Key Points
Question
What are the growth rates and patterns of small abdominal aortic aneurysms surveilled by computed tomography over 2 years?
Findings
In this cohort analysis of a randomized trial of 254 patients with 3.5- to 5.0-cm abdominal aortic aneurysms, average annual growth was 0.19 cm, and 70% of patients displayed linear growth.
Meaning
Based on linear low growth patterns, surveillance of abdominal aortic aneurysms less than 4.25 cm can be safely extended to at least 2 years.
Abstract
Importance
Small abdominal aortic aneurysms (AAAs) are common in the elderly population. Their growth rates and patterns, which drive clinical surveillance, are widely disputed.
Objective
To assess the growth patterns and rates of AAAs as documented on serial computed tomography (CT) scans.
Design, Setting, and Participants
Cohort study and secondary analysis of the Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT), a randomized, double-blind placebo-controlled clinical trial conducted from 2013 to 2018, with CT imaging every 6 months for 2 years. The trial was a multicenter, observational secondary analysis, not related to treatment hypotheses of data collected in the N-TA3CT. Participants included 254 patients with baseline AAA diameter between 3.5 and 5.0 cm.
Exposures
Patients received serial CT scan measurements, analyzed for maximum transverse diameter, at 6-month intervals.
Main Outcomes and Measures
The primary study outcome was AAA annual growth rate. Secondary analyses included characterizing AAA growth patterns, assessing likelihood of AAA diameter to exceed sex-specific intervention thresholds over 2 years.
Results
A total of 254 patients, 35 women with baseline AAA diameter 3.5 to 4.5 cm and 219 men with baseline diameter 3.5 to 5.0 cm, were included. Yearly growth rates of AAA diameters were a median of 0.17 cm/y (interquartile range [IQR], 0.16) and a mean (SD), 0.19 (0.14) cm/y. Ten percent of AAAs displayed minimal to no growth (<0.05 cm/y), 62% displayed low growth (0.05-0.25 cm/y), and 28% displayed high growth (>0.25 cm/y). Baseline AAA diameter accounted for 5.4% of variance of growth rate (P < .001; R2, 0.054). Most AAAs displayed linear growth (70%); large variations in interval growth rates occurred infrequently (3% staccato growth and 4% exponential growth); and some patients’ growth patterns were not clearly classifiable (23% indeterminate). No patients with a maximum transverse diameter less than 4.25 cm exceeded sex-specific repair thresholds at 2 years (men, 0 of 92; 95% CI, 0.00-0.055; women, 0 of 25 ; 95% CI, 0.00-0.247). Twenty-six percent of patients with a maximum transverse diameter of at least 4.25 cm exceeded sex-specific repair thresholds at 2 years (n = 12 of 83 men with diameter ranging from 4.25 to <4.75 cm; 95% CI, 0.091-0.264; n = 21 of 44 men with diameter ranging from 4.75-5.0 cm; 95% CI, 0.362-0.669; n = 3 of 10 women with diameter ≥4.25 cm; 95% CI, 0.093-0.726).
Conclusions and Relevance
Most small AAAs showed linear growth; large intrapatient variations in interval growth rates were infrequently observed over 2 years. Linear growth modeling of AAAs in individual patients suggests smaller AAAs (<4.25 cm) can be followed up with a CT scan in at least 2 years with little chance of exceeding interventional thresholds.
Trial Registration
ClinicalTrials.gov Identifier: NCT01756833
This secondary analysis of a randomized clinical trial assesses the growth patterns and rates of abdominal aortic aneurysms as documented on serial computed tomography scans.
Introduction
Abdominal aortic aneurysm (AAA) affects 1.1 million people in the United States.1 Most of these are small aneurysms without indication for immediate repair; they are managed expectantly with imaging surveillance until maximum transverse diameter (MTD) predicts a substantial enough risk of rupture to merit repair. Abdominal aortic aneurysm rupture or complication of repair were the primary cause of nearly 10 000 deaths in 2014 and a contributing cause of more than 17 000 deaths in 2009.2,3 Ruptured AAA was the 17th leading cause of death in people older than 65 years in 2017.4 Understanding AAA growth patterns informs appropriate monitoring intervals. Discrepancies exist in the literature on the most common patterns of AAA growth. Some studies suggest staccato growth patterns5,6; others suggest exponential growth7,8,9,10; and others present growth as linear.11,12
The Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT), a double-blind, placebo-controlled clinical trial of doxycycline randomized 261 participants with AAAs between 3.5 and 5.0 cm and measured the MTD of the AAA approximately every 6 months. The primary objective of this secondary cohort analysis is to describe growth rates and growth patterns of AAAs in this patient population. Secondarily, we seek to inform surveillance intervals by assessing likelihood of AAA diameter to exceed sex-specific intervention thresholds over 2 years and to clarify the association between baseline diameter and annual growth rates.
Methods
Study Design
This study adhered to the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) statement and checklist (http://www.strobe-statement.org). Data in this study were collected from the N-TA3CT (n = 261); patients were enrolled from 22 US clinical centers between May 2013 and January 2017. The N-TA3CT enrolled patients 55 years or older with AAAs between 3.5 to 5.0 cm for men and 3.5 to 4.5 cm for women. Notable exclusion criteria included patients with AAAs less than 4.0 cm with documented failure to grow over the 2 years prior to enrollment, known connective tissue diseases, and current or planned treatment with systemic immunosuppressive agents. Institutional review boards at all clinical sites, core laboratories, and coordinating centers approved the protocol. Written consent was obtained from all patients. The study design, methods, and findings of N-TA3CT have been previously published.13,14 The primary analysis indicated doxycycline did not affect aneurysm growth.14 Based on this finding and secondary analyses showing MTD growth rates (eFigure 1 in the Supplement) and patterns not differing significantly between treatment groups, our analysis pooled both treatment arms.
