Key Points
Question
What is the effect of a single high dose of vitamin D3 on hospital length of stay among hospitalized patients with moderate to severe coronavirus disease 2019 (COVID-19)?
Findings
In this randomized clinical trial that involved 240 hospitalized patients with moderate to severe COVID-19, a single dose of 200 000 IU of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay (median of 7.0 vs 7.0 days; unadjusted hazard ratio for hospital discharge, 1.07).
Meaning
The study does not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19 in hospitalized patients.
Abstract
Importance
The efficacy of vitamin D3 supplementation in coronavirus disease 2019 (COVID-19) remains unclear.
Objective
To investigate the effect of a single high dose of vitamin D3 on hospital length of stay in patients with COVID-19.
Design, Setting, and Participants
This was a multicenter, double-blind, randomized, placebo-controlled trial conducted in 2 sites in Sao Paulo, Brazil. The study included 240 hospitalized patients with COVID-19 who were moderately to severely ill at the time of enrollment from June 2, 2020, to August 27, 2020. The final follow-up was on October 7, 2020.
Interventions
Patients were randomly assigned to receive a single oral dose of 200 000 IU of vitamin D3 (n = 120) or placebo (n = 120).
Main Outcomes and Measures
The primary outcome was length of stay, defined as the time from the date of randomization to hospital discharge. Prespecified secondary outcomes included mortality during hospitalization; the number of patients admitted to the intensive care unit; the number of patients who required mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D, total calcium, creatinine, and C-reactive protein.
Results
Of 240 randomized patients, 237 were included in the primary analysis (mean [SD] age, 56.2 [14.4] years; 104 [43.9%] women; mean [SD] baseline 25-hydroxyvitamin D level, 20.9 [9.2] ng/mL). Median (interquartile range) length of stay was not significantly different between the vitamin D3 (7.0 [4.0-10.0] days) and placebo groups (7.0 [5.0-13.0] days) (log-rank P = .59; unadjusted hazard ratio for hospital discharge, 1.07 [95% CI, 0.82-1.39]; P = .62). The difference between the vitamin D3 group and the placebo group was not significant for in-hospital mortality (7.6% vs 5.1%; difference, 2.5% [95% CI, –4.1% to 9.2%]; P = .43), admission to the intensive care unit (16.0% vs 21.2%; difference, –5.2% [95% CI, –15.1% to 4.7%]; P = .30), or need for mechanical ventilation (7.6% vs 14.4%; difference, –6.8% [95% CI, –15.1% to 1.2%]; P = .09). Mean serum levels of 25-hydroxyvitamin D significantly increased after a single dose of vitamin D3 vs placebo (44.4 ng/mL vs 19.8 ng/mL; difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001). There were no adverse events, but an episode of vomiting was associated with the intervention.
Conclusions and Relevance
Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19.
Trial Registration
ClinicalTrials.gov Identifier: NCT04449718
This randomized trial compares the effects of a single 200 000-IU dose of vitamin D3 vs placebo on length of stay in patients hospitalized with moderate to severe COVID-19.
Introduction
Vitamin D may enhance innate1,2,3 and adaptive immunity.4,5 Because antigen-presenting cells have the ability to synthesize 1,25-dihydroxyvitamin D from 25-hydroxyvitamin D, it has been postulated that vitamin D3 supplementation could improve the function of macrophages and dendritic cells, thereby ameliorating overall immune response.6 Vitamin D insufficiency is a potential risk factor for noncommunicable7 and acute respiratory tract diseases,8,9 including viral infections.10
It has been suggested that optimal serum levels of 25-hydroxyvitamin D may have immunomodulatory and anti-inflammatory properties, and could possibly benefit patients with coronavirus disease 2019 (COVID-19).11,12 However, the benefits of supplementary vitamin D3 to patients with COVID-19 remain speculative and only partially supported by observational studies and 1 small-scale nonrandomized trial.13,14,15
The objective of this randomized clinical trial was to investigate the effect of vitamin D3 administration on hospital length of stay and other relevant clinical outcomes and adverse events in hospitalized patients with moderate to severe COVID-19. The main hypothesis was that a single dose of 200 000 IU of vitamin D3 would increase 25-hydroxyvitamin D levels and shorten hospital length of stay.
Methods
This was a multicenter, double-blind, parallel-group, randomized, placebo-controlled trial. The study was approved by the ethics committee of the Clinical Hospital of the School of Medicine of the University of Sao Paulo and by the ethics committee of the Ibirapuera field hospital. Patients provided written informed consent before participation. The trial protocol and statistical analysis plan are included in Supplement 1.
Participants
Patients were recruited from the Clinical Hospital of the School of Medicine of the University of Sao Paulo (a quaternary referral teaching hospital) and from the Ibirapuera field hospital, both located in Sao Paulo, Brazil. Patients were enrolled from June 2, 2020, to August 27, 2020. The final follow-up was on October 7, 2020. To provide a comprehensive demographic characterization, self-reported race/ethnicity data were also collected based on the following fixed categories: White, Black, Asian, and Pardo (the latter refers to people of mixed ethnicities). All patients had COVID-19 diagnosis confirmed by polymerase chain reaction (PCR) testing at the time of enrollment or by serology assay (ELISA) to detect IgG against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) throughout the study.
