This cohort study assesses if the performance of magnetic resonance imaging in the presence of an abandoned cardiac implantable electronic device lead is safe and whether there are deleterious effects on concomitant active cardiac implantable electronic device leads.
Key Points
Question
Can patients with abandoned cardiac implantable electronic device (CIED) leads safely undergo magnetic resonance imaging (MRI)?
Findings
In this cohort study of 139 patients undergoing 200 MRIs of various anatomic regions including the thorax, no serious adverse events were noted. CIED parameter changes included transient decrease in lead sensing in 5 patients and subjective sternal heating in 1 patient with an abandoned subcutaneous array and sternal wires.
Meaning
The findings of this study suggest that the presence of abandoned CIED leads should not necessarily preclude MRI, regardless of the anatomic region being studied.
Abstract
Importance
Magnetic resonance imaging (MRI) is the modality of choice for many conditions. Conditional devices and novel protocols for imaging patients with legacy cardiac implantable electronic devices (CIEDs) have increased access to MRI in patients with devices. However, the presence of abandoned leads remains an absolute contraindication.
Objective
To assess if the performance of an MRI in the presence of an abandoned CIED lead is safe and whether there are deleterious effects on concomitant active CIED leads.
Design, Setting, and Participants
This cohort study included consecutive CIED recipients undergoing 1.5-T MRI with at least 1 abandoned lead between January 2013 and June 2020. MRI scans were performed at the Hospital of the University of Pennsylvania. No patients were excluded.
Exposures
CIEDs were reprogrammed based on patient-specific pacing needs. Electrocardiography telemetry and pulse oximetry were monitored continuously, and live contact with the patient throughout the scan via visual and voice contact was performed if possible. After completion of the MRI, CIED evaluation was repeated and programming returned to baseline or to a clinically appropriate setting.
Main Outcomes and Measures
Variation in pre- and post-MRI capture threshold of 50% or more, ventricular sensing 40% or more, and lead impedance 30% or more, as well as clinical sequelae such as pain and sustained tachyarrhythmia were considered significant. Long-term follow-up lead-related data were analyzed if available.
Results
A total of 139 consecutive patients (110 men [79%]) with a mean (SD) age of 65.6 (13.4) years underwent 200 MRIs of various anatomic regions including the thorax. Repeat examinations were common with a maximum of 16 examinations for 1 patient. There was a total of 243 abandoned leads with a mean (SD) of 1.22 (0.45) per patient. The mean (SD) number of active leads was 2.04 (0.78) and 64 patients (46%) were pacemaker dependent. A transmit-receive radiofrequency coil was used in 41 patients (20.5%), all undergoing MRI of the brain. There were no abnormal vital signs or sustained tachyarrhythmias. No changes in battery voltage, power-on reset events, or changes of pacing rate were noted. CIED parameter changes including decreased right atrial sensing in 4 patients and decreased left ventricular R-wave amplitude in 1 patient were transiently noted. One patient with an abandoned subcutaneous array experienced sternal heating that subsided on premature cessation of the study.
Conclusions and Relevance
The risk of MRI in patients with abandoned CIED leads was low in this large observational study, including patients who underwent examination of the thorax. The growing aggregate of data questions the absolute contraindication for MRI in patients with abandoned CIED leads.
Introduction
Magnetic resonance imaging (MRI) remains the test of choice for many conditions owing to its safety profile and excellent soft tissue contrast without associated exposure to iodinated contrast or ionizing radiation.1 One estimate is that 50% to 75% of patients with cardiac implantable electronic devices (CIEDs) will have an indication for MRI during their lifetime.2 While the development of MRI-conditional devices has the potential to enhance access to MRI for patients with such devices, there are a large number of patients with nonconditional or legacy devices. Recent data suggesting safety of scanning patients with these devices3,4,5 has led to supportive specialty society guidelines and expert consensus documents, as well as changes in Centers for Medicare & Medicaid Services (CMS) reimbursement policies for MRI in patients with legacy CIED systems.6,7,8 However, abandoned leads, which are theoretically associated with higher risk to adjacent structures including functional leads due to greater lead tip heating compared with leads attached to a generator,9 remain a contraindication to MRI examination in many institutions and are excluded from CMS reimbursement due to a paucity of safety data.
As the population of patients with CIEDs increases, so too does the number of patients with abandoned leads, making accurate risk assessment essential. Recent work has suggested safety of MRI with abandoned leads,10,11 but these data are from a single center. Additionally, there is a dearth of data regarding the safety of thoracic examination in these patients, where the theoretical risk for heating and current induction is greatest. We evaluated the safety of MRI examination in patients with abandoned CIED leads.