Patients were included in our analysis if they had follow-up data beyond baseline imaging. Of the 261 patients enrolled in N-TA3CT, 254 had data allowing for their inclusion in the clinical outcomes analyses, 250 had 2 or more computed tomography (CT) scans enabling their inclusion in growth rate analyses, 241 had 3 or more CT scans enabling their inclusion in maximum segmental slope difference analysis, and 214 had 4 or more enabling their inclusion in growth pattern analyses (eFigure 2 in the Supplement).
Computed Tomography Acquisition Protocol and Analysis
Abdominal and pelvic CT scans were obtained at baseline and approximately every 6 months thereafter for 18 to 30 months. A total of 204 patients (80%) completed imaging through the 24-month window; median period of follow-up was 26 months (interquartile range [IQR], 24-31 months). The AAA MTD was assessed via CT imaging processed at AortaCore Imaging Core Laboratory at the University of Wisconsin, Madison on postprocessing workstations (Aquarius Intuition, version 4.4.12; TeraRecon). The protocol included masking of patient identity, digital magnification, standardized windowing, and quality control with blind replicates and twice-yearly proficiency testing. A single reader, with many years of aortic CT analysis experience, used the double oblique technique to measure the MTD; intraobserver and interobserver intraclass correlation coefficients were greater than 0.99 for this technique.13 The double oblique technique selects the multiplanar reconstruction image of the largest section of the AAA, following a manually corrected centerline, in a plane perpendicular to the aneurysm walls. The largest diameter of the aneurysm was measured with electronic calipers from outer wall to outer wall. This study used the double oblique method to measure MTD because it is more reproducible and accurate than measuring diameters on axial CT or ultrasonography images.15,16
Statistical Analysis
Growth Rate Assessment
Each patient’s diameter growth rate was estimated by simple linear regression of MTD on time using all available CT scans performed in the study; growth rate (slope of linear regression) was expressed as centimeters of growth per year. Annual growth rates were grouped into 3 categories: minimal to no growth (<0.05 cm/y), low growth (0.05-0.25 cm/y), or high growth (>0.25 cm/y). These categories were established a posteriori based on (1) ±0.05 cm as an acceptable difference for reproducibility of AAA diameter measurements on CT scans17,18 and (2) data from prior surveillance studies of AAAs comparable with N-TA3CT, which reported mean annual growth rates ranging from 0.19 cm19 to 0.28 cm.20 The Shapiro-Wilk test was used to assess whether distribution of growth rates was approximately normal.21
Growth Pattern Assessment
Besides the simple linear regression modeling of MTD on time (linear model), regressions of MTD on etime (exponential model) were performed. Linear growth was defined as an R2 value of at least 0.90 for the linear model. Patients with nonlinear growth were subcategorized into exponential growth, staccato growth, or indeterminate growth patterns. Exponential growth was defined by an R2value of at least 0.95 for the exponential model. Staccato growth pattern was defined as a pattern of 1 or more “no significant growth intervals” (≤0.05 cm/interval) accompanied by 1 or more “fast growth intervals” (≥0.6 cm/y).5 Growth patterns that did not fit the definitions here were classified as indeterminate and further categorized as indeterminate–not growing (annual growth rate <0.05 cm/y), and indeterminate-growing (≥0.05 cm/y). Two investigators (S.O. and J.M.), individually and then jointly, reviewed every individual patient’s growth pattern to qualitatively confirm that calculated growth rates and pattern assessments were appropriate; no growth patterns were changed on review.
Maximum Segment Slope Difference
The observed growth rate between each successive pair of measurements was calculated and the range (maximum minus minimum) of sequential growth rate differences, referred to here as the maximum segmental slope difference (max-SSD), was also calculated. Perfect, undeviating linear growth would result in a max-SSD of 0. Thus, max-SSD was used to further describe linear growth, and was calculated for each patient for whom there were at least 3 images (n = 241). The Shapiro-Wilk test was used to assess whether distribution of max-SSD was approximately normal.21
Baseline Diameter vs Growth Rate Assessment
Association of growth rate, calculated as described in previous sections, with baseline aneurysm diameter was assessed by simple linear regression of a patient’s growth rate based on their baseline MTD.
Growth to Surgical Repair Thresholds
Probabilities of meeting the MTD threshold for repair (5.5 cm for men and 5.0 cm for women) within 2 years or having AAA repair within 2 years were estimated by the life table method to include data for patients with less than 2 years of follow-up. Confidence intervals were calculated using exact binomial calculation for proportions. Results with P at or less than .05 were considered statistically significant, and all P values were 2-sided. No correction for multiple comparisons was made. Statistical analysis was performed using SAS, version 9.4 (SAS Institute and NCSS 10 and NCSS 2020 (Number Cruncher Statistical Systems).
