boesch-Acherman, 2000; Asensio et al., 2008) while maintaining the number of encountered social learning models. In addition, this reduction in within-group competition will increase social tolerance and, therefore, increase social learning opportunities (Coussi-Korbel and Fragaszy, 1995; Sterck, Watts and van Schaik, 1997; van Schaik, 2003).
Indeed, chimpanzees and bonobos (Pan paniscus), which are well-known examples of species with fission-fusion dynamics, show one of the highest levels of social learning found in primates (Whiten et al., 1999; Hohmann and Fruth, 2003). Moreover, spider-monkeys (Ateles geoffroyi), which are the other genus of primates known to show clear fission-fusion dynamics, possess multiple traditions, for example, customary drinking habits, resulting from social learning (Santorelli et al., 2011a, 2011b). In fission-fusion societies, most socially learned behaviors are found when individuals have high encounter rates with conspecifics (van Schaik, 2003).
In conclusion, fission-fusion dynamics seems to increase socially learned behavior by decreasing within-group competition while maintaining a relatively large number of social learning models. Accordingly, the few primate species that show fission-fusion dynamics exhibit high levels of social learning.
Group composition
When different classes of individuals show different levels of social tolerance, then the relative presence of these different classes of group members influences social learning opportunities. These classes may concern kin, sex, or age (Silk et al., 2006; Widdig et al., 2001). In a nepotistic society, the social learning opportunities of an individual depend on the distribution of kin versus non-kin individuals in a specific group. The sex of the learning model may also influence social tolerance. In primate societies, the sex ratio in groups of the same species can vary considerably, as well as the average sex ratio between different species (Kappeler and van Schaik, 2002; Mitani et al., 2012). If the sexes consistently show a different level of social tolerance, the sex ratio in a group or species will influence social learning opportunities. Similarly, the age of the learner or model may influence social tolerance. Juveniles of different primate species across the taxa receive higher levels of close proximity and social tolerance from all other group members compared with older primates, which can lead to more social learning opportunities (vervet monkeys: Fairbanks, 1993; long-tailed macaques: van Noordwijk et al., 1993; muriquis, Brachyteles arachnoides: Strier, 1993; great apes: Watts and Pusey, 1993). Groups that are composed of more juveniles will therefore have more social learning opportunities.
Although kinship affects tolerance, group effects of different degrees of relatedness on social learning have not been studied. In addition, as far as we are aware, no study has found a difference in social tolerance dependent on the sex of the model that is not explained by kinship, dominance, or dispersal (Lonsdorf, 2005; van de Waal et al., 2010). Furthermore, although no study has looked at the effect of the group composition of juveniles in relation to social learning, studies have shown that innovations can diffuse via juveniles using social learning (Kawai, 1965) and that younger individuals are more influenced by social information (Barret et al., 2017).
Altogether, group composition may influence social learning opportunities if social tolerance varies between different classes. However, data to test this proposition are lacking.
Discussion
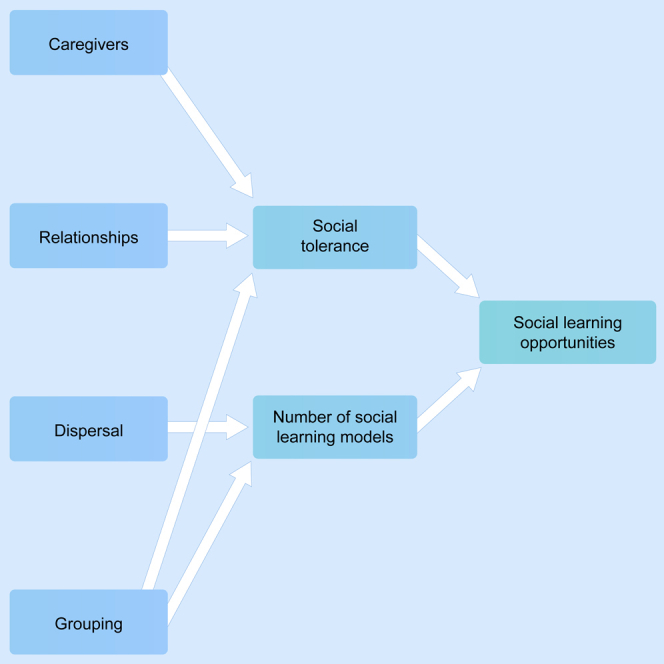
This review explores how different features of social structure can influence the social learning opportunities present for an individual that is part of that social unit. The review highlighted that social learning opportunities can indeed be influenced by two important factors: the number of social learning models (quantity) and social tolerance (quality). The number of social learning models an individual has access to in its lifetime is characterized by group size and dispersal. Simultaneously, the social tolerance an individual will encounter in its lifetime is influenced by many factors: primary caretakers, dominance hierarchy, nepotism, social bonds, group size, fission-fusion dynamics, and group composition (Figure 3). Although for some predictions empirical data are lacking, the data presented in this review in general do support the proposal that social structure influences social learning opportunities. Features of social structure that resulted in a higher number of social learning models and/or higher levels of social tolerance were connected to more socially learned behaviors, indicating more social learning opportunities.
Figure 3.
General overview of the different features of social structure that influence social learning opportunities via the number of potential social learning models and social tolerance
Features are grouped (dashed lines) along the subsections of the article. Pluses and minuses stand for a, respectively, positive or negative correlation. Arrows are empty (no evidence), lightly filled (only modeling evidence), darker filled (mixed empirical evidence), or fully filled (supporting empirical evidence) depending on the empirical evidence supporting the correlation.