Inclusion Criteria
Inclusion criteria were age 18 years or older; diagnosis of COVID-19 via PCR testing for SARS-CoV-2 from nasopharyngeal swabs or computed tomography scan findings compatible with the disease (bilateral multifocal ground-glass opacities ≥50%); and diagnosis of flu syndrome with institutional criteria for hospitalization on hospital admission, presenting respiratory rate greater than 24/min, saturation less than 93% while breathing room air, or risk factors for complications (eg, heart disease, diabetes, systemic arterial hypertension, neoplasms, immunosuppression, pulmonary tuberculosis, obesity) followed by COVID-19 confirmation. Patients who met these criteria were considered to have moderate to severe COVID-19.
Exclusion Criteria
Patients were excluded if they were unable to read and sign the written informed consent form, were already admitted and receiving invasive mechanical ventilation, received previous vitamin D3 supplementation (>1000 IU/d), had kidney failure requiring dialysis or creatinine of at least 2.0 mg/dL, had hypercalcemia (total calcium >10.5 mg/dL), were pregnant or lactating, or had expected hospital discharge in less than 24 hours.
Randomization and Study Interventions
Patients were assigned in a 1:1 ratio to the vitamin D3 group or the placebo group. The randomization list was created using a computer-generated code with block sizes of 20. A staff member who had no role in the study managed the randomization. Outcomes were assessed at baseline and on hospital discharge.
The vitamin D3 group received a single, oral dose of 200 000 IU of vitamin D3 dissolved in a 10-mL peanut oil solution. This selected dose is in the recommended range for effectively treating patients with 25-hydroxyvitamin D deficiency.16 Patients from the placebo group received 10 mL of a peanut oil solution. The solutions were identical in color, taste, smell, consistency, and container. They were prepared by the pharmacy unit of the Clinical Hospital and labeled by a staff member who did not participate in the study. Patients and investigators remained blinded to randomization until the final analysis.
Outcome Measures
The primary outcome was hospital length of stay, defined as the total number of days that patients remained hospitalized from the date of randomization until the date of hospital discharge. The criteria used for patient discharge were no need for supplemental oxygen in the past 48 hours, no fever in the past 72 hours, and oxygen saturation greater than 93% without supplemental oxygen and without respiratory distress.
The prespecified secondary outcomes were mortality, defined as the number of patients who died during hospitalization; the number of patients admitted to the intensive care unit; the number of patients who needed mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D (assessed by a chemiluminescent immunoassay), total calcium (assessed by a 5-nitro-5'-methyl-[1,2-bis[o-aminophenoxy]ethan-N,N,N',N'-tetraacetic acid method), creatinine (assessed by a colorimetric assay based on the kinetic Jaffe reaction), and C-reactive protein (assessed by an immunoturbidimetric assay). In addition, a set of exploratory health-related laboratory markers (eTable 1 and eTable 2 in Supplement 2) were assessed. All of the laboratory assessments were analyzed in an accredited laboratory from the Clinical Hospital and were performed on the day of randomization and on hospital discharge. Thus, follow-up blood samples were not collected for patients who died during the trial.
Serum D-dimer was included as an outcome post hoc because the investigators believed that this outcome would provide further exploratory data on the effects of the intervention. Cytokines analysis was originally planned, but sufficient financial resources were not available. Physical activity was assessed for a separate prospective cohort study nested in this clinical trial; therefore, those results are not presented in this article.
Statistical Analysis
The number of participants was chosen on the basis of feasibility, based on resources, capacity of research staff and facility, and available patients, in line with current recommendations.17,18 Approximately 200 patients were expected to be enrolled, with the expectation of 16 to 17 eligible patients per week in both centers. Although the actual enrollment was approximately 20 patients per week, the planned date for ending enrollment was not changed to increase the study power, resulting in a larger final sample size than originally anticipated. The minimal clinically important difference between groups for length of stay among patients with COVID-19 is unknown.
The log-rank test was used to compare the Kaplan-Meier estimate curves for length of stay, with deaths being right-censored in the analysis. Post hoc adjusted analyses for the primary outcome of length of stay were performed using Cox regression models to estimate hazard ratios (HRs) with corresponding 2-sided 95% CIs, considering potential confounders that were not fully balanced by randomization, prespecified as P < .20 for baseline comparisons between groups. These confounders were joint pain, sore throat, hypertension, diabetes, parathyroid hormone, and creatinine. The proportionality assumption for Cox regression models was confirmed by assessing Schoenfeld residuals.
Generalized estimating equations for repeated measures were used for testing possible differences in laboratory parameters and duration of mechanical ventilation (using death as a covariate for the latter), assuming group and time (when applicable) as fixed factors, with marginal distribution, and a first-order autoregressive correlation matrix to test the main and interaction effects. Bonferroni adjustment was performed for generalized estimating equation analyses to maintain a family-wise 2-sided significance threshold of .05, considering 6 pairwise comparisons for all secondary end points. Percentages were compared between groups using χ2 and Fisher exact tests for mortality, admission to the intensive care unit, and mechanical ventilation requirement.