Results
Baseline Characteristics
The N-TA3CT randomization occurred between May 2013 and January 2017. Of 261 patients randomized, 254 had follow-up beyond baseline that could be used in these analyses. The baseline characteristics of the 254 patients are summarized in Table 1. The mean (SD) age of patients was 70.9 (7.4) years and 86% (n = 219) were men. Ninety-two percent (n = 235) were current or former smokers. Comorbidities included hypercholesterolemia (n = 196; 77%), coronary artery disease (n = 104; 41%), cancer (n = 82; 32%), and chronic obstructive pulmonary disease (n = 59; 23%). Baseline AAA MTDs ranged from 3.50 to 4.52 cm for women and 3.50 to 5.04 cm for men.
Table 1. Baseline Characteristics.
| Demographics | No. (%) |
|---|---|
| No. | 254 |
| Age, mean (SD), y | 70.9 (7.4) |
| Sex | |
| Female | 35 (14) |
| Male | 219 (86) |
| Health status | |
| Smoking status | |
| Current | 87 (34) |
| Former | 148 (58) |
| Never | 19 (7) |
| Hypercholesteremia | 196 (77) |
| Coronary artery disease | 104 (41) |
| Cancer | 82 (32) |
| Chronic obstructive pulmonary disease | 59 (23) |
| Diabetes | 57 (22) |
| Family history of abdominal aortic aneurysm | 47 (19) |
| Atrial fibrillation | 33 (13) |
| Stroke | 28 (11) |
| Congestive heart failure | 20 (8) |
| Medication | |
| Statin | 207 (82) |
| Any antihypertensive | 199 (78) |
| β-Blocker | 131 (52) |
| Diuretics | 83 (33) |
| Angiotensin-converting enzyme inhibitor | 88 (35) |
| Calcium channel blocker | 60 (24) |
| Angiotensin receptor blocker | 45 (18) |
| Aspirin or other antiplatelet | 187 (74) |
| Daily aspirin | 175 (69) |
| Other antiplatelet | 44 (17) |
| Baseline MTD, cm | |
| Men, No. | 219 |
| 3.50-3.99 | 49 (22) |
| 4.00-4.49 | 75 (34) |
| 4.50-5.04 | 95 (43) |
| Women, No. | 35 |
| 3.50-3.99 | 19 (54) |
| 4.00-4.52 | 16 (46) |
Abbreviation: MTD, maximum transverse diameter.
Yearly Growth Rates
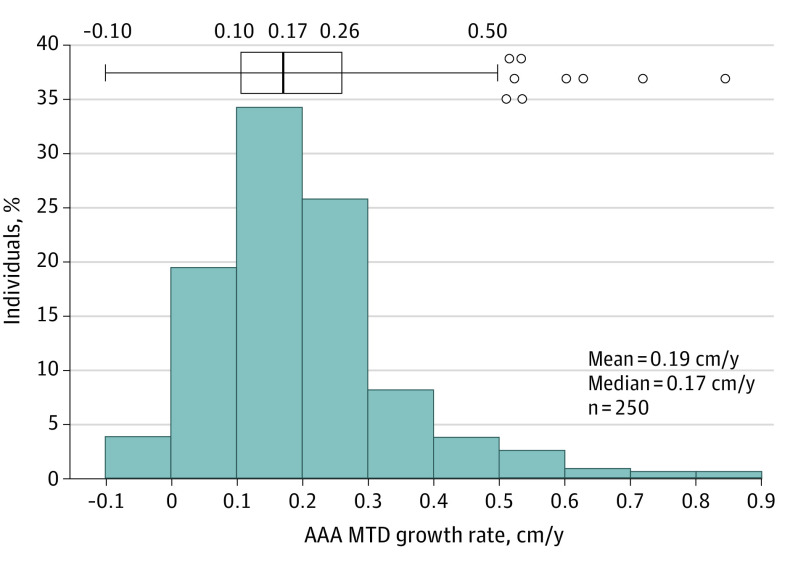
Distribution of yearly growth rates shows mean growth of 0.19 cm/y and median growth of 0.17 cm/y (IQR, 0.16) (Figure 1). The Shapiro-Wilk test showed AAA MTD annual growth rate to be not normally distributed, with a right skew. Annual growth rates in the 3 assigned groups were: minimal to no growth (<0.05 cm/y), 10% of patients (n = 25); low growth (0.05-0.25cm/y), 62% (n = 156); and high growth (>0.25 cm/y), 28% (n = 69) (eFigure 3 in the Supplement). eFigure 4 in the Supplement displays 3 individual patient examples that are representative of growth rates of minimal to no growth (eFigure 3A), low growth (eFigure 3B), and high growth (eFigure 3C). On linear regression, 10 patients had negative slopes (eFigure 5 in the Supplement) consistent in absolute magnitude with minimal to no growth or low growth.
Figure 1. Distribution of Yearly Abdominal Aortic Aneurysm (AAA) Maximum Transverse Diameter (MTD) Growth Rates.
Histogram and box plots showing distribution of yearly MTD growth rates. Patients were included if they had measurement data from at least 2 computed tomography scans (n = 250). Growth rates were calculated in centimeters per year based on the linear regression lines calculated for each patient. Shapiro-Wilk test for normal distribution showed data to be not normally distributed (P < .001).