This review showed that some social structures facilitate more or better social learning opportunities compared with other social structures. Two possible pathways can explain how these social structures evolved over time. One pathway could be that the selection pressure for increased social learning pushed for social structures that promote social learning. Another neutral pathway assumes that social structures evolved by selection pressures unrelated to social learning and an increase in social learning was a consequence of this evolution. These pathways are not mutually exclusive, and some interplay between both pathways is possible. To determine if increased social learning was a consequence or cause of the evolution of certain social structures, studies need to combine data about social structure, social learning, and phylogenetics. Currently, not enough systematic data are available for such an exercise.
The examples in this review are drawn from a wide variety of studies when exploring the effect of features of social structure on social learning opportunities. Still, this review has several limitations. First, most examples are only available for a few species. Most studies were conducted with a limited number of species, namely, great apes, capuchins, or marmosets, whereas most other species were overlooked (Hopper et al., 2013). To better understand the effect of social structure and to fill the gaps, social learning in more species needs to be studied. Second, although this review focused on primates, these connections are most likely not limited to primates. Social learning and variation in social structure are found in several taxa (Allen, 2019). Thus, the influences discussed in this review can be extended to other taxa. Third, we did not focus on the content or the mechanism of social learning. Different mechanisms of social learning may be differently influenced by features of social structure. Fourth, most comparisons made in this review are between different species. Therefore, the effect of genetics or ecology as a confounding variable cannot be ignored. To exclude the effect of genetics or ecology, different groups within a species should be studied. Different groups within the same population often show some variation in their social structure (Sapolsky and Share, 2004; Wikberg et al., 2013; van de Waal, 2018). Research on the behavioral variation between neighboring groups of a species has been neglected and is needed to grasp the diversity of socially learned behavior within a population (McGrew et al., 2011; van Leeuwen et al., 2012; Luncz and Boesch, 2015; van de Waal, 2018). This review conceptualizes a new framework that explores the influence of social structure on social learning opportunities, but more systematic research is needed.
For some of the presented features of social structure the evidence for an effect on social learning opportunities is clear, whereas for others, like group size and composition, studies are scarce or the findings are more ambiguous. To fully explore the relationship between social structure and social learning we suggest that further research needs to be conducted in four different directions. The first direction is to look at how variation in social tolerance affects social learning. Some studies have focused on social learning based on the rank of the model (Horner et al., 2010; Kendal et al., 2015; Botting et al., 2018; Canteloup, Hoppitt and van de Waal, 2020), but did not look at the effect of the dominance hierarchy system itself on the amount of social learning. This field is of extra interest, because the small amount of evidence present has conflicting outcomes, e.g., by showing that both despotic and egalitarian societies can possess tool-use. Systematic data on the effect of nepotism and social bonds are lacking as well. A second interesting direction for future research is testing the effect that group size has on social learning opportunities, as so far only theoretical models have explored this. This effect could be studied at the species level, as group size can vary a lot within a population (Mitani et al., 2012; van de Waal, 2018). Studies in captivity could test this experimentally by actively changing group size or tolerance due to food availability and testing the influence on social learning. In addition, studies in wild populations could use the effect of seasonality on food availability and social tolerance, to test its influence on social learning. The third interesting direction for future research is studying effects of group composition like sex ratio on social learning. Several studies have showed that the sex of an individual has an effect on its learning opportunities and tolerance (Lonsdorf, Eberly and Pusey, 2004; Kulik et al., 2015; Bono et al., 2018; Watson et al., 2018). However, no study as yet has looked at the effect of the sex and age of the model on the social tolerance between model and observer. The fourth direction is to look at other possible group structures. Although this review treated fission-fusion dynamics as a social system promoting social learning opportunities, it is not the only way a group can organize itself. One other group structure of possible interest would be the multi-level society of, for example, geladas, where core units are small but total group size is large (Grueter, Chapais & Zinner, 2012; Kawai et al., 1983). Individuals that are part of these enormous groups can potentially observe a lot of different individuals, which may lead to more social learning. Therefore, it would be of interest to study whether individuals are able to socially learn from individuals not part of their core unit. One promising tool that can aid in studying these four directions is social network analysis, which can quantify both elements of social structure and social learning (Claidière et al., 2013; Hoppit and Laland, 2011; Kasper and Voelkl, 2009). Social network analyses, in particular Network-Based Diffusion Analysis and Bayesian dynamic learning models, have already been successfully used to explore the relationship between social structure and social learning both with primates (Barret et al., 2017; Canteloup, et al., 2020; Carter et al., 2016) and other taxa (Aplin, 2016). In these four directions, substantial progress can be made, as they have eluded the focus of any previous research.
In addition, we did not systematically address the effect of the number of learning models and tolerance on the complexity of the learned behavior. However, this is an interesting avenue of research. We expect that prolonged access to a learning model is required to learn complex behavior. This would predict that rather than the number of learning models encountered, the tolerance shown by learning models is most relevant. However, groups with a rich cultural repertoire probably need both multiple models and high tolerance from these models. How these two are intertwined and affect social learning of complex behavior requires further attention. To do this, future studies need to define how to determine the complexity of a learned behavior. This definition needs to address the multiple components of behavior that can increase its complexity. Potential components of interest that we suggest are the presence of a temporal component, if the behavior is sequential in nature, or if it requires a broad array of knowledge.