Post hoc analyses that included patients with 25-hydroxyvitamin D deficiency (ie, <20 ng/mL) were performed for the primary outcome and some secondary outcomes, using the same statistical procedures aforementioned. Post hoc analyses were also performed to examine the potential site effect on the primary outcome, by including site as strata and using the same procedures previously described, and to test whether deaths were noninformative for lengths of stay as initially assumed. To that end, the 90th-percentile hospital length of stay for each group for those who died were imputed and data were then reanalyzed.
All analyses were performed according to patient randomization group, with retention of all patients in the analyses except for those who withdrew consent before receiving the intervention. There was no imputation for missing data. For laboratory parameters, missingness was handled by generalized estimating equation models, assuming that missingness was at random based on the nonsignificant differences between groups for the proportion of missing data. Statistical analyses were performed with IBM-SPSS software, version 20.0. The significance level was set at 2-sided α = .05.
Results
Patients
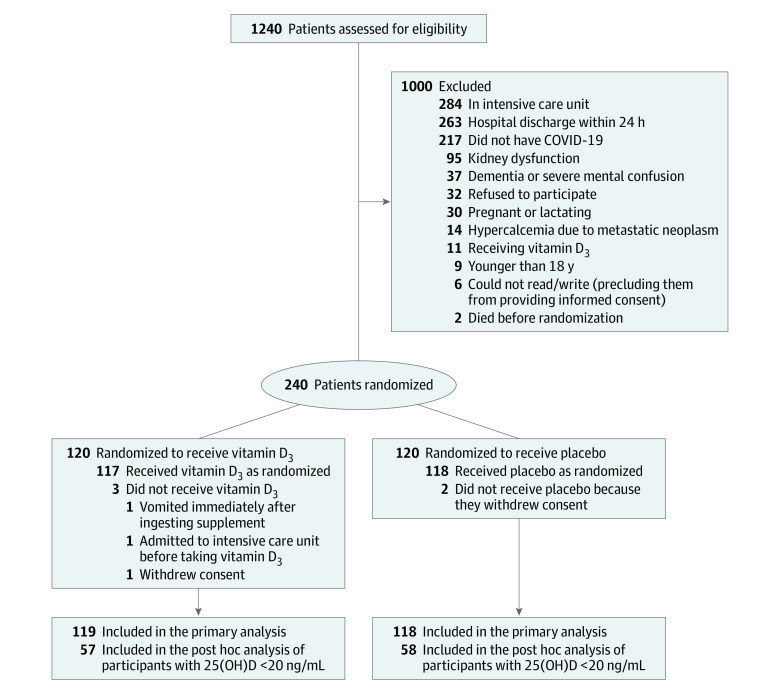
Of 1240 patients assessed for eligibility, 240 were eligible and randomized to either the vitamin D3 group or the placebo group. Patients were not eligible for inclusion due to the following reasons: 284 were in the intensive care unit, 263 had hospital discharge within 24 hours, 217 did not have COVID-19, 95 had kidney dysfunction, 37 had dementia or severe mental confusion precluding them from providing consent for participation, 32 refused to participate, 30 were pregnant or lactating women, 14 had hypercalcemia, 11 were receiving vitamin D3 (≥1000 IU/d), 9 were younger than 18 years, 6 could not read/write to provide consent, and 2 died before randomization.
Of the 240 patients eligible for participation, 122 were recruited at the Clinical Hospital of the School of Medicine of the University of Sao Paulo and 118 were recruited at the Ibirapuera field hospital. Of the 120 patients who were randomized to the vitamin D3 group, 3 did not receive the intervention (1 withdrew the consent before receiving the intervention, 1 vomited immediately after ingesting the supplement, and 1 was admitted to the intensive care unit before receiving the intervention). During the follow-up period, 1 patient received an extra dose of vitamin D3 as part of a fracture treatment. Of the 120 patients who were randomized to the placebo group, 2 did not receive the intervention because they withdrew consent. Of the 240 patients, only 3 who withdrew consent were excluded from the analysis, corresponding to 1.25% of missing data (Figure 1).
Figure 1. Flow of Patients in a Study of the Effect of a High Dose of Vitamin D3 on Patients With Moderate to Severe Coronavirus Disease 2019 (COVID-19).
All analyses were completed according to the patients’ randomization group. There was no imputation for missing data, except for laboratory parameters, in which missingness appeared to be at random and was modeled using generalized estimating equations. 25(OH)D indicates 25-hydroxyvitamin D.
Overall, 125 of 210 patients (59.5%) had computed tomography scan findings suggestive of COVID-19 and 147 of 237 (62.0%) had a PCR test result positive for SARS-CoV-2 at the time of enrollment. All remaining patients had the diagnosis confirmed by serology assay to detect IgG against SARS-CoV-2 at some point during the hospital stay. The mean (SD) time from the onset of symptoms to randomization was 10.3 (4.3) days and from hospitalization to randomization was 1.4 (0.9) days. The mean (SD) age of the patients was 56.2 (14.4) years, the mean (SD) body mass index was 31.7 (7.1), 104 patients (43.9%) were women, and 212 (89.5%) required supplemental oxygen at baseline (181 were receiving oxygen therapy and 31 were receiving noninvasive ventilation). Baseline characteristics of both groups are shown in Table 1.