Growth Patterns
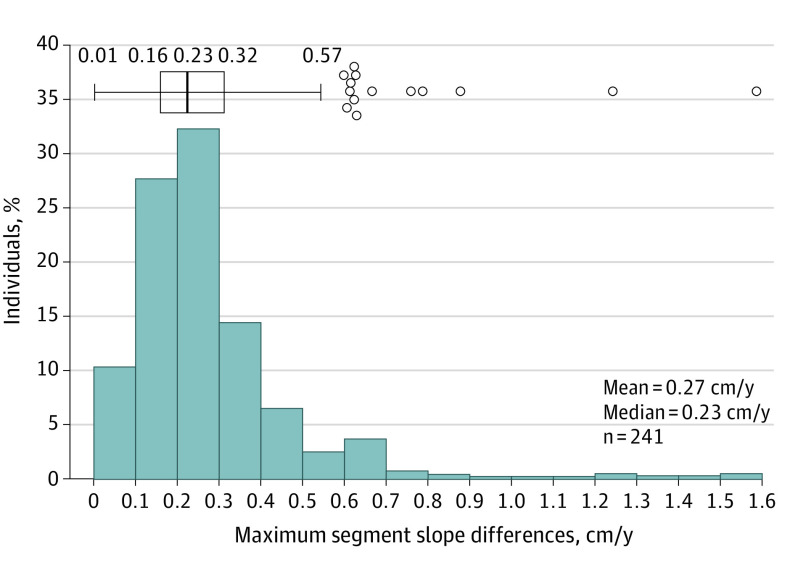
Maximum segment slope difference ranged from negligible to 1.6 cm/y, with a mean (SD) of 0.27 (0.18) cm/y; median, 0.23 (IQR, 0.16) (Figure 2). The Shapiro-Wilk test for normal distribution showed max-SSD to be not normally distributed; there is a right skew. The small differences between slope segments in most patients support the linear model fit of MTD growth rates per patient over 2 years. eFigure 6 in the Supplement displays 3 individual patient examples, with corresponding max-SSDs, that are representing undeviating linear growth, linear growth with some variation over time, and nonlinear growth. The distribution of growth patterns was not significantly different between N-TA3CT treatment groups (placebo [n = 107]: 67% linear, 5% exponential, 6% staccato, 11% indeterminate-growing, and 11% indeterminate–not growing; doxycycline [n = 107]: 72% linear, 3% exponential, 1% staccato, 8% indeterminate-growing, and 16% indeterminate–not growing).
Figure 2. Distribution of Maximum Segment Slope Difference Supports Most Abdominal Aortic Aneurysms (AAAs) Grow Linearly.
Histogram and box plot distribution of maximum segment slope difference values for all patients with at least 3 computed tomography scans (n = 241). The maximum segment slope difference values for each patient measure the difference between the highest growth rate (centimeters per year) between 2 consecutive scans and the lowest growth rate between 2 consecutive scans. Shapiro-Wilk test for normal distribution showed data to be not normally distributed (P < .001). Low differences between slope segments in the majority of patients emphasize that linear modeling of maximum transverse diameter growth rates per patient is appropriate.
Linear growth was observed in 70% of patients (95% CI, 0.630-0.757) assessed for growth pattern (16.1% of patients with 4 scans, 32.2% with 5 scans, and 51.7% with 6 scans). Nonlinear growth patterns were infrequent: staccato was observed in 7 patients (3%; 95% CI, 0.03-0.066) (14.3% of patients with 4 scans, 57.1% with 5 scans, and 28.6% with 6 scans) and exponential in 8 patients (4%; 95% CI, 0.016-0.072) (87.5% of patients with 5 scans and 12.5% with 6 scans). Twenty-three percent of patients had indeterminate growth patterns: 29 patients with indeterminate-growing (13%; 95% CI, 0.093-0.189) (20.7% of patients with 4 scans, 41.4% with 5 scans, and 37.9% with 6 scans) and 21 patients with indeterminate–not growing (10%; 95% CI, 0.062-0.146) patterns (19.0% of patients with 4 scans, 19.0% with 5 scans, and 61.9% with 6 scans). Patients with linear growth had lower max-SSDs than patients with nonlinear growth (eFigure 7 in the Supplement). eFigure 8 in the Supplement displays 3 individual patient examples representing nonlinear growth patterns of staccato growth (eFigure 8A), exponential growth (eFigure 8B), and indeterminate growing (eFigure 8C). Applying a previously published staccato growth definition, we identified 22 patients (10%) with staccato growth.5
Baseline AAA MTD Compared With Growth Rates and Surgical Repair Thresholds
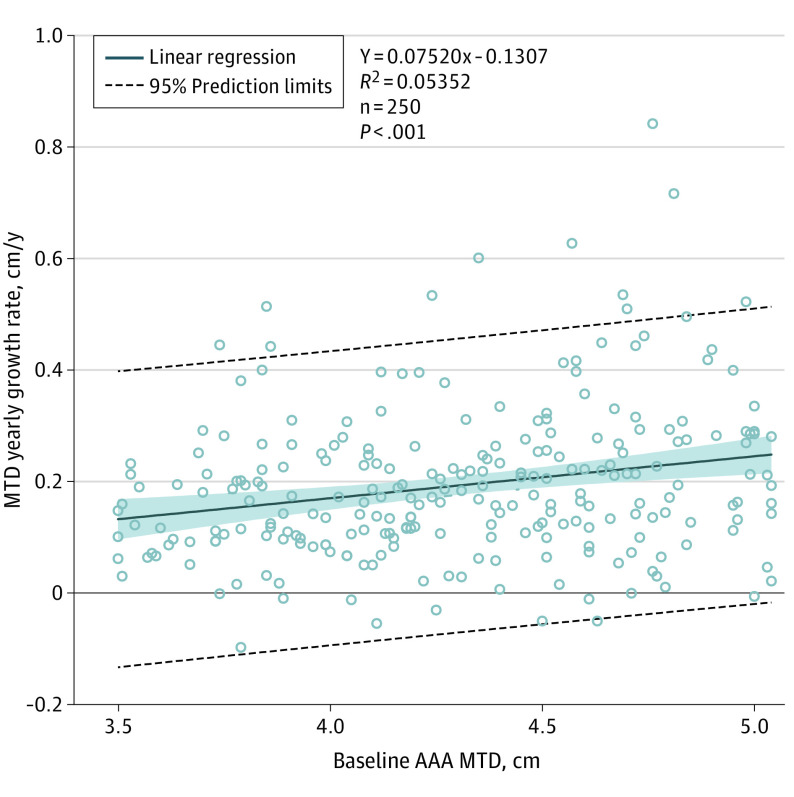
Pretreatment AAA MTD was significantly associated with yearly MTD growth rate but accounted for only 5.4% of the variance of growth rate (Figure 3). A 1-cm increase in baseline diameter was associated with 0.08-cm/y increase in annual growth rate (95% CI, 0.04-0.11). Minimal to no growth and high growth rates were observed across the range of baseline MTDs. For patients with baseline MTD less than 4.25 cm, 0% exceeded surgical repair thresholds. Among men whose baseline MTD was less than 4.25 cm, the 95% CI upper limit to the frequency of reaching threshold over 2 years was 5.5%. Specific frequencies of surpassing repair thresholds are given for each size range in Table 2. No patients in N-TA3CT had ruptured AAAs. Of the 24 patients repaired over the study, only 4 exceeded the MTD threshold for repair on the CT imaging preceding the repair.
Figure 3. Association of Yearly Maximum Transverse Diameter (MTD) Growth Rates With Baseline Abdominal Aortic Aneurysm (AAA) Diameter.
Linear regressions were calculated for each patient based on measurement and study day. Patients were included if they had measurement data from at least 2 computed tomography scans (n = 250). Linear regression growth rates in centimeters per year are plotted vs baseline MTD in centimeters. The shaded blue area represents 95% confidence limits. Linear regression trendlines show a small but significant association between baseline aneurysm size and yearly growth rate.