When combining all the features of social structure discussed in this review that promote social learning opportunities, it is possible to theorize what kind of social structure would support the maximum social learning opportunities and at the same time allow for all the possible social transmission pathways (i.e., vertical, oblique, horizontal) to occur. First of all, in this optimal social structure parental investment should be high, preferably including allocaretakers. Second, it should be an egalitarian society without any nepotism but with many social bonds. Third, dispersal should be common and, to increase the exchange of behavior even more, not limited to one sex. Fourth, the group size would be such that the benefits of increased social learning models and the cost of within-group competition are balanced, which may be optimal when a group has fission-fusion dynamics. Of all possible primate species there is only one species that shows all requirements of the optimal social structure: humans (parental investment and allocare, Larke and Crews, 2006; Burkart, Hrdy and Van Schaik, 2009; Hrdy, 2009; van Schaik, 2015; egalitarian, Dyble et al., 2015; Erdal et al., 1994; Hill and Dunbar, 2003; Hill et al., 2011; van Schaik, 2015; dispersal by both sexes, Dyble et al., 2015; Hill et al., 2011; van Schaik, 2015; large group size, Hill and Dunbar, 2003; van Schaik, 2015; fission-fusion dynamics, Marlowe, 2005). So, the human social structure maximizes our social learning opportunities. These combined human social features may have allowed the evolution of or may even have been selected for promoting human social learning abilities.
This review shows that social learning does not take place in a vacuum. Although social learning is, in its essence, an interaction between at least two individuals, the quality and quantity of social learning opportunities are shaped by the social structure these individuals live in. Future research into social learning and culture should not overlook the importance of the social context in which it takes place.
Acknowledgments
E.v.d.W. is grateful for the support of the Swiss National Science Foundation (PP00P3_170624) during the writing of this review. We thank Francisca van Hassel for her help with figures and Rachel Harrison for comments on this manuscript. We also thank two anonymous reviewers for their constructive feedback.
Author contributions
B.v.B. conceptualized and wrote the original draft. B.v.B., E.v.d.W., and E.H.M.S. reviewed and edited the draft and E.v.d.W. provided the funding.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity statement
One or more of the authors of this article received support from a program designed to increase minority representation in science.
References
- Allen J.A. Community through culture: from insects to whales. BioEssays. 2019;41:1900060–1900068. doi: 10.1002/bies.201900060. [DOI] [PubMed] [Google Scholar]
- Anderson J.R. Development of tool-use to obtain food in a captive group of Macaca tonkeana. J. Hum. Evol. 1985;14:637–645. doi: 10.1016/S0047-2484(85)80072-5. [DOI] [Google Scholar]
- Aplin L. Understanding the multiple factors governing social learning and the diffusion of innovations. Curr. Opin. Behav. Sci. 2016;12:59–65. doi: 10.1016/j.cobeha.2016.09.003. [DOI] [Google Scholar]
- Asensio N., Aureli F., Schaffner C., Korstjens A. Intragroup aggression, fission-fusion dynamics and feeding competition in spider monkeys. Behaviour. 2008;145:983–1001. doi: 10.1163/156853908784089234. [DOI] [Google Scholar]
- Aureli F., Schaffner C.M., Boesch C., Bearder S.K., Call J., Chapman C.A., Connor R., Fiore A.D., Dunbar R.I.M., Henzi S.P. Fission-fusion dynamics: new research Frameworks. Curr. Anthropol. 2008;49:627–654. doi: 10.1086/586708. [DOI] [Google Scholar]
- Barrett B.J., McElreath R.L., Perry S.E. Pay-off-biased social learning underlies the diffusion of novel extractive foraging traditions in a wild primate. Proc. Biol. Sci. 2017;284:1–10. doi: 10.1098/rspb.2017.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko A.O., Russon A.E. Social learning opportunities in captive orangutans (Pongo abelii) and mandrills (Mandrillus sphinx) Int. J. Primatol. 2015;36:1014–1035. doi: 10.1007/s10764-015-9870-0. [DOI] [Google Scholar]
- Belisle P., Chapais B. Tolerated co-feeding in relation to degree of kinship in Japanese macaques. Behaviour. 2001;138:487–509. doi: 10.1163/156853901750382124. [DOI] [Google Scholar]
- Bentley-Condit V., Smith E.O. Animal tool use: current definitions and an updated comprehensive catalog. Behaviour. 2010;147:185–232A. doi: 10.1163/000579509X12512865686555. [DOI] [Google Scholar]
- Bernstein I.S., Gordon T.P., Rose R.M. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol. 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Biro D., Inoue-Nakamura N., Tonooka R., Yamakoshi G., Sousa C., Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cogn. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Boesch C. Teaching among wild chimpanzees. Anim. Behav. 1991;41:530–532. doi: 10.1016/S0003-3472(05)80857-7. [DOI] [Google Scholar]
- Boesch C., Boesch-Acherman H. 2000. The Chimpanzees of the Taï-Forest: Behavioural Ecology and Evolution. [Google Scholar]