Table 1. Baseline Characteristics in a Study of the Effect of a High Dose of Vitamin D3 on Patients With Moderate to Severe Coronavirus Disease 2019 (COVID-19).
| Characteristic | Vitamin D3 group (n = 119) | Placebo group (n = 118) |
|---|---|---|
| Age, mean (SD), y | 56.5 (13.8) | 56.0 (15.0) |
| Sex, No. (%) | ||
| Men | 70 (58.8) | 63 (53.4) |
| Women | 49 (41.2) | 55 (46.6) |
| Race, No. (%) | ||
| White | 62 (52.1) | 68 (57.6) |
| Pardoa | 37 (31.1) | 36 (30.5) |
| Black | 19 (16.0) | 14 (11.9) |
| Asian | 1 (0.8) | 0 |
| Time from symptom onset to enrollment, mean (SD), d | 10.2 (3.9) | 10.4 (4.7) |
| Time from hospital admission to enrollment, mean (SD), d | 1.3 (0.9) | 1.4 (0.9) |
| Body mass index, mean (SD) | 31.9 (6.5) | 31.4 (7.6) |
| <18.5, No. (%) | 0 | 2 (1.9) |
| 18.5-24.9, No. (%) | 9 (8.3) | 19 (17.6) |
| 25.0-29.9, No. (%) | 37 (33.9) | 31 (26.9) |
| ≥30, No. (%) | 63 (57.8) | 58 (53.7) |
| Acute COVID-19 symptoms, No. (%) | ||
| Cough | 102 (85.7) | 97 (82.2) |
| Fatigue | 97 (81.5) | 99 (83.9) |
| Fever | 85 (71.4) | 79 (66.9) |
| Myalgia | 68 (57.1) | 70 (59.3) |
| Sore throat | 45 (37.8) | 29 (24.6) |
| Joint pain | 45 (37.8) | 34 (28.8) |
| Runny nose | 43 (36.1) | 44 (37.3) |
| Diarrhea | 41 (34.5) | 46 (39.0) |
| Nasal congestion | 38 (31.9) | 42 (35.6) |
| Coexisting diseases, No. (%) | ||
| Hypertension | 67 (56.3) | 58 (49.2) |
| Diabetes | 49 (41.2) | 35 (29.7) |
| Cardiovascular disease | 16 (13.4) | 16 (13.6) |
| Rheumatic disease | 13 (10.9) | 10 (8.5) |
| Asthma | 7 (5.9) | 7 (5.9) |
| Chronic obstructive pulmonary disease | 7 (5.9) | 5 (4.2) |
| Chronic kidney disease | 2 (1.7) | 0 |
| Concomitant medications, No. (%) | ||
| Anticoagulant | 109 (91.6) | 101 (85.6) |
| Antibiotic | 101 (84.9) | 103 (87.3) |
| Corticosteroids | 77 (64.7) | 73 (61.9) |
| Antihypertensive | 67 (56.3) | 57 (48.3) |
| Proton-pump inhibitor | 47 (39.5) | 49 (41.5) |
| Antiemetic | 45 (37.8) | 55 (46.6) |
| Analgesic | 45 (37.5) | 52 (43.7) |
| Hypoglycemic | 26 (21.8) | 24 (20.3) |
| Hypolipidemic | 15 (12.6) | 18 (15.3) |
| Thyroid | 10 (8.4) | 10 (8.5) |
| Antiviralb | 4 (3.4) | 4 (3.4) |
| Oxygen supplementation, No. (%) | ||
| No oxygen therapy | 16 (13.4) | 9 (7.6) |
| Oxygen therapy | 86 (72.3) | 95 (80.5) |
| Noninvasive ventilation | 17 (14.3) | 14 (11.9) |
| Ground-glass opacities on computed tomography findings, No. (%) | ||
| <50% | 47 (43.9) | 38 (36.9) |
| ≥50% | 60 (56.1) | 65 (63.1) |
| Laboratory values | ||
| 25-Hydroxyvitamin D, mean (SD), ng/mL | 21.2 (10.1) | 20.6 (8.1) |
| Total calcium, mean (SD), mg/dL | 8.7 (0.5) | 8.7 (0.5) |
| Creatinine, mean (SD), mg/dL | 0.90 (0.33) | 0.85 (0.25) |
| C-reactive protein, median (IQR), mg/L | 57.9 (23.3-100.5) | 68.4 (31.5-111.5) |
| D-dimer, median (IQR), ng/mL | 823 (566-1769) | 840 (497-1490) |
SI conversion factors: To convert 25-hydroxyvitamin D to nmol/L, multiply values by 2.496; calcium to mmol/L, multiply values by 0.25; creatinine to μmol/L, multiply values by 88.4; D-dimer to nmol/L, multiply values by 5.476.
Pardo is the exact term used in Brazilian Portuguese, meaning “mixed ethnicity,” according to the Brazilian Institute of Geography and Statistics.
Included 3 patients from the vitamin D3 group and 3 patients from the placebo group receiving 75 mg of oseltamivir twice per day for 5 days, 1 patient from the vitamin D3 group receiving 400 mg of acyclovir twice per day for herpes zoster prophylaxis, and 1 patient from the placebo group receiving highly active antiretroviral therapy for HIV (atazanavir [300 mg] + tenofovir + ritonavir [100 mg] + lamivudine [300 mg]).