Table 2. Rates of AAA Repair and Growth to Repair Threshold at 2 Yearsa.
| Baseline MTD, cm | No.b | Average yearly growth rate, mean (SD), cm/yc | No. (%) [95% CI]d | No. (%) [95% Upper CI] | |
|---|---|---|---|---|---|
| MTD repair threshold met within 2 ye | AAA repaired within 2 y | MTD repair threshold met or AAA repaired by 2 y | |||
| Men | |||||
| <4.25 | 92 | 0.16 (0.11) | 0 [0-5.5] | 0 [0-4.0] | 0 [0-5.5] |
| 4.25 to <4.75 | 83 | 0.20 (0.14) | 2 (3.3) [0.3-9.2] | 10 (12.4) [6.1-20.4] | 12 (16.9) [9.1-26.4] |
| ≥4.75 | 44 | 0.24 (0.18) | 12 (36.6) [20.9-53.9] | 13 (30.0) [17.4-44.3] | 21 (51.6) [36.2-66.9] |
| Women | |||||
| <4.25 | 25 | 0.19 (0.12) | 0 [0-24.7] | 0 [0-14.2] | 0 [0-24.7] |
| 4.25 to <4.75 | 10 | 0.26 (0.06) | 3 (38.1) [9.3-72.6] | 0 [0-30.8] | 3 (38.1) [9.3-72.6] |
Abbreviations: AAA, abdominal aortic aneurysm affects; MTD, maximum transverse diameter.
A category with fewer events can have a higher estimate of event rate than a category with more events, owing to different censoring patterns for different categories.
Number of patients at baseline.
Includes 250 patients.
Estimation of event rate by life table method and 95% CI using arcsine square root transformation; for 0 events, 95% CI estimated for repair by exact binomial calculations based on number of patients with 2 years of follow-up and for MTD threshold with censoring at the time of the last computed tomography scan.
Male threshold for repair of at least 5.5 cm; female threshold of at least 5.0 cm.
Discussion
These data demonstrate that most small AAAs exhibit low growth in a linear pattern. The annual growth rate of AAA diameter was lower than anticipated, with a mean of 0.19 cm/y and median of 0.17 cm/y.13 A small proportion of aneurysms with high growth rates gives the annual growth rates a nonnormal distribution with a right skew, a finding seen in a prior retrospective analysis of AAAs.22,23
Ten percent of patients displayed minimal to no growth over 2 years. A surveillance study by Thompson et al20 of small AAAs in England (median baseline size 3.5 cm, IQR 3.1-4.2) reported a bimodal distribution of growth with around half of AAAs remaining quiescent with little growth and the other half continuing to expand. A similar surveillance study of 3.0- to 3.9-cm aneurysms from the US Veteran Affairs system reported 25% of aneurysms did not grow at all over a median follow-up of 3.5 years.23 These studies’ findings raise questions about whether the aortas with small, nongrowing MTDs are truly aneurysmal or have a different etiology/pathophysiology.20 The N-TA3CT design excluded patients with AAA diameters less than 4.0 cm who had documented failure of the aneurysm to grow over the 2 years prior to enrollment, which could result in this study underestimating the proportion of aneurysms that are stable or very slow growers. Likely, the true proportion of small AAAs that do not grow lies somewhere between our reported 10% no growth and the Thompson et al 50%.20
Ten patients exhibited small “negative” growth rates. These patients have stable nonshrinking aneurysms (eFigure 5 in the Supplement); small biologic fluctuations (cardiac cycle, blood pressure, and hydration),24 measurement variability, and serial surveillance captured a trivial regression from baseline imaging to a stable mean diameter. While drug interventions that regress preformed AAAs in animal models are an active field of study,25,26 spontaneous regression of human AAAs has been reported in only a small number of cases.27
The data support an association between initial MTD and growth rate, although the effect size is small. Over 2 years, we observed growth rates with “minimal to no growth” and “high growth” over the range of baseline MTDs. Based on these 2-year follow-up data, our study supports prior findings that baseline MTD is not a strong predictor of future growth of small AAAs.11,15,20 Importantly, the association of a cross-sectional measure of MTD at baseline with growth rate should not be taken as a basis for inferring exponential growth. To support an exponential growth pathophysiology, growth within a patient proportional to diameter over time would have to be observed.