Methods
The study is a descriptive study of consecutive CIED recipients undergoing a 1.5-T MRI at the Hospital of the University of Pennsylvania. Those with abandoned CIED leads imaged between January 2013 and June 2020 formed the study cohort. Patients provided written consent for the procedure, and possible increased risk was discussed. The study was approved by the institutional review board at the Hospital of the University of Pennsylvania. All available imaging and other records were reviewed prior to the MRI to identify abandoned hardware including a discussion between the ordering health care professional and a radiologist in each case to assess the risks and benefits of MRI compared with alternative imaging modalities or other testing. An external defibrillator with capacity for cardiac pacing and a manufacturer-specific device programming system was maintained in the patient-holding area adjacent to the MRI control room while the patient was in the scanner. Physicians and mid-level health care professionals from radiology and cardiac electrophysiology were identified to oversee the safety of the MRI environment and all CIED-related issues, respectively. Prior to MRI, the CIED was interrogated with evaluation of baseline battery voltage, sensing, pacing thresholds, and lead impedances. Devices were reprogrammed based on patient-specific pacing needs, and tachyarrhythmia detection and therapies were deactivated. Attempts were made during reprogramming to avoid potential competition from an underlying rhythm and to minimize the risk of a pacing-mediated arrhythmia. Patients who were permanent pacemaker (PPM) dependent, defined as no significant intrinsic rhythm above 40 beats per minute, were reprogrammed to an asynchronous mode. Patients who were not PPM dependent were programmed to either a nontracking mode, programmed to an asynchronous mode, or pacing was turned off. Manufacturer-specific MRI conditional device programming was used for applicable devices, although the presence of an abandoned lead renders the system nonconditional and the protocol used for these patients was the same as used for those with legacy devices. During the time that the CIED was reprogrammed to accommodate the MRI environment, electrocardiography telemetry and pulse oximetry were monitored continuously. Live contact with the patient throughout the scan via visual and voice contact was performed if possible, and patients were instructed to report symptoms of any kind within the chest or device pocket during the study. Specialized medical personnel (advanced cardiac life support and a CIED-trained electrophysiology fellow, physician assistant, or physician) able to recognize and treat a significant change in cardiac rhythm or hemodynamic stability, perform advanced cardiac life support, and perform transcutaneous pacing or cardioversion/defibrillation, were present in the MRI control area throughout the scan. After completion of the MRI, CIED evaluation was repeated and programming returned to baseline or to a clinically appropriate setting.
Related adverse event criteria were based on the CMS decision memo for MRI in patients with CIEDs8 and defined as a variation in pre- and post-MRI capture threshold of 50% or more, sensing 40% or more, and lead impedance of 30% or more, as well as burning or pulling sensations in the chest or device pocket, sustained tachyarrhythmia during MRI, changes in vital signs determined to be attributed to MRI-related programming changes, power-on resets, or a change in the pacing rate. Long-term follow-up data were collected if present in the electronic heath record.
Continuous variables are expressed as means (SDs) or as medians with interquartile ranges. Categorical variables are expressed as numbers and percentages. Analyses were performed using JMP version 12 (SAS Institute). This study was a descriptive case series, and no statistical comparisons were made.
Results
Patient and CIED characteristics are presented in Table 1. From 2013 to 2020, 139 patients (110 men [79%]) with a mean (SD) age of 65.6 (13.4) years underwent 200 MRIs of multiple different anatomic regions including the brain (84 [42%]), heart (50 [25%]), lumbar spine (27 [14%]), cervical spine (16 [8%]), abdomen (11 [6%]), thoracic spine (6 [3%]), head (4 [2%]), prostate (4 [2%]), pelvis (2 [1%]), knee (2 [1%]), shoulder (2 [1%]), foot (2 [1%]), rectum (2 [1%]), hip (2 [1%]), orbits (1 [0.5%]), ankle (1 [0.5%]), face (1 [0.5%]), chest (1 [0.5%]), and neck (1 [0.5%]) (Table 2). There were a total of 219 anatomic regions included as 14 studies involving MRIs of multiple body parts including 2 regions (10 [71%]), 3 regions (3 [21%]), and 4 regions (1 [7%]). Thirteen patients (9%) underwent 2 separate MRIs, 2 patients (1%) had 3 studies, 2 patients (1%) had 4, 2 patients (1%) had 7, 1 patient had 9, and 1 patient (0.7%) underwent 16 MRIs. There were a total 243 abandoned leads with a mean (SD) of 1.22 (0.45) leads per patient (Figure 1). Thirty-seven patients (26.6%) had 2 abandoned leads, and 3 patients (2%) had 3 (Figure 2). Abandoned leads included right ventricular PPM (70 [29%]), implantable cardioverter-defibrillator (ICD) (65 [27%]), right atrial PPM (50 [21%]), right ventricular PPM epicardial (EPI) (18 [7%]), ICD coil (8 [3%]), pace-sense portion of ICD (11 [5%]), coronary sinus (6 [3%]), right atrial PPM EPI (5 [2%]), left ventricular endocardial PPM (1 [0.4%]), left ventricular PPM EPI (3 [1%]), subcutaneous array (1 [0.4%]), and various lead fragments (5 [2%]) (including PPM tip in lung [1], partial ICD right ventricular lead tip [2], and partial right ventricular PPM lead tip [2]) (Table 2). There were no patients with abandoned CIED leads who were excluded from the study.