Primary Outcome
The median (interquartile range [IQR]) hospital length of stay was not significantly different between the vitamin D3 group (7.0 [4.0-10.0] days) and the placebo group (7.0 [5.0-13.0] days) (log-rank P = .59; unadjusted HR for hospital discharge, 1.07 [95% CI, 0.82-1.39]; P = .62; adjusted HR, 0.99 [95% CI, 0.71-1.37]; P = .94) (Figure 2).
Figure 2. Hospital Discharge in a Study of the Effect of a High Dose of Vitamin D3 on Patients With Moderate to Severe Coronavirus Disease 2019.
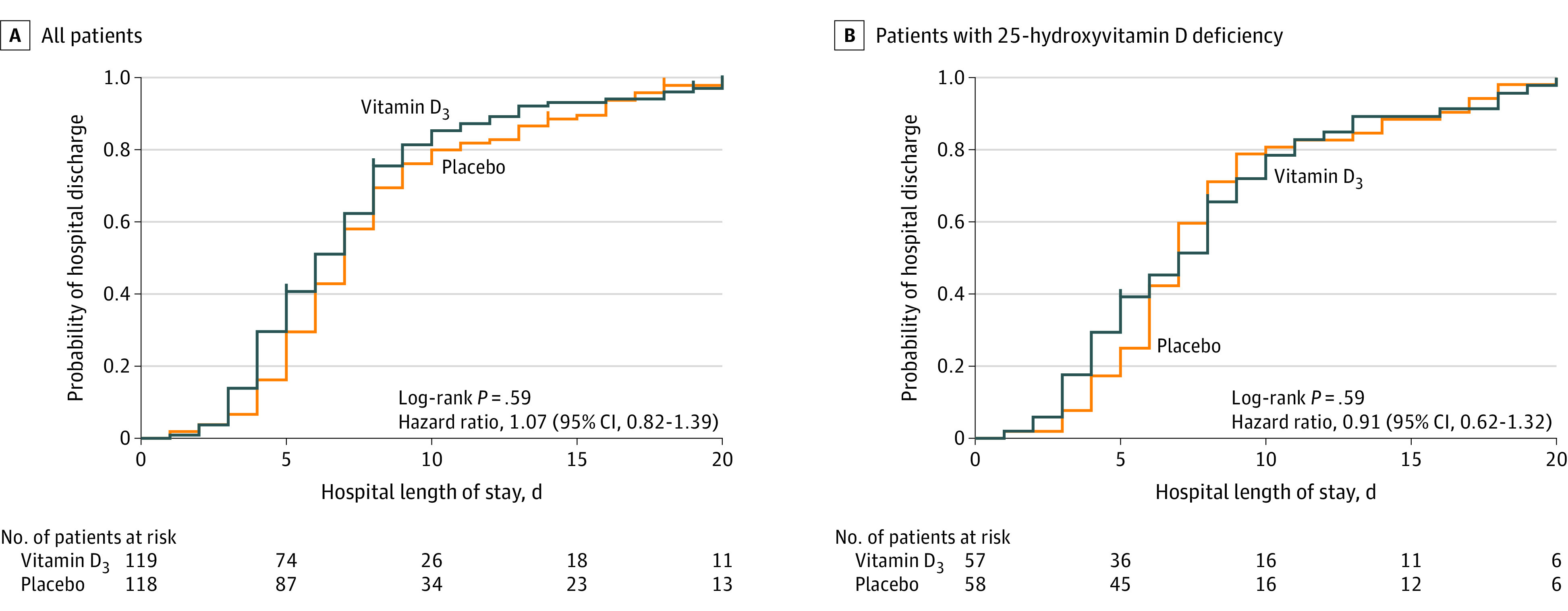
Vertical bars represent single censored events. A, The median (interquartile range) observation time was not significantly different between the vitamin D3 group (7.0 [4.0-10.0] d) and the placebo group (7.0 [5.0-13.0] d). B, Among the patients with 25-hydroxyvitamin D deficiency, there was no significant difference observed in the median (interquartile range) observation time between the vitamin D3 group (8.0 [4.0-11.5] d) and the placebo group (7.0 [6.0-13.3] d).
Secondary Outcomes
There were no significant differences between the vitamin D3 and placebo groups for in-hospital mortality (7.6% vs 5.1%; difference, 2.5% [95% CI, –4.1% to 9.2%]; P = .43), admission to the intensive care unit (16.0% vs 21.2%; difference, –5.2% [95% CI, –15.1% to 4.7%]; P = .30), or need for mechanical ventilation (7.6% vs 14.4%; difference, –6.8% [95% CI, –15.1% to 1.2%]; P = .09) (Table 2). The mean duration of mechanical ventilation was not significantly different between the vitamin D3 and the placebo group (15.0 vs 12.8 days; difference, 2.2 [95% CI, –8.4 to 12.8]; P = .69).