- Bono A.E.J., Whiten A., van Schaik C., Krützen M., Eichenberger F., Schnider A., van de Waal E. Payoff- and sex-biased social learning interact in a wild primate population. Curr. Biol. 2018;28:2800–2805. doi: 10.1016/j.cub.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Botting J., Whiten A., Grampp M., van de Waal E. Field experiments with wild primates reveal no consistent dominance-based bias in social learning. Animal Behaviour. 2018;136:1–12. doi: 10.1016/j.anbehav.2017.11.025. [DOI] [Google Scholar]
- Brown G.R., Almond R.E.A., van Bergen Y. Begging, stealing, and offering: food transfer in nonhuman primates. In: Slater P.J.B., Rosenblatt J.S., Roper T.J., Snowdon C.T., Brockmann H.J., Naguib M., editors. Advances in the Study of Behavior. Elsevier academic press; 2004. pp. 265–295. [DOI] [Google Scholar]
- Burkart J.M., van Schaik C.P. Revisiting the consequences of cooperative breeding. J. Zool. 2016;299:77–83. doi: 10.1111/jzo.12322. [DOI] [Google Scholar]
- Burkart J.M., Hrdy S.B., Van Schaik C.P. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 2009;18:175–186. doi: 10.1002/evan.20222. [DOI] [Google Scholar]
- Canteloup C., Hoppitt W., van de Waal E. Wild primates copy higher-ranked individuals in a social transmission experiment. Nat. Commun. 2020;11:459. doi: 10.1038/s41467-019-14209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro T.M., Hauser M.D. Is there teaching in nonhuman animals? Q. Rev. Biol. 1992;67:151–174. doi: 10.1086/417553. [DOI] [PubMed] [Google Scholar]
- Carter A.J., Torrents Ticó M., Cowlishaw G. Sequential phenotypic constraints on social information use in wild baboons. eLife. 2016;5:1–21. doi: 10.7554/eLife.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapais B. Competence and the evolutionary origins of status and power in humans. Hum. Nat. 2015;26:161–183. doi: 10.1007/s12110-015-9227-6. [DOI] [PubMed] [Google Scholar]
- Chapman C.A., Chapman L.J., Wrangham R.W. Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav. Ecol. Sociobiol. 1995;36:59–70. doi: 10.1007/BF00175729. [DOI] [Google Scholar]
- Chapman C.A., Rothman J.M., Lambert J.E. Food as selective force in primates. In: Mitani J.C., Call J., Kappeler P.M., Palombit R.A., Silk J.B., editors. The Evolution of Primate Societies. The University of Chigaco Press; 2012. pp. 149–168. [Google Scholar]
- Claidière N., Messer E.J., Hoppitt W., Whiten A. Diffusion dynamics of socially learned foraging techniques in squirrel monkeys. Curr. Biol. 2013;23:1251–1255. doi: 10.1016/j.cub.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Coelho C.G., Falótico T., Izar P., Mannu M., Resende B.D., Siqueira J.O., Ottoni E.B. Social learning strategies for nut-cracking by tufted capuchin monkeys (Sapajus spp.) Anim. Cogn. 2015;18:911–919. doi: 10.1007/s10071-015-0861-5. [DOI] [PubMed] [Google Scholar]
- Coussi-Korbel S., Fragaszy D.M. On the relation between social dynamics and social learning. Anim. Behav. 1995;50:1441–1453. doi: 10.1016/0003-3472(95)80001-8. [DOI] [Google Scholar]
- Dyble M., Salali G.D., Chaudhary N., Page A., Smith D., Thompson J., Vinicius L., Mace R., Migliano A.B. Human behavior. Sex equality can explain the unique social structure of hunter-gatherer bands. Science. 2015;348:796–798. doi: 10.1126/science.aaa5139. [DOI] [PubMed] [Google Scholar]
- Enquist M., Strimling P., Eriksson K., Laland K., Sjostrand J. One cultural parent makes no culture. Anim. Behav. 2010;79:1353–1362. doi: 10.1016/j.anbehav.2010.03.009. [DOI] [Google Scholar]
- Erdal D., Whiten A., Boehm C., Knauft B. On human egalitarianism: an evolutionary product of machiavellian status escalation? Curr. Anthropol. 1994;35:175–183. doi: 10.1086/204255. [DOI] [Google Scholar]
- Fairbanks L.A. Juvenile vervet monkeys: establishing relationships and practicing skills for the future. In: Pereira M.E., Fairbanks L.A., editors. Juvenile Primates: Life History, Development, and Behavior. Oxford University Press; 1993. pp. 211–227. [Google Scholar]
- Feistner A.T.C., Price E.C. Food offering in new world primates: two species added. Folia Primatol. 1991;57:165–168. doi: 10.1159/000156579. [DOI] [Google Scholar]
- Fernandez-Duque E., Di Fiore A., Huck M. The behavior, ecology, and social evolution of new world monkeys. In: Mitani J.C., Call J., Kappeler P.M., Palombit R.A., Silk J.B., editors. The Evolution of Primate Societies. The University of Chigaco Press; 2012. pp. 43–65. [Google Scholar]
- Fragaszy D., Visalberghi E. Socially biased learning in monkeys. Anim. Learn. Behav. 2004;32:24–35. doi: 10.3758/BF03196004. [DOI] [PubMed] [Google Scholar]
- Frisch K.V. Die tänze der Bienen. Öesterr. Zool. Zeit. 1946;1:1–48. [Google Scholar]
- Griffin A.S. Social learning about predators: a review and prospectus. Learn Behav. 2004;32:131–140. doi: 10.3758/BF03196014. [DOI] [PubMed] [Google Scholar]
- Grueter C.C., Chapais B., Zinner D. Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 2012;33:1002–1037. doi: 10.1007/s10764-012-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B., Melis A.P., Woods V., Hastings S., Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Henrich J., Gil-White F.J. The evolution of prestige: freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol. Hum. Behav. 2001;22:165–196. doi: 10.1016/S1090-5138(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Heyes C.M. Social learning in animals: categories and mechanisms. Biol. Rev. 1994;69:207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Hill R.A., Dunbar R.I. Social network size in humans. Hum. Nat. 2003;14:53–72. doi: 10.1007/s12110-003-1016-y. [DOI] [PubMed] [Google Scholar]