Table 1. Patient Characteristics.
| Characteristic | No. (%) |
|---|---|
| No. of patients | 139 |
| Age, mean (SD), y | 64.8 (14.1) |
| Male | 110 (79) |
| Active CIED systems | |
| Single-chamber PPM | 5 (2.5) |
| Dual chamber | |
| PPM | 43 (21.5) |
| PPM EPI | 1 (0.5) |
| PPM with 1 EPI lead | 2 (1) |
| ICD | |
| Single chamber | 39 (19.5) |
| Dual chamber | 42 (21) |
| Biventricular | |
| PPM | 1 (0.5) |
| ICD | 59 (29.5) |
| ICD EPI | 1 (0.5) |
| Subcutaneous ICD | 4 (2) |
| None | 3 (1.5) |
| Abandoned leads | |
| Right atrial | |
| PPM | 50 (21) |
| PPM EPI | 5 (2) |
| Right ventricular | |
| PPM | 70 (29) |
| PPM EPI | 18 (7) |
| ICD | 65 (27) |
| ICD coil | 8 (3) |
| Pace-sense portion of ICD | 11 (5) |
| Coronary sinus | 6 (2) |
| Left ventricular | |
| Endocardial | 1 (0.4) |
| EPI | 3 (1) |
| Lead fragments | 5 (2) |
| PPM tip (lung) | 1 (0.4) |
| PPM (right ventricular) | 2 (1) |
| ICD (right ventricular) | 2 (1) |
| Subcutaneous array | 1 (0.4) |
Abbreviations: CIED, cardiac implantable electronic device; EPI, epicardial; ICD, implantable cardioverter-defibrillator; PPM, permanent pacemaker.
Table 2. Magnetic Resonance Imaging (MRI) Region and Frequency.
| Region | No. (%) |
|---|---|
| Brain | 84 (42) |
| Heart | 50 (25) |
| Spine | |
| Lumbar | 27 (13.5) |
| Cervical | 16 (8) |
| Abdomen | 11 (5.5) |
| Thoracic spine | 6 (3) |
| Head | 4 (2) |
| Prostate | 4 (2) |
| Pelvis | 2 (1) |