Table 2. Secondary Outcomes in a Study of the Effect of a High Dose of Vitamin D3 on Patients With Moderate to Severe Coronavirus Disease 2019.
| Outcome | Patients (95% CI), % | Between-group difference (95% CI), % | P value | |
|---|---|---|---|---|
| Vitamin D3 group | Placebo group | |||
| All patients | n = 119 | n = 118 | ||
| In-hospital mortality | 7.6 (3.5 to 13.9) | 5.1 (1.9 to 10.7) | 2.5 (–4.1 to 9.2) | .43 |
| Admission to intensive care unit | 16.0 (9.9 to 22.5) | 21.2 (14.2 to 29.7) | –5.2 (–15.1 to 4.7) | .30 |
| Mechanical ventilation requirement | 7.6 (3.5 to 13.9) | 14.4 (8.6 to 22.1) | –6.8 (–15.1 to 1.2) | .09 |
| Patients with 25-hydroxyvitamin D deficiency (<20 ng/mL) | n = 57 | n = 58 | ||
| In-hospital mortality | 7.0 (1.9 to 17.0) | 1.7 (0.04 to 9.2) | 5.3 (–3.3 to 15.1) | .21 |
| Admission to intensive care unit | 19.3 (10.0 to 31.9) | 15.5 (7.4 to 27.4) | 3.8 (–10.3 to 17.8) | .59 |
| Mechanical ventilation requirement | 7.0 (1.9 to 17.0) | 8.6 (2.9 to 19.0) | –1.6 (–12.5 to 9.2) | >.99 |
Mean (SD) 25-hydroxyvitamin D was significantly increased from baseline after a single high dose of vitamin D3 (from 21.2 [10.1] ng/mL to 44.4 [15.0] ng/mL) vs placebo (from 20.6 [8.1] ng/mL to 19.8 [10.5] ng/mL ) (between-group postintervention difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001) (Figure 3). After receiving the intervention, 91 of 105 patients (86.7%) in the vitamin D3 group had 25-hydroxyvitamin D levels above 30 ng/mL (compared with 11 of 101 [10.9%] in the placebo group) and only 7 patients (6.7%) in the vitamin D3 group had 25-hydroxyvitamin D deficiency (compared with 52 [51.5%] in the placebo group).
Figure 3. Serum 25-Hydroxyvitamin D Levels in a Study of the Effect of a High Dose of Vitamin D3 on Patients With Moderate to Severe Coronavirus Disease 2019.
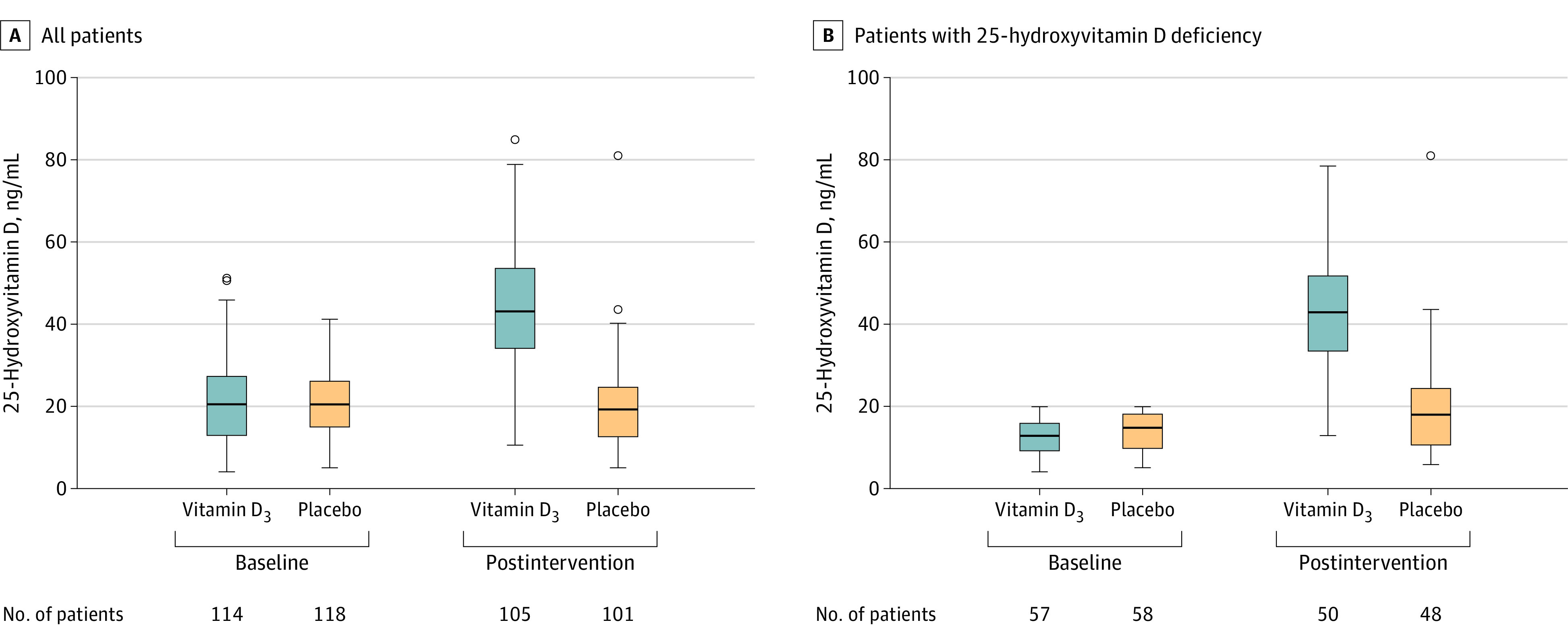
Serum 25-hydroxyvitamin D levels were measured on the day of randomization (baseline) and on hospital discharge (postintervention). A, For all patients, a single high dose of vitamin D3 significantly increased 25-hydroxyvitamin D levels compared with placebo (difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001). Median (interquartile range) observation time of the postintervention period was 7.0 (4.0-10.0) days for the vitamin D3 group and 7.0 (5.0-13.0) days for the placebo group. B, For patients with 25-hydroxyvitamin D deficiency, a single high dose of vitamin D3 significantly increased 25-hydroxyvitamin D levels compared with placebo (difference, 22.7 ng/mL [95% CI, 19.3-26.1]; P < .001). Median (interquartile range) observation time of the postintervention period was 8.0 (4.0-11.5) days for the vitamin D3 group and 7.0 (6.0-13.3) days for the placebo group. Boxes represent median and interquartile range and whiskers extend to the highest and lowest values within 1.5 times the interquartile range of the 25th and 75th percentiles. Circles represent outliers.