- Hill K.R., Walker R.S., Bozicević M., Eder J., Headland T., Hewlett B., Hurtado A.M., Marlowe F., Wiessner P., Wood B. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- Hohmann G., Fruth B. Culture in bonobos? Between-species and within-species variation in behavior. Curr. Anthropol. 2003;44:563–571. doi: 10.1086/377649. [DOI] [Google Scholar]
- Hopper L., Holmes A., Williams L., Brosnan S. Dissecting the mechanisms of squirrel monkey (Saimiri boliviensis) social learning. PeerJ. 2013;1:e13–e21. doi: 10.7717/peerj.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppit W.J.E., Laland K.N. Detecting social learning using networks: a users guide. Am. J. Primatology. 2011;73:834–844. doi: 10.1002/ajp.20920. [DOI] [PubMed] [Google Scholar]
- Horner V., Proctor D., Bonnie K.E., Whiten A., de Waal F.B. Prestige affects cultural learning in chimpanzees. PLoS One. 2010;5:e1–e5. doi: 10.1371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy S.B. 1st edn. Belknap Press of Harvard University Press; 2009. Mothers and Others: The Evolutionary Origins of Mutual Understanding. [Google Scholar]
- Inoue-Nakamura N., Matsuzawa T. Development of stone tool use by wild chimpanzees (Pan troglodytes) J. Comp. Psychol. 1997;111:159–173. doi: 10.1037/0735-7036.111.2.159. [DOI] [PubMed] [Google Scholar]
- Jaeggi A.V., van Noordwijk M.A., van Schaik C.P. Begging for information: mother-offspring food sharing among wild bornean orangutans. Am. J. Primatol. 2008;70:533–541. doi: 10.1002/ajp.20525. [DOI] [PubMed] [Google Scholar]
- Jaeggi A.V., Dunkel L.P., Van Noordwijk M.A., Wich S.A., Sura A.A., Van Schaik C.P. Social learning of diet and foraging skills by wild immature Bornean orangutans: implications for culture. Am. J. Primatol. 2010;72:62–71. doi: 10.1002/ajp.20752. [DOI] [PubMed] [Google Scholar]
- Janson C.H., Goldsmith M.L. Predicting group size in primates: foraging costs and predation risks. Behav. Ecol. 1995;6:326–336. doi: 10.1093/beheco/6.3.326. [DOI] [Google Scholar]
- Joly M., Micheletta J., De Marco A., Langermans J.A., Sterck E.H.M., Waller B.M. Comparing physical and social cognitive skills in macaque species with different degrees of social tolerance. Proc. Biol. Sci. 2017;284:284. doi: 10.1098/rspb.2016.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler P.M., van Schaik C.P. Evolution of primate social systems. Int. J. Primatol. 2002;23:707–740. [Google Scholar]
- Kasper C., Voelkl B. A social network analysis of primate groups. Primates. 2009;50:343–356. doi: 10.1007/s10329-009-0153-2. [DOI] [PubMed] [Google Scholar]
- Kawai M. Newly-acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima islet. Primates. 1965;6:1–30. doi: 10.1007/BF01794457. [DOI] [Google Scholar]
- Kawai M., Ohsawa H., Mori U., Dunbar R. Social organization of gelada baboons: social units and definitions. Primates. 1983;24:13–24. doi: 10.1007/BF02381450. [DOI] [Google Scholar]
- Kendal R.L., Boogert N.J., Rendell L., Laland K.N., Webster M., Jones P.L. Social learning strategies: bridge-building between fields. Trends Cogn. Sci. 2018;22:651–665. doi: 10.1016/j.tics.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Kendal R.L., Hopper L.M., Whiten A., Brosnan S.F., Lambeth S.P., Schapiro S.J., Hoppitt W.J.E. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evol. Hum. Behav. 2015;36:65–72. doi: 10.1016/j.evolhumbehav.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L., Amici F., Langos D., Widdig A. Sex differences in the development of social relationships in rhesus macaques (Macaca mulatta) Int. J. Primatol. 2015;36:353–376. doi: 10.1007/s10764-015-9826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larke A., Crews D.E. Parental investment, late reproduction, and increased reserve capacity are associated with longevity in humans. J. Physiol. Anthropol. 2006;25:119–131. doi: 10.2114/jpa2.25.119. [DOI] [PubMed] [Google Scholar]
- van Leeuwen E.J.C., Cronin K.A., Haun D.B.M., Mundry R., Bodamer M.D. Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proc. R. Soc. B. 2012;279:4362–4367. doi: 10.1098/rspb.2012.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Ross C. Baboon (Papio anubis) social complexity-a network approach. Am. J. Primatol. 2011;73:775–789. doi: 10.1002/ajp.20967. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E.V. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim. Behav. 2005;70:673–683. doi: 10.1016/j.anbehav.2004.12.014. [DOI] [Google Scholar]
- Lonsdorf E.V. What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Anim. Cogn. 2006;9:36–46. doi: 10.1007/s10071-005-0002-7. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E.V., Eberly L.E., Pusey A.E. Sex differences in learning in chimpanzees. Nature. 2004;428:715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E.V., Anderson K.E., Stanton M.A., Shender M., Heintz M.R., Goodall J., Murray C.M. Boy will be boys: sex differences in wild infant chimpanzee social interactions. Anim. Behav. 2014;88:79–83. doi: 10.1016/j.anbehav.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luncz L.V., Boesch C. Tradition over trend: neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. Am. J. Primatol. 2014;76:649–657. doi: 10.1002/ajp.22259. [DOI] [PubMed] [Google Scholar]