For male patients with small AAAs who meet the enrollment criteria of N-TA3CT, we conclude that a small AAA (3.5-4.24 cm) can be monitored at a frequency of at least 2 years, with little chance of exceeding the interventional MTD threshold (5.5 cm). Our data on women are lesser in amount and cannot be used to support a definitive conclusion on their own, but they are consistent with the data on men. Our findings are consistent with the RESCAN collaboration, a collection of 15 471 patients from 18 clinical data sets with repeated ultrasonography measurements, which concluded that surveillance intervals for AAA could be safely extended.28 The present study capitalized on the detailed and precise serial CT imaging to ascertain patterns of growth, which are predominately linear and predictable. Our recommendation for extending imaging intervals based on the linearity and predictability of AAA growth measured via CT scans supports the conclusions of RESCAN based on longer-term ultrasonography surveillance and clinical outcomes.
The finding that 70% of AAAs undergo linear and predictable growth behavior has 3 clinical implications. First, patients with early-stage disease may be reassured that their disease is unlikely to suddenly alter course. Second, along with others’ findings,20 eg, the RESCAN collaboration,10,28 recommended intervals for surveillance29 could be extended safely to reduce costly and unnecessary CT scan imaging. Third, monitoring of small AAAs is safe because sudden unpredictable AAA growth is infrequent.30,31,32 Of 116 patients with baseline AAA MTD less than 4.25 cm, none progressed to repair thresholds at 2 years. Obtaining CT images at 2-year intervals is reasonable. Additionally, the N-TA3CT findings14 are consistent with 4 randomized trials (UKSAT,33 ADAM,30 PIVOTAL,34 and CAESAR19) that found no benefit for intervention on small aneurysms and are discordant with the suggestion from a epidemiologic study,35 vulnerable to the ecological fallacy, that practice guidelines should be reevaluated in favor of indicating earlier intervention.35
This study adds to and can clarify the different perspectives on AAA growth patterns. The max-SSD statistic identifies AAAs with nonlinear growth and displays a right skew in its distribution. Most patients display linear growth, with few patients showing markedly nonlinear patterns of growth that could be described as staccato or exponential. A 2004 study by Kurvers et al5 reported staccato growth in 65% of 52 patients with AAAs who were too high risk for open repair and unsuitable for endovascular repair. That study and others reporting large frequencies of staccato growth compare unequal time intervals between images, which could artificially create the appearance of growth arrest and/or high growth intervals.5,36 Those studies have suggested that aneurysm growth is unpredictable and shows large variations over time. Applying the Kurvers definition to the patients in this study, 22 patients (10%) qualified for staccato growth, although 15 did not appear staccato on review; 5 of these 15 demonstrated high growth over 12 months or longer and 10 were classified as staccato on the basis of a single measurement deviating from a linear pattern making the analysis vulnerable to random biologic variation (eg, owing to changes in blood pressure) and CT scan performance/interpretation.37,38,39 Using a definition of staccato growth with consistent time intervals between CT scans, there were 7 patients with staccato growth patterns (3%) in our data. There were 8 patients (4%) whose growth might be better described as exponential rather than linear. The patients with “minimal to no growth” rates were classified as having an “indeterminate–not growing” pattern because, although there was no significant growth over the 2 years we observed, at some point these aneurysms must have been developing and growing, and therefore we are unable to identify a clear growth pattern with measurements for only 2 years.
The aneurysm diameter growth pattern categories we observed inform 2 major conclusions and future directions. First, longer-term follow-up is necessary to confirm growth patterns and trends over the course of aneurysmal disease that may not be apparent within 2 years of surveillance. Second, although nonlinear growth is infrequent, it could be clinically important and justifies investigation of biomarkers to predict future growth or intervals without growth; such studies could provide information to shape future therapeutic approaches for small aneurysms.
Limitations
This study is limited by its sparse follow-up beyond 2 years; growth patterns or variability that manifest more clearly over longer periods may have been missed. Additionally, this study analyzed data of patients with AAAs 5.0 cm or less in diameter at baseline; AAAs with diameters greater than 5.0 cm may demonstrate different growth patterns. As previously noted, eligibility criteria of N-TA3CT limited the inclusion of patients and also insisted on well-controlled blood pressure and encouraged smoking cessation. A greater proportion of patients with diabetes (22%) than enrolled in previous study populations could have altered growth trends in the direction of more predictable slow growth. The small number of female patients (35; 14%) reduces the precision of our observations about AAA growth among women, particularly evident in subgroup analyses. Fast-growing AAAs may be slightly underrepresented in our data based on accelerated progression reducing the window in which they would meet MTD eligibility criteria for N-TA3CT and because faster growth rates lead surgeons to select out patients for early repair. Comprehensive address of confounders or predictors of growth rates are beyond the scope of this article and are important future directions.