There were no significant differences between the vitamin D3 group and the placebo group in total calcium (0.02 mg/dL [95% CI, –0.17 to 0.22]; P > .99), creatinine (0.06 mg/dL [95% CI, –0.17 to 0.29]; P > .99), C-reactive protein (–0.66 mg/L [95% CI, –5.34 to 4.00]; P = .99), and D-dimer (a post hoc outcome; 30.4 ng/mL [95% CI, –255.4 to 316.2]; P >.99) (eTable 2 in Supplement 2).
Post Hoc Analyses
In a post hoc analysis imputing the 90th-percentile hospital length of stay for those who died, the median (IQR) hospital length of stay was not significantly different between the vitamin D3 group (7.0 [4.0-10.0] days) and the placebo group (7.0 [5.0-13.0] days) (log-rank P = .33; unadjusted HR for hospital discharge, 1.13 [95% CI, 0.87-1.45]; P = .36; adjusted HR, 1.03 [95% CI, 0.75-1.41]; P = .88). The median (IQR) time to death did not significantly differ between the vitamin D3 (26.0 [13.5-48.5] days) and placebo group (26.5 [17.0-32.2] days) (P = .69 for Mann-Whitney test).
In a post hoc analysis involving patients with 25-hydroxyvitamin D deficiency at baseline (n = 115), a single high dose of vitamin D3 significantly increased mean (SD) 25-hydroxyvitamin D levels from baseline (from 12.8 [3.9] ng/mL to 35.7 [11.1] ng/mL) vs placebo (from 13.9 [4.7] ng/mL to 13.0 [4.4] ng/mL) (between-group postintervention difference, 22.7 ng/mL [95% CI, 19.3-26.1]; P < .001) (Figure 3; eTable 3 in Supplement 2). Among the patients with 25-hydroxyvitamin D deficiency at baseline, no significant differences were observed in the median (IQR) hospital length of stay between the vitamin D3 (8.0 [4.0-11.5] days) and placebo group (7.0 [6.0-13.3] days) (log-rank P = .59; unadjusted HR for hospital discharge, 0.91 [95% CI, 0.62-1.32]; P = .61; adjusted HR, 0.77 [95% CI, 0.46-1.27]; P = .30) (Figure 2). In addition, there were no significant differences between the vitamin D3 group and the placebo group for in-hospital mortality (7.0% vs 1.7%; difference, 5.3% [95% CI, –3.3% to 15.1%]; P = .21), admission to the intensive care unit (19.3% vs 15.5%; difference, 3.8% [95% CI, –10.3% to 17.8%]; P = .59), or need for mechanical ventilation (7.0% vs 8.6%; difference, –1.6% [95% CI, –12.5% to 9.2%]; P > .99) (Table 2). The mean duration of mechanical ventilation was not significantly different between the vitamin D3 and placebo group (12.2 vs 16.0 days; difference –3.8 [95% CI, –19.0 to 11.4]; P = .63).
A post hoc analysis showed no site effect in the median length of stay between the vitamin D3 and the placebo group (log-rank P = .51; unadjusted HR for hospital discharge, 1.09 [95% CI, 0.83-1.42]; P = .54; adjusted HR, 1.00 [95% CI, 0.72-1.38]; P = .97).
Adverse Events
A single high dose of vitamin D3 was well tolerated and no severe adverse events were reported throughout the trial, with the exception of 1 patient who vomited after vitamin D3 administration. There were no significant between-group differences in any health-related laboratory markers after the intervention (eTable 2 in Supplement 2).
Discussion
In this randomized, double-blind, placebo-controlled clinical trial, a single high dose of vitamin D3 did not significantly reduce hospital length of stay or improve any other clinically relevant outcomes among hospitalized patients with moderate to severe COVID-19. To our knowledge, this is the first randomized clinical trial to demonstrate these findings.