- Luncz L.V., Boesch C. The extent of cultural variation between adjacent chimpanzee (Pan troglodytes verus) communities; A microecological approach. Am. J. Phys. Anthropol. 2015;156:67–75. doi: 10.1002/ajpa.22628. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Ross S.K., Megna N.L. Mother-infant interactions in western Lowland Gorillas (Gorilla gorilla gorilla): spatial relationships, communication, and opportunities for social learning. J. Comp. Psychol. 2002;116:219–227. doi: 10.1037/0735-7036.116.3.219. [DOI] [PubMed] [Google Scholar]
- Malaivijitnond S., Lekprayoon C., Tandavanittj N., Panha S., Cheewatham C., Hamada Y. Stone-tool usage by Thai long-tailed macaques (Macaca fascicularis) Am. J. Primatol. 2007;69:227–233. doi: 10.1002/ajp.20342. [DOI] [PubMed] [Google Scholar]
- Marlowe F.W. Hunter-gatherers and human evolution. Evol. Anthropol. 2005;14:54–67. doi: 10.1002/evan.20046. [DOI] [PubMed] [Google Scholar]
- Massen J., Sterck E., De Vos H. Close social associations in animals and humans: functions and mechanisms of friendship. Behav. 2010;147:1379–1412. doi: 10.1163/000579510X528224. [DOI] [Google Scholar]
- Matsumura S. Relaxed dominance relations among female moor macaques (Macaca maurus) in their natural Habitat, South sulawesi, Indonesia. Folia Primatol. 1998;69:346–356. doi: 10.1159/000021650. [DOI] [PubMed] [Google Scholar]
- Mitani J.C., Call J., Kappeler P.M., Palombit R.A., Silk J.B. The University of Chicago Press; 2012. The Evolution of Primate Societies. [Google Scholar]
- Mitani J.C., Watts D. The evolution of non-maternal caretaking among anthropoid primates: do helpers help? Behav. Ecol. Sociobiol. 1997;40:213–220. doi: 10.1007/s002650050335. [DOI] [Google Scholar]
- McGrew W.C., Marchant M.F., Scott S.E., Tutin C.E.G. Intergroup differences in a social custom of wild chimpanzees: the grooming Hand-clasp of the mahale mountains. Curr. Anthropol. 2011;42:148–153. https://www.jstor.org/stable/10.1086/318441 [Google Scholar]
- Moore J. Female transfer in primates. Int. J. Primatol. 1984;5:537–589. doi: 10.1007/BF02692285. [DOI] [Google Scholar]
- Mueller T., O'Hara R.B., Converse S.J., Urbanek R.P., Fagan W.F. Social learning of migratory performance. science. 2013;341:999–1002. doi: 10.1126/science.1237139. [DOI] [PubMed] [Google Scholar]
- Musgrave S., Morgan D., Lonsdorf E., Mundry R., Sanz C. Tool transfers are a form of teaching among chimpanzees. Sci. Rep. 2016;6:34783. doi: 10.1038/srep34783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Uehara S. Proximate factors of different types of grooming Hand-clasp in mahale chimpanzees: implications for chimpanzee social customs. Curr. Anthropol. 2004;45:108–114. doi: 10.1086/381007. [DOI] [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahali Mountains. Primates. 1968;9:167–224. doi: 10.1007/BF01730971. [DOI] [Google Scholar]
- Nishida T., Turner L.A. Food transfer between mother and infant chimpanzees of the Mahale Mountains National Park, Tanzania. Int. J. Primatol. 1996;17:947–968. doi: 10.1007/BF02735296. [DOI] [Google Scholar]
- Nunn C.L., Thrall P.H., Bartz K., Dasgupta T., Boesch C. Do transmission mechanisms or social systems drive cultural dynamics in socially structured populations? Anim. Behav. 2009;77:1515–1524. doi: 10.1016/j.anbehav.2009.02.023. [DOI] [Google Scholar]
- O’Malley R.C., Wallauer W., Murray C.M., Goodall J. The appearance and spread of ant fishing among the Kasekela chimpanzees of Gombe: a possible case of intercommunity cultural transmission. Curr. Anthropol. 2012;53:650–663. doi: 10.1086/666943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoni E.B., de Resende B.D., Izar P. Watching the best nutcrackers: what capuchin monkeys (Cebus apella) know about others tool-using skills. Anim. Cogn. 2005;8:215–219. doi: 10.1007/s10071-004-0245-8. [DOI] [PubMed] [Google Scholar]
- Perry S.E. Conformism in the food processing techniques of white-faced capuchin monkeys (Cebus capucinus) Anim. Cogn. 2009;12:705–716. doi: 10.1007/s10071-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E.C., Feistner A.T.C. Food sharing in lion tamarins: tests of three hypotheses. Am. J. Primatol. 1993;31:211–221. doi: 10.1002/ajp.1350310306. [DOI] [PubMed] [Google Scholar]
- Rapaport L.G. Provisioning of young in golden lion tamarins (callitrichidae, Leontopithecus rosalia): a test of the information hypothesis. Ethology. 1999;105:619–636. doi: 10.1046/j.1439-0310.1999.00449.x. [DOI] [Google Scholar]
- Rapaport L.G., Brown G.R. Social influences on foraging behavior in young nonhuman primates: learning what, where, and how to eat. Evol. Anthropol. 2008;17:189–201. doi: 10.1002/evan.20180. [DOI] [Google Scholar]
- Rapaport L.G., Ruiz-Miranda C.R. Tutoring in wild golden lion tamarins. Int. J. Primatology. 2002;23:1063–1070. doi: 10.1023/A:1019650032735. [DOI] [Google Scholar]