| Knee | 2 (1) |
| Shoulder | 2 (1) |
| Foot | 2 (1) |
| Rectum | 2 (1) |
| Hip | 2 (1) |
| Orbits | 1 (0.5) |
| Ankle | 1 (0.5) |
| Face | 1 (0.5) |
| Chest | 1 (0.5) |
| Neck | 1 (0.5) |
| No. of MRIs per patient | |
| 1 | 121 |
| 2 | 13 |
| 3 | 2 |
| 4 | 2 |
| 7 | 2 |
| 9 | 1 |
| 16 | 1 |
Figure 1. Cardiac Magnetic Resonance Images (MRIs) of 2 Patients With Active and Abandoned Implantable Cardioverter-Defibrillator (ICD) Leads Performed Prior to Ventricular Tachycardia Ablation Procedures.
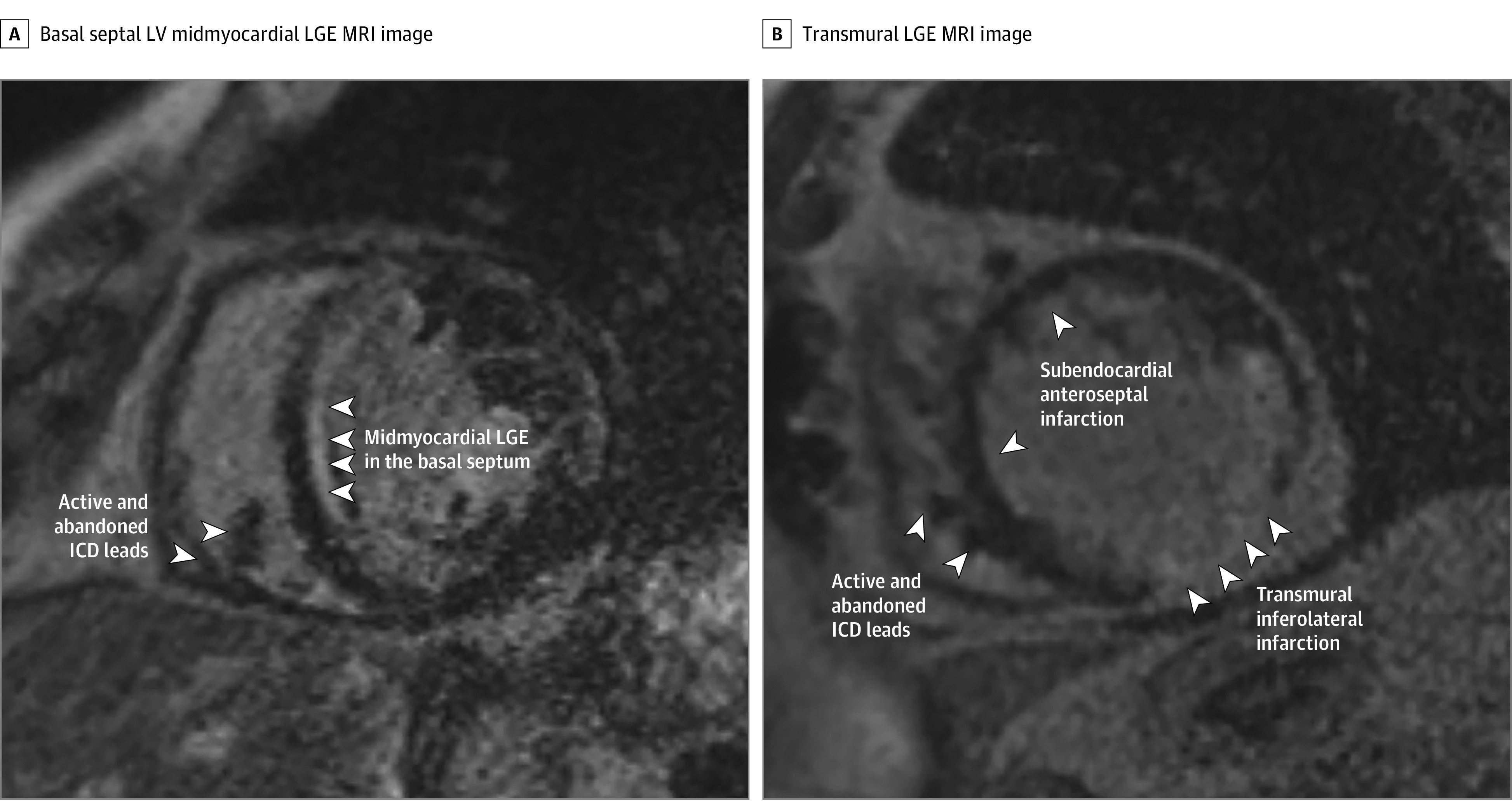
A, Basal septal left ventricular (LV) midmyocardial late gadolinium enhancement (LGE) consistent with nonischemic cardiomyopathy. B, Transmural basal inferolateral and subendocardial anteroseptal LGE consistent with prior infarctions.
Figure 2. Representative Chest Radiographs of Patients With Abandoned Cardiac Implantable Electronic Device Leads.
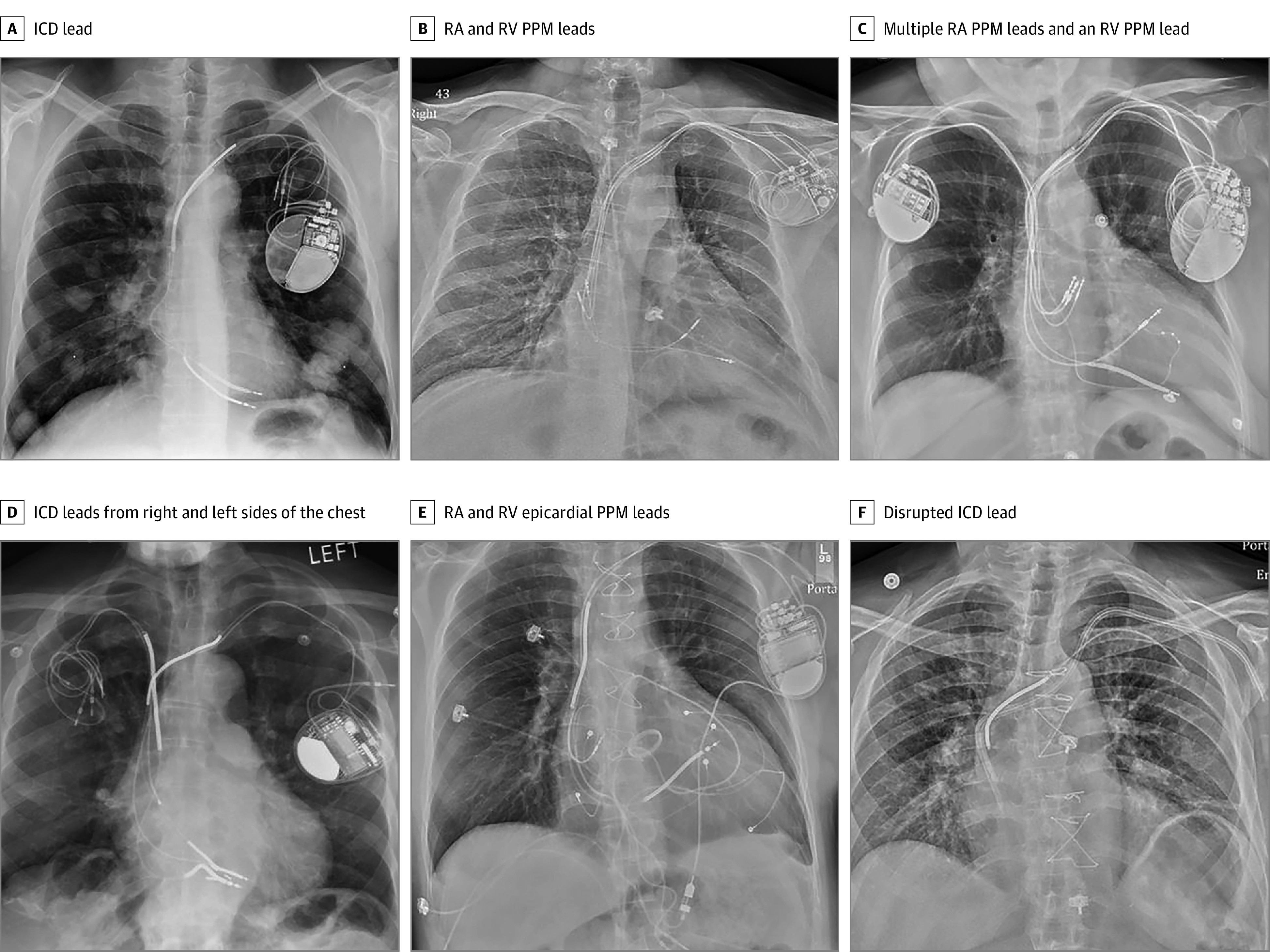
A, An implantable cardioverter-defibrillator (ICD) lead (note the multiple lung masses). B, Right atrial (RA) permanent pacemaker (PPM) and right ventricular (RV) PPM leads. C, Multiple RA PPM leads and an RV PPM lead (note the additional leads attached to a contralateral PPM). D, ICD leads from the right and left sides of the chest. E, RA and RV epicardial PPM leads from the abdomen. F, A disrupted ICD lead including the superior vena cava high-voltage coil adjacent to a hemodialysis catheter.