Conclusions
Most small AAAs (3.5-5.0 cm) exhibit “minimal to no” or “low” yearly growth (0.05-0.25 cm/y). Growth is usually linear. Great variability in growth over a 2-year period is infrequent. Linear growth modeling of AAAs in individual patients suggests small AAAs (<4.25 cm) can be followed up with a CT scan 2 years later, with a small chance of exceeding interventional MTD thresholds of 5.5 cm for men.
eFigure 1. Distribution of yearly MTD growth rates by N-TA3CT treatment assignment.
eFigure 2. Subject inclusion flow chart.
eFigure 3. Proposed clinical imaging definitions for describing small abdominal aortic aneurysm growth rates.
eFigure 4. MTD growth rate individual examples.
eFigure 5. Subjects with negative growth rates per linear regression modeling.
eFigure 6. Distribution of maximum segment slope difference (max-SSD) individual examples.
eFigure 7. Maximum segmental slope differences (max-SSD) of AAA growth patterns.
eFigure 8. Individual Examples of Non-linear Growth Patterns
References
- 1.Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(3):539-548. doi: 10.1016/j.jvs.2010.05.090 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Accessed February 3, 2015. https://wonder.cdc.gov/ucd-icd10.html.
- 3.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245. doi: 10.1161/CIR.0b013e31828124ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, National Center for Health Statistics . Underlying Cause of Death 1999-2017 on CDC WONDER Online Database, released December 2018. Accessed January 7, 2020. https://wonder.cdc.gov/ucd-icd10.html
- 5.Kurvers H, Veith FJ, Lipsitz EC, et al. Discontinuous, staccato growth of abdominal aortic aneurysms. J Am Coll Surg. 2004;199(5):709-715. doi: 10.1016/j.jamcollsurg.2004.07.031 [DOI] [PubMed] [Google Scholar]
- 6.Vega de Céniga M, Gómez R, Estallo L, et al. Analysis of expansion patterns in 4-4.9 cm abdominal aortic aneurysms. Ann Vasc Surg. 2008;22(1):37-44. doi: 10.1016/j.avsg.2007.07.036 [DOI] [PubMed] [Google Scholar]
- 7.Badger SA, Jones C, McClements J, Lau LL, Young IS, Patterson CC. Surveillance strategies according to the rate of growth of small abdominal aortic aneurysms. Vasc Med. 2011;16(6):415-421. doi: 10.1177/1358863X11423971 [DOI] [PubMed] [Google Scholar]
- 8.Vardulaki KA, Prevost TC, Walker NM, et al. Growth rates and risk of rupture of abdominal aortic aneurysms. Br J Surg. 1998;85(12):1674-1680. doi: 10.1046/j.1365-2168.1998.00946.x [DOI] [PubMed] [Google Scholar]
- 9.Vega de Céniga M, Gómez R, Estallo L, et al. Growth rate and associated factors in small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2006;31(3):231-236. doi: 10.1016/j.ejvs.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Thompson SG, Brown LC, Sweeting MJ, et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013;17(41):1-118. doi: 10.3310/hta17410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MA3RS Study Investigators . Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation. 2017;136(9):787-797. doi: 10.1161/CIRCULATIONAHA.117.028433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen KL, Dahl M, Rasmussen LM, Lindholt JS. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms: a substudy from the VIVA (Viborg Vascular) randomized screening trial. Arterioscler Thromb Vasc Biol. 2017;37(4):730-736. doi: 10.1161/ATVBAHA.116.308874 [DOI] [PubMed] [Google Scholar]
- 13.Baxter BT, Matsumura J, Curci J, et al. ; N-TA(3)CT Investigators . Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): design of a Phase IIb, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemp Clin Trials. 2016;48:91-98. doi: 10.1016/j.cct.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxter BT, Matsumura J, Curci JA, et al. ; N-TA3CT Investigators . Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: a randomized clinical trial. JAMA. 2020;323(20):2029-2038. doi: 10.1001/jama.2020.5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhak RH, Wininger M, Johnson GR, et al. ; Aneurysm Detection and Management (ADAM) Study Group . Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150(1):44-50. doi: 10.1001/jamasurg.2014.2025 [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, Wilson SE, Johnson GR, et al. ; Abdominal Aortic Aneurysm Detection and Management Veterans Administration Cooperative Study Group . Variability in measurement of abdominal aortic aneurysms. J Vasc Surg. 1995;21(6):945-952. doi: 10.1016/S0741-5214(95)70222-9 [DOI] [PubMed] [Google Scholar]
- 17.Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12(2):230-237. doi: 10.1016/S1078-5884(96)80112-2 [DOI] [PubMed] [Google Scholar]
- 18.Sprouse LR II, Meier GH III, Parent FN, DeMasi RJ, Glickman MH, Barber GA. Is ultrasound more accurate than axial computed tomography for determination of maximal abdominal aortic aneurysm diameter? Eur J Vasc Endovasc Surg. 2004;28(1):28-35. doi: 10.1016/j.ejvs.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 19.Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E; CAESAR Trial Group . Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41(1):13-25. doi: 10.1016/j.ejvs.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 20.Thompson AR, Cooper JA, Ashton HA, Hafez H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br J Surg. 2010;97(1):37-44. doi: 10.1002/bjs.6779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3/4):591-611. doi: 10.2307/2333709 [DOI] [Google Scholar]