- Reader S.M., Laland K.N. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl. Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende B.D.de, Ottoni E.B., Fragaszy D.M. Ontogeny of manipulative behavior and nut-cracking in young tufted capuchin monkeys (Cebus apella): a Perception-action perspective. Dev. Sci. 2008;11:828–840. doi: 10.1111/j.1467-7687.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Santorelli C.J., Schaffner C.M., Campbell C.J., Notman H., Pavelka M.S., Weghorst J.A., Aureli F. Traditions in spider monkeys are biased towards the social domain. PLoS One. 2011;6:e16863. doi: 10.1371/journal.pone.0016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Miranda C.R., Kleiman D.G., Dietz J.M., Moraes E., Grativol A.D., Baker A.J., Beck B.B. Food transfers in wild and reintroduced golden lion tamarins, Leontopithecus rosalia. Am. J. Primatol. 1999;48:305–320. doi: 10.1002/(SICI)1098-2345(1999)48:4<305::AID-AJP6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Santorelli C.J., Schaffner C.M., Aureli F. Universal behaviors as candidate traditions in wild spider monkeys. PLoS One. 2011;6:e24400. doi: 10.1371/journal.pone.0024400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Endocrine aspects of social instability in the olive baboon (Papio anubis) Am. J. Primatol. 1983;5:365–379. doi: 10.1002/ajp.1350050406. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Share L.J. A pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 2004;2:E106. doi: 10.1371/journal.pbio.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordwijk M., Hemelrijk C.K., Herremans L.A., Sterck E.H.M. Spatial position and behavioral sex differences in juvenile long-tailed macaques. In: Pereira M.E., Fairbanks L.A., editors. Juvenile Primates: Life History, Development, and Behavior. Oxford University Press; New York: 1993. pp. 77–85. [Google Scholar]
- van Schaik C.P. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- van Schaik C.P. Local traditions in orangutans and chimpanzees: social learning and social tolerance. In: Fragaszy D.M., Perry S., editors. The Biology of Traditions: Models and Evidence. Cambridge University Press; New York: 2003. pp. 297–328. [DOI] [Google Scholar]
- van Schaik C.P. John Wiley & Sons, Ltd; 2015. The Primate Origins of Human Nature, the Primate Origins of Human Nature. [DOI] [Google Scholar]
- van Schaik C.P., Burkart J.M. Social learning and evolution: the cultural intelligence hypothesis. Phil. Trans. R. Soc. B. 2011;366:1008–1016. doi: 10.1098/rstb.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik C.P., Knott C.D. Geographic variation in tool use on Neesia fruits in orangutans. Am. J. Phys. Anthropol. 2001;114:331–342. doi: 10.1002/ajpa.1045. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P., Pradhan G.R. A model for tool-use traditions in primates: implications for the coevolution of culture and cognition. J. Hum. Evol. 2003;44:645–664. doi: 10.1016/S0047-2484(03)00041-1. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P., Deaner R.O., Merrill M.Y. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P., Ancrenaz M., Borgen G., Galdikas B., Knott C.D., Singleton I., Suzuki A., Utami S.S., Merrill M. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Schiel N., Huber L. Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus) Am. J. Primatol. 2006;68:1150–1160. doi: 10.1002/ajp.20284. [DOI] [PubMed] [Google Scholar]
- Schuppli C., van Schaik C.P. Animal cultures: how weve only seen the tip of the iceberg. Evolut. Hum. Sci. 2019;1:1–13. doi: 10.1017/ehs.2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppli C., Meulman E.J.M., Forss S.I.F., Aprilinayati F., van Noordwijk M.A., van Schaik C.P. Observational social learning and socially induced practice of routine skills in immature wild orang-utans. Anim. Behav. 2016;119:87–98. doi: 10.1016/j.anbehav.2016.06.014. [DOI] [Google Scholar]
- Silk J. Using the F-word in primatology. Behav. 2002;139:421–446. doi: 10.1163/156853902760102735. [DOI] [Google Scholar]
- Silk J.B., Altmann J., Alberts S.C. Social relationships among adult female baboons (papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 2006;61:183–195. doi: 10.1007/s00265-006-0249-2. [DOI] [Google Scholar]
- Smith J.E. Hamiltons legacy: kinship, cooperation and social tolerance in mammalian groups. Anim. Behav. 2014;92:291–304. doi: 10.1016/j.anbehav.2014.02.029. [DOI] [Google Scholar]
- Snowdon C.T. Social processes in communication and cognition in callitrichid monkeys: a review. Anim. Cogn. 2001;4:247–257. doi: 10.1007/s100710100094. [DOI] [PubMed] [Google Scholar]
- Sterck E.H.M., Korstjens A.H. Female dispersal and infanticide avoidance in primates. In: Van Schaik C.P., Janson C.H., editors. Infanticide by Males. Cambridge University Press; 2000. p. 569. [Google Scholar]
- Sterck E.H.M., Watts D.P., van Schaik C.P. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 1997;41:291–309. [Google Scholar]
- Strier K.B. Growing up in a patrifocal society: sex differences in the spatial relations of immature muriquis. In: Pereira M.E., Fairbanks L.A., editors. Juvenile Primates: Life History, Development, and Behavior. Oxford University Press; 1993. pp. 138–147. [Google Scholar]
- Strier K.B. Myth of the typical primate. Am. J. Phys. Anthropol. 1994;37:233–271. doi: 10.1002/ajpa.1330370609. [DOI] [Google Scholar]
- Tan A.W.Y., Hemelrijk C.K., Malaivijitnond S., Gumert M.D. Young macaques (Macaca fascicularis) preferentially bias attention towards closer, older, and better tool users. Anim. Cogn. 2018;21:551–563. doi: 10.1007/s10071-018-1188-9. [DOI] [PubMed] [Google Scholar]