Active CIED systems included single-chamber PPM (5 [2.5%]), dual-chamber PPM (43 [21.5%]), dual-chamber PPM EPI (1 [0.5%]), dual-chamber PPM (with 1 EPI lead) (2 [1%]), single-chamber ICD (39 [19.5%]), dual-chamber ICD (42 [21%]), biventricular PPM (1 [0.5%]), biventricular ICD (59 [29.5%]), biventricular ICD (with 1 EPI PPM lead) (1 [0.5%]), subcutaneous ICD (4 [2%]), and no active system (3 [1.5%]) (Figure 2 and Table 1). The mean (SD) number of active leads was 2.04 (0.78), and 64 patients (46%) were PPM dependent. A transmit-receive radiofrequency coil was used in 41 studies (21%), all of which involved MRI of the brain, and 40 patients (29%) had history of sternotomy. There were no power-on resets, sustained tachyarrhythmias, or clinically relevant changes in vital signs, battery voltage, or pacing rates during MRI. All studies were performed in normal specific absorption rate mode and did not exceed 2.0 Watts per kg whole body or 3.2 Watts per kg during any study performed using the body coil for radiofrequency transmission.
There were 6 adverse events (Table 3), all occurring in men, including a significant decrease in right atrial sensing in 4 patients with a mean (SD) decrease in amplitude of 57.8% (7.8%). One patient was in permanent atrial fibrillation and was appropriately programmed to ventricular demand pacing mode. Three patients underwent appropriate sensitivity reprogramming without clinical sequelae. Sensing returned to normal in all 4 patients at the first available follow-up appointment (range, 5 days to 20 months). One patient had a 48% decrease in R-wave sensing of a coronary sinus lead from 6.0 to 3.1 millivolts, which improved to 5.0 millivolts the following day. One patient with an abandoned subcutaneous array experienced sternal heating that subsided on cessation of the study, representing the only study that was terminated prematurely. Long-term lead-related follow-up data were available for 83 patients after a total of 143 MRIs showing no adverse events throughout a mean (SD) of 15.77 (14.4) months.
Table 3. Related Adverse Events After MRI in Patients With CIEDs With Abandoned Leads.
| Patient No. | Age, y | Sex | MRI region | Active CIED | Abandoned lead | Adverse event | Follow-up result (first available) |
|---|---|---|---|---|---|---|---|
| 1 | 60s | M | Cardiac | DC PPM (EPI RV) | RV PPM | RA 0.3 to 0.1 mV | Normalization at 1 mo |
| 2 | 50s | M | Knee | DC ICD | RV PPM | RA 6 to 2.1 mV | Normalization at 9 mo |
| 3 | 50s | M | Brain | BiV ICD | RV PPM | RA 6 to 3 mV | Normalization at 20 mo |
| 4 | 80s | M | Entire spine | DC PPM (EPI RV) | RA and RV PPM | RA 2 to 1 mV | Normalization at 5 d |
| 5 | 50s | M | Cardiac | BiV ICD | CS | LV 6 to 3.1 mV | Improvement to 5 mV at 1 d |
| 6 | 60s | M | Cardiac | DC ICD | Subcutaneous array | Sternal burning | Subsided on cessation of MRI |
Abbreviations: BiV, biventricular; CIED, cardiac implantable electronic device; CS, coronary sinus; DC, dual-chamber; EPI, epicardial; ICD, implantable cardioverter-defibrillator; LV, left ventricle; M, male; MRI, magnetic resonance imaging; mV, millivolts; PPM, permanent pacemaker; RA, right atrial; RV, right ventricle.
Discussion
CIED leads have been shown to heat when exposed to a magnetic field in an MRI environment,12,13 potentially resulting in myocardial thermal injury, arrhythmias, damage to adjacent leads, and changes in capture thresholds and sensing parameters. As such, CIEDs have historically been considered a relative contraindication to MRI, to be done on a case-by-case and site-by-site basis with appropriate radiology and cardiology support, and in centers conducting or participating in clinical studies designed to assess the utility and safety of MRI exposure.14 The development of MRI-conditional devices, managed according to specific labeling requirements, has enhanced access to MRI when dedicated protocols are followed. However, imaging of patients with legacy devices, who make up the bulk of the CIED patients worldwide,13 has been restricted.