- 22.Tajima Y, Goto H, Ohara M, et al. Oral steroid use and abdominal aortic aneurysm expansion: positive association. Circ J. 2017;81(12):1774-1782. doi: 10.1253/circj.CJ-16-0902 [DOI] [PubMed] [Google Scholar]
- 23.Santilli SM, Littooy FN, Cambria RA, et al. Expansion rates and outcomes for the 3.0-cm to the 3.9-cm infrarenal abdominal aortic aneurysm. J Vasc Surg. 2002;35(4):666-671. doi: 10.1067/mva.2002.121572 [DOI] [PubMed] [Google Scholar]
- 24.Lortz J, Tsagakis K, Rammos C, et al. Hemodynamic changes lead to alterations in aortic diameters and may challenge further stent graft sizing in acute aortic syndrome. J Thorac Dis. 2018;10(6):3482-3489. doi: 10.21037/jtd.2018.05.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake T, Aoki M, Masaki H, et al. Regression of abdominal aortic aneurysms by simultaneous inhibition of nuclear factor kappaB and ets in a rabbit model. Circ Res. 2007;101(11):1175-1184. doi: 10.1161/CIRCRESAHA.107.148668 [DOI] [PubMed] [Google Scholar]
- 26.Sharma N, Dev R, Ruiz-Rosado JD, et al. Pharmacological inhibition of Notch signaling regresses pre-established abdominal aortic aneurysm. Sci Rep. 2019;9(1):13458. doi: 10.1038/s41598-019-49682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellows PH, Anaya-Ayala JE, Younes HK, et al. Spontaneous regression of an abdominal aortic aneurysm in an immunocompromised patient. Vasc Med. 2010;15(4):315-319. doi: 10.1177/1358863X10375331 [DOI] [PubMed] [Google Scholar]
- 28.Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG; RESCAN Collaborators . Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA. 2013;309(8):806-813. doi: 10.1001/jama.2013.950 [DOI] [PubMed] [Google Scholar]
- 29.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 30.Lederle FA, Wilson SE, Johnson GR, et al. ; Aneurysm Detection and Management Veterans Affairs Cooperative Study Group . Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437-1444. doi: 10.1056/NEJMoa012573 [DOI] [PubMed] [Google Scholar]
- 31.Powell JT, Brady AR, Brown LC, et al. ; United Kingdom Small Aneurysm Trial Participants . Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445-1452. doi: 10.1056/NEJMoa013527 [DOI] [PubMed] [Google Scholar]
- 32.Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms: the UK Small Aneurysm Trial Participants. Lancet. 1998;352(9141):1649-1655. doi: 10.1016/S0140-6736(98)10137-X [DOI] [PubMed] [Google Scholar]
- 33.Powell JT, Brown LC, Forbes JF, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007;94(6):702-708. doi: 10.1002/bjs.5778 [DOI] [PubMed] [Google Scholar]
- 34.Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators . Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51(5):1081-1087. doi: 10.1016/j.jvs.2009.10.113 [DOI] [PubMed] [Google Scholar]
- 35.Karthikesalingam A, Vidal-Diez A, Holt PJ, et al. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med. 2016;375(21):2051-2059. doi: 10.1056/NEJMoa1600931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adovasio R, Calvagna C, Sgorlon G, et al. Growth rate of small abdominal aortic aneurysms and genetic polymorphisms of matrix metalloproteases-1, -3, and -9. Int J Angiol. 2016;25(2):93-98. doi: 10.1055/s-0035-1563603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grøndal N, Bramsen MB, Thomsen MD, Rasmussen CB, Lindholt JS. The cardiac cycle is a major contributor to variability in size measurements of abdominal aortic aneurysms by ultrasound. Eur J Vasc Endovasc Surg. 2012;43(1):30-33. doi: 10.1016/j.ejvs.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 38.Muhs BE, Vincken KL, van Prehn J, et al. Dynamic cine-CT angiography for the evaluation of the thoracic aorta; insight in dynamic changes with implications for thoracic endograft treatment. Eur J Vasc Endovasc Surg. 2006;32(5):532-536. doi: 10.1016/j.ejvs.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 39.van Herwaarden JA, Bartels LW, Muhs BE, et al. Dynamic magnetic resonance angiography of the aneurysm neck: conformational changes during the cardiac cycle with possible consequences for endograft sizing and future design. J Vasc Surg. 2006;44(1):22-28. doi: 10.1016/j.jvs.2006.03.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Distribution of yearly MTD growth rates by N-TA3CT treatment assignment.
eFigure 2. Subject inclusion flow chart.
eFigure 3. Proposed clinical imaging definitions for describing small abdominal aortic aneurysm growth rates.
eFigure 4. MTD growth rate individual examples.
eFigure 5. Subjects with negative growth rates per linear regression modeling.
eFigure 6. Distribution of maximum segment slope difference (max-SSD) individual examples.
eFigure 7. Maximum segmental slope differences (max-SSD) of AAA growth patterns.
eFigure 8. Individual Examples of Non-linear Growth Patterns