Vitamin D appears to regulate both innate and adaptative immune responses.6,19 Observational studies have shown that higher 25-hydroxyvitamin D levels are associated with better clinical outcomes in respiratory diseases.20 Positive associations between low 25-hydroxyvitamin D levels and poor prognosis among patients with COVID-19 have also been observed.21 Furthermore, a small nonrandomized trial demonstrated that administration of regular boluses of vitamin D3 before the infection was associated with better survival and less severe disease among older frail patients with COVID-19.22 However, in the current trial, a single dose of 200 000 IU of vitamin D3 did not result in any clinically relevant effects among hospitalized patients with moderate to severe COVID-19, contesting the use of supplementary vitamin D3 as a treatment for patients with this disease.
The lack of clinical benefits seen in this study was independent of the ability of vitamin D3 to increase serum 25-hydroxyvitamin D levels. After the intervention, 86.7% of the patients in the vitamin D3 group achieved 25-hydroxyvitamin D sufficiency (≥30 ng/mL) vs 10.9% in the placebo group. In a post hoc analysis confined to the patients exhibiting 25-hydroxyvitamin D deficiency, a single high dose of vitamin D3 remained effective in increasing 25-hydroxyvitamin D levels compared with placebo, yet no clinical improvements were noted. These analyses indicate that a single oral dose of 200 000 IU of vitamin D3 can rapidly increase 25-hydroxyvitamin levels, so the present null findings cannot be attributed to the failure of increasing serum 25-hydroxyvitamin D levels.
The strengths of this study include the randomized, double-blind, placebo-controlled, experimental design; the very low attrition rate (1.25%); the concomitant assessment of 25-hydroxyvitamin D levels along with clinical outcomes; and the assessment of hospitalized patients with moderate to severe COVID-19.
Limitations
This trial has several limitations. First, the minimal clinically important difference in hospital length of stay among patient with COVID-19 remains to be determined. Although the HR for the primary outcome indicates that the intervention was ineffective, the relatively low sample size in this trial could have had inadequate power to exclude small, but clinically meaningful, differences between the groups. Second, because the patients had several coexisting diseases and were subjected to a diverse medication regimen, the results could have been affected by the heterogeneity of the sample and its treatment. Third, the percentage of patients with 25-hydroxyvitamin D deficiency enrolled in this study was considerably lower than those reported in other cohorts,23 possibly as a consequence of differences in geographic locations. Therefore, caution should be exercised in generalizing these findings to patients from other geographical regions. Fourth, the patients were given a dose of vitamin D3 after a relatively long time from symptom onset to randomization (ie, mean of 10.3 days). Further studies should determine whether preventive or early vitamin D3 supplementation could be useful in the treatment of patients with COVID-19, especially those with mild or moderate disease.
Conclusions
Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of vitamin D3 for treatment of moderate to severe COVID-19.
Trial protocol and statistical analysis plan
eTable 1. Baseline laboratory values
eTable 2. Laboratory values
eTable 3. Baseline demographic and clinical characteristics from the patients with 25-hydroxyvitamin D deficiency (< 20 ng/mL)
Data sharing statement
References
- 1.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-1773. doi: 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 2.Aglipay M, Birken CS, Parkin PC, et al. ; TARGet Kids! Collaboration . Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245-254. doi: 10.1001/jama.2017.8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523-1525. doi: 10.4161/auto.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1-2):93-101. doi: 10.1016/j.jsbmb.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Laplana M, Royo JL, Fibla J. Vitamin D receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. 2018;678:384-394. doi: 10.1016/j.gene.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 6.Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133-R147. doi: 10.1530/EJE-20-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76-89. doi: 10.1016/S2213-8587(13)70165-7 [DOI] [PubMed] [Google Scholar]
- 8.Aibana O, Huang CC, Aboud S, et al. Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019;16(9):e1002907. doi: 10.1371/journal.pmed.1002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. doi: 10.1371/journal.pone.0011088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8(9):735-736. doi: 10.1016/S2213-8587(20)30268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195-1198. doi: 10.1007/s40520-020-01570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341-351. doi: 10.4158/EP13265.RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacchetti P. Current sample size conventions: flaws, harms, and alternatives. BMC Med. 2010;8:17. doi: 10.1186/1741-7015-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacchetti P, McCulloch CE, Segal MR. Simple, defensible sample sizes based on cost efficiency. Biometrics. 2008;64(2):577-585. doi: 10.1111/j.1541-0420.2008.01004_1.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco AS, Freitas TQ, Bernardo WM, Pereira RMR. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(23):e7024. doi: 10.1097/MD.0000000000007024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129-1140. doi: 10.1017/S0950268806007175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpagnano GE, Di Lecce V, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. Published online August 9, 2020. doi: 10.1007/s40618-020-01370-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annweiler G, Corvaisier M, Gautier J, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):e3377. doi: 10.3390/nu12113377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández JL, Nan D, Fernandez-Ayala M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020;dgaa733. doi: 10.1210/clinem/dgaa733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eTable 1. Baseline laboratory values
eTable 2. Laboratory values
eTable 3. Baseline demographic and clinical characteristics from the patients with 25-hydroxyvitamin D deficiency (< 20 ng/mL)
Data sharing statement