Recently, MRI protocols have been developed and tested showing safety in patients with legacy devices.15 This was followed by larger multi-institutional observational studies that have replicated these safety findings including the MagnaSafe registry, which included 1500 patients with non-MRI–conditional PPMs or ICDs who underwent nonthoracic MRIs at 1.5 T with appropriate reprogramming.16 A similar study by Nazarian et al4 of 1509 patients that included thoracic MRIs showed similar safety4 and has since been replicated on a smaller scale.17 These data ultimately led to refinement of guidelines and protocols for MRI, which eased restrictions on patients with legacy devices.6,7
However, MRI in the setting of abandoned leads is theoretically associated with higher risk leading to exclusion of many of these patients from studies. In a cylindrical phantom model, for clinical lead lengths between 40 and 60 cm, abandoned leads exhibited greater lead tip heating compared with leads attached to a generator.9 In a similar study using a phantom human trunk simulator, abandoned leads were found to modify the radiofrequency-heating profile of adjacent MRI-conditional leads, strongly dependent on the termination condition of the lead, with a maximum temperature rise of 17.6 °C of the active lead.18 Because of these data, access to MRI in this population has been limited, with patients and clinicians required to consider a different imaging modality or performance of transvenous lead extraction.19 As transvenous lead extraction carries both short-20,21 and long-term22 complication sequelae, this option has remained unappealing.
Recent studies have demonstrated clinical safety of MRIs in patients with abandoned leads,10,11,23,24 the largest of which included 80 patients with 90 abandoned leads who underwent 97 MRIs with no clinical or electrical evidence of CIED dysfunction, arrhythmias, or pain. Additionally, preimaging and postimaging paired cardiac troponin T measurements showed no evidence of myocardial injury. In this study, 24% of the MRIs were thoracic. Similar data have been shown for MRIs of patients with CIED lead fragments.25,26 Our study adds to the accumulating safety data in patients with abandoned leads and represents the largest description thus far, including endocardial and epicardial PPM, ICD, subcutaneous high-voltage coils, and lead fragments. The 6 adverse events (3%) that were seen included mostly (5 of 6) reductions in lead sensing with the ability to successfully reprogram the sensitivity of the affected lead until normalization occurred. Of those 5 patients, 4 (2%) involved the right atrial lead, slightly higher than the 1% incidence of comparable events seen in the largest experience of patients undergoing MRI with legacy devices but without abandoned leads,27 likely explained by the higher change in sensing threshold of 50% used in that study. One patient experienced subjective chest heating during the MRI that subsided after premature termination of the study. This patient had an abandoned subcutaneous array that coursed inferiorly and posteriorly around his back, distant from his pain. He also had sternal chest wires that presumably could have heated during the MRI. This phenomenon of chest burning or pulling, which was only seen in 1 of 41 patients with previous sternotomy in our study, has been reported before without a conclusive determination of cause.27,28
This study also represents the largest cohort of patients with abandoned CIED leads undergoing MRI of the thoracic region with 57 total MRIs (28.5%) including 50 (25%) of the heart. As the device and leads reside within the isocenter of the magnetic field, the risk of lead heating increases.29 Multiple studies have described the safety of thoracic MRI in this scenario, but the majority of these did not include abandoned leads.5,28,30 Our study, with a heterogeneous population all with abandoned CIED leads, showed no serious adverse events, suggesting an overall positive safety profile in this scenario. The implications of these data relate to the utility of cardiac MRI for diagnostic and prognostic purposes in patients with cardiomyopathy31 (Figure 1) as well as prior to complex procedures, including ventricular tachycardia32,33,34,35,36,37,38,39 and atrial fibrillation40,41,42 ablation.
Despite the relative safety seen in this study, appropriate monitoring precautions and protocols consistent with the 2017 Heart Rhythm Society Expert Consensus Statement on MRI and Radiation Exposure in Patients with CIEDs6 remains prudent. Other risk mitigation strategies have been proposed in patients with legacy devices with or without abandoned leads such as the use of a transmit-receive radiofrequency coil9,43 in patients undergoing MRI of the brain or extremities to avoid a central location of the leads in relation to the radiofrequency coil, as well as a change in the MRI landmark.44
Limitations
This is a single-center observational study. All MRI scans were performed at 1.5 T, and thus, our results cannot be extrapolated to different MRI field strengths. While our adverse event criteria were guided by the widely accepted CMS decision document,8 a different threshold for CIED-related phenomena may have changed our findings. Although our patient population was heterogenous with a wide array of active and abandoned leads, this does not represent all available leads, and the extent of heating and current induction is likely a function of lead fragment length as well as the presence or absence of end caps. Although we specifically looked for evidence of abandoned leads during our MRI screening procedure, it is possible that some patients with abandoned leads were not known at the time of MRI. Finally, this cohort only includes a limited number of patients with abandoned leads without an active CIED because these were not tracked routinely in our CIED database. Therefore, we cannot draw definitive conclusions about MRIs in patients with abandoned leads but no active system, for example, after generator explant.