- Tan A., Tan S.H., Vyas D., Malaivijitnond S., Gumert M.D. There Is More than One Way to Crack an Oyster: Identifying Variation in Burmese Long-Tailed Macaque (Macaca fascicularis aurea) Stone-Tool Use. PLOS ONE. 2015;10(5):1–25. doi: 10.1371/journal.pone.0124733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B. Feedback loop between kinship and dominance: the macaque model. J. Theor. Biol. 1990;145:511–522. doi: 10.1016/S0022-5193(05)80485-0. [DOI] [PubMed] [Google Scholar]
- Thierry B. Covariation of conflict management patterns across macaque species. In: Aureli F., de Waal F.B.M., editors. Natural Conflict Resolution. University of California Press; Berkeley and Los Angeles: 2000. pp. 106–128. [Google Scholar]
- Thierry B. Unity in diversity: lessons from macaque societies. Evol. Anthropol. 2007;16:224–238. doi: 10.1002/evan.20147. [DOI] [Google Scholar]
- Thornton A., Raihani N.J. Identifying teaching in wild animals. Learn Behav. 2010;38:297–309. doi: 10.3758/LB.38.3.297. [DOI] [PubMed] [Google Scholar]
- Tokida E., Tanaka I., Takefushi H., Hagiwara T. Tool-using in Japanese macaques: use of stones to obtain fruit from a pipe. Anim. Behav. 1994;47:1023–1030. doi: 10.1006/anbe.1994.1140. [DOI] [Google Scholar]
- Vermande M.M., Sterck E.H.M. How to get the biggest slice of the cake. A comparative view of social behaviour and resource access in human children and nonhuman primates. Front. Psychol. 2020;11:1–14. doi: 10.3389/fpsyg.2020.584815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B., Schrauf C., Huber L. Social contact influences the response of infant marmosets towards novel food. Anim. Behav. 2006;72:365–372. doi: 10.1016/j.anbehav.2005.10.013. [DOI] [Google Scholar]
- de Waal F.B. The integration of dominance and social bonding in primates. Q. Rev. Biol. 1986;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- van de Waal E. On the neglected behavioural variation among neighbouring primate groups, Ethology. In: Bshary R., editor. Vol. 124. 2018. pp. 845–854. [DOI] [Google Scholar]
- van de Waal E., Renevey N., Favre C.M., Bshary R. Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proc. R. Soc. B. 2010;277:2105–2111. doi: 10.1098/rspb.2009.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Waal E., Krützen M., Hula J., Goudet J., Bshary R. Similarity in food cleaning techniques within matrilines in wild vervet monkeys. PLoS One. 2012;7:e35694. doi: 10.1371/journal.pone.0035694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Waal E., Borgeaud C., Whiten A. Potent social learning and conformity shape a wild primates foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- Watson S.K., Vale G.L., Hopper L.M., Dean L.G., Kendal R.L., Price E.E., Wood L.A., Davis S.J., Schapiro S.J., Lambeth S.P. Chimpanzees demonstrate individual differences in social information use. Animal Cognition. 2018;21(5):639–650. doi: 10.1007/s10071-018-1198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.P. The apes: taxonomy, biogeography, life histories, and behavioral ecology. In: Mitani J.C., Call J., Kappeler P.M., Palombit R.A., Silk J.B., editors. The Evolution of Primate Societies. The University of Chigaco Press; Chicago: 2012. pp. 113–142. [Google Scholar]
- Watts D.P., Pusey A.E. Behavior of juvenile and adolescent great apes. In: Pereira M.E., Fairbanks L.A., editors. Juvenile Primates: Life History, Development, and Behavior. Oxford University Press; 1993. pp. 148–167. [Google Scholar]
- Westergaard G.C. Lion-tailed macaques (Macaca silenus) manufacture and use tools. J. Comp. Psychol. 1988;102:152–159. doi: 10.1037/0735-7036.102.2.152. [DOI] [PubMed] [Google Scholar]
- White D.J. Influences of social learning on mate-choice decisions. Learn Behav. 2004;32:105–113. doi: 10.3758/bf03196011. [DOI] [PubMed] [Google Scholar]
- Whitehead J.M. Development of feeding selectivity in mantled howling monkeys; Alouatta palliatta. In: Else D.G., Lee P.C., editors. Primate Ontogeny; Cognition and Social Behaviour. Cambridge University Press; 1986. pp. 105–117. [Google Scholar]
- Whiten A. Primate culture and social learning. Cogn. Sci. 2000;24:477–508. doi: 10.1207/s15516709cog2403_6. [DOI] [Google Scholar]
- Whiten A., van de Waal E. The pervasive role of social learning in primate lifetime development. Behav. Ecol. Sociobiol. 2018;72:80. doi: 10.1007/s00265-018-2489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A., Goodall J., McGrew W.C., Nishida T., Reynolds V., Sugiyama Y., Tutin C.E., Wrangham R.W., Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Wich S.A., Utami-Atmoko S.S., Setia T.M., Rijksen H.D., Schürmann C., van Hooff J.A., van Schaik C.P. Life history of wild Sumatran orangutans (Pongo abelii) J. Hum. Evol. 2004;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Widdig A., Nürnberg P., Krawczak M., Streich W.J., Bercovitch F.B. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc. Natl. Acad. Sci. USA. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikberg E.C., Teichroeb J.A., Bǎdescu I., Sicotte P. Individualistic female dominance hierarchies with varying strength in a highly folivorous population of black-and-white colobus. Behaviour. 2013;150:295–320. doi: 10.1163/1568539X-00003050. [DOI] [Google Scholar]
- Wrangham R.W., Koops K., Machanda Z.P., Worthington S., Bernard A.B., Brazeau N.F., Donovan R., Rosen J., Wilke C., Otali E., Muller M.N. Distribution of a chimpanzee social custom is explained by matrilineal relationship rather than conformity. Curr. Biol. 2016;26:3033–3037. doi: 10.1016/j.cub.2016.09.005. [DOI] [PubMed] [Google Scholar]