Conclusions
In this study of patients with abandoned CIED leads undergoing MRI, including those who underwent MRI of the thorax, a low rate of arrhythmia, patient symptoms, or change in device settings was observed. The growing aggregate of data calls into question current institutional and CMS reimbursement policies concerning MRI in patients with abandoned CIED leads.
References
- 1.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. doi: 10.1001/jama.2012.5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28(4):326-328. doi: 10.1111/j.1540-8159.2005.50024.x [DOI] [PubMed] [Google Scholar]
- 3.Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376(8):755-764. doi: 10.1056/NEJMoa1603265 [DOI] [PubMed] [Google Scholar]
- 4.Nazarian S, Halperin HR. Magnetic resonance imaging and cardiac devices. N Engl J Med. 2018;378(17):1652-1653. doi: 10.1056/NEJMc1802623 [DOI] [PubMed] [Google Scholar]
- 5.Padmanabhan D, Kella DK, Deshmukh AJ, et al. Safety of thoracic magnetic resonance imaging for patients with pacemakers and defibrillators. Heart Rhythm. 2019;16(11):1645-1651. doi: 10.1016/j.hrthm.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 6.Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14(7):e97-e153. doi: 10.1016/j.hrthm.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 7.Jabehdar Maralani P, Schieda N, Hecht EM, et al. MRI safety and devices: an update and expert consensus. J Magn Reson Imaging. 2020;51(3):657-674. doi: 10.1002/jmri.26909 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. Decision memo for magnetic resonance imaging (MRI) (CAG-00399R2). Accessed January 12, 2021. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=246&ver=11&NcaName=Magnetic+Resonance+Imaging+(MRI)+(2nd+Recon)&bc=BEAAAAAAEAAA
- 9.Langman DA, Goldberg IB, Finn JP, Ennis DB. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging. 2011;33(2):426-431. doi: 10.1002/jmri.22463 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol. 2014;37(10):1284-1290. doi: 10.1111/pace.12419 [DOI] [PubMed] [Google Scholar]
- 11.Padmanabhan D, Kella DK, Mehta R, et al. Safety of magnetic resonance imaging in patients with legacy pacemakers and defibrillators and abandoned leads. Heart Rhythm. 2018;15(2):228-233. doi: 10.1016/j.hrthm.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 12.Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26(4):376-383. doi: 10.1093/eurheartj/ehi009 [DOI] [PubMed] [Google Scholar]
- 13.Beinart R, Nazarian S. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation. 2013;128(25):2799-2809. doi: 10.1161/CIRCULATIONAHA.113.005697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine GN, Gomes AS, Arai AE, et al. ; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Radiology and Intervention . Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116(24):2878-2891. doi: 10.1161/CIRCULATIONAHA.107.187256 [DOI] [PubMed] [Google Scholar]
- 15.Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155(7):415-424. doi: 10.7326/0003-4819-155-7-201110040-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo RJ. Determining the risks of clinically indicated nonthoracic magnetic resonance imaging at 1.5 T for patients with pacemakers and implantable cardioverter-defibrillators: rationale and design of the MagnaSafe Registry. Am Heart J. 2013;165(3):266-272. doi: 10.1016/j.ahj.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Mason S, Osborn JS, Dhar R, et al. Real world MRI experience with nonconditional and conditional cardiac rhythm devices after MagnaSafe. J Cardiovasc Electrophysiol. 2017;28(12):1468-1474. doi: 10.1111/jce.13351 [DOI] [PubMed] [Google Scholar]
- 18.Mattei E, Gentili G, Censi F, Triventi M, Calcagnini G. Impact of capped and uncapped abandoned leads on the heating of an MR-conditional pacemaker implant. Magn Reson Med. 2015;73(1):390-400. doi: 10.1002/mrm.25106 [DOI] [PubMed] [Google Scholar]
- 19.Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503-e551. doi: 10.1016/j.hrthm.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 20.Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55(6):579-586. doi: 10.1016/j.jacc.2009.08.070 [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh A, Patel N, Noseworthy PA, et al. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation. 2015;132(25):2363-2371. doi: 10.1161/CIRCULATIONAHA.114.013801 [DOI] [PubMed] [Google Scholar]
- 22.Poole JE, Gleva MJ, Mela T, et al. ; REPLACE Registry Investigators . Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553-1561. doi: 10.1161/CIRCULATIONAHA.110.976076 [DOI] [PubMed] [Google Scholar]
- 23.Morris MF, Verma DR, Sheikh H, Su W, Pershad A. Outcomes after magnetic resonance imaging in patients with pacemakers and defibrillators and abandoned leads. Cardiovasc Revasc Med. 2018;19(6):685-688. doi: 10.1016/j.carrev.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 24.Horwood L, Attili A, Luba F, et al. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace. 2017;19(5):812-817. [DOI] [PubMed] [Google Scholar]
- 25.Austin CO, Landolfo K, Parikh PP, Patel PC, Venkatachalam KL, Kusumoto FM. Retained cardiac implantable electronic device fragments are not associated with magnetic resonance imaging safety issues, morbidity, or mortality after orthotopic heart transplant. Am Heart J. 2017;190:46-53. doi: 10.1016/j.ahj.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 26.Alvarez PA, Sperry BW, Perez AL, et al. Burden and consequences of retained cardiovascular implantable electronic device lead fragments after heart transplantation. Am J Transplant. 2018;18(12):3021-3028. doi: 10.1111/ajt.14755 [DOI] [PubMed] [Google Scholar]
- 27.Nazarian S, Hansford R, Rahsepar AA, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377(26):2555-2564. doi: 10.1056/NEJMoa1604267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dandamudi S, Collins JD, Carr JC, et al. The safety of cardiac and thoracic magnetic resonance imaging in patients with cardiac implantable electronic devices. Acad Radiol. 2016;23(12):1498-1505. doi: 10.1016/j.acra.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 29.Mattei E, Triventi M, Calcagnini G, et al. Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations. Biomed Eng Online. 2008;7:11. doi: 10.1186/1475-925X-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyotowidjojo IS, Skinner K, Shah AS, et al. Thoracic versus nonthoracic MR imaging for patients with an MR nonconditional cardiac implantable electronic device. Pacing Clin Electrophysiol. 2018;41(6):589-596. doi: 10.1111/pace.13340 [DOI] [PubMed] [Google Scholar]
- 31.Patel AR, Kramer CM. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2017;10(10 pt A):1180-1193. doi: 10.1016/j.jcmg.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahida S, Sacher F, Dubois R, et al. Cardiac imaging in patients with ventricular tachycardia. Circulation. 2017;136(25):2491-2507. doi: 10.1161/CIRCULATIONAHA.117.029349 [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee RK, Whitaker J, Williams SE, Razavi R, O’Neill MD. Magnetic resonance imaging guidance for the optimization of ventricular tachycardia ablation. Europace. 2018;20(11):1721-1732. doi: 10.1093/europace/euy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piers SR, Zeppenfeld K. Imaging-guided ventricular tachycardia ablation. Arrhythm Electrophysiol Rev. 2013;2(2):128-134. doi: 10.15420/aer.2013.2.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njeim M, Yokokawa M, Frank L, et al. Value of cardiac magnetic resonance imaging in patients with failed ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol. 2016;27(2):183-189. doi: 10.1111/jce.12848 [DOI] [PubMed] [Google Scholar]
- 36.Siontis KC, Kim HM, Sharaf Dabbagh G, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017;14(10):1487-1493. doi: 10.1016/j.hrthm.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 37.Nelson T, Garg P, Clayton RH, Lee J. The role of cardiac MRI in the management of ventricular arrhythmias in ischaemic and non-ischaemic dilated cardiomyopathy. Arrhythm Electrophysiol Rev. 2019;8(3):191-201. doi: 10.15420/aer.2019.5.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muser D, Santangeli P, Liang JJ, et al. Characterization of the electroanatomic substrate in cardiac sarcoidosis: correlation with imaging findings of scar and inflammation. JACC Clin Electrophysiol. 2018;4(3):291-303. doi: 10.1016/j.jacep.2017.09.175 [DOI] [PubMed] [Google Scholar]
- 39.Zghaib T, Ipek EG, Hansford R, et al. Standard ablation versus magnetic resonance imaging-guided ablation in the treatment of ventricular tachycardia. Circ Arrhythm Electrophysiol. 2018;11(1):e005973. doi: 10.1161/CIRCEP.117.005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fochler F, Yamaguchi T, Kheirkahan M, Kholmovski EG, Morris AK, Marrouche NF. Late gadolinium enhancement magnetic resonance imaging guided treatment of post-atrial fibrillation ablation recurrent arrhythmia. Circ Arrhythm Electrophysiol. 2019;12(8):e007174. doi: 10.1161/CIRCEP.119.007174 [DOI] [PubMed] [Google Scholar]
- 41.Akoum N, Wilber D, Hindricks G, et al. MRI assessment of ablation-induced scarring in atrial fibrillation: analysis from the DECAAF Study. J Cardiovasc Electrophysiol. 2015;26(5):473-480. doi: 10.1111/jce.12650 [DOI] [PubMed] [Google Scholar]
- 42.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311(5):498-506. doi: 10.1001/jama.2014.3 [DOI] [PubMed] [Google Scholar]
- 43.Naehle CP, Meyer C, Thomas D, et al. Safety of brain 3-T MR imaging with transmit-receive head coil in patients with cardiac pacemakers: pilot prospective study with 51 examinations. Radiology. 2008;249(3):991-1001. doi: 10.1148/radiol.2493072195 [DOI] [PubMed] [Google Scholar]
- 44.Nordbeck P, Ritter O, Weiss I, et al. Impact of imaging landmark on the risk of MRI-related heating near implanted medical devices like cardiac pacemaker leads. Magn Reson Med. 2011;65(1):44-50. doi: 10.1002/mrm.22592 [DOI] [PubMed] [Google Scholar]


