This follow-up study of a randomized clinical trial evaluates the survival, complications, and risk factors associated with hybrid minimally invasive esophagectomy and open esophagectomy among adults with esophagus cancer.
Key Points
Question
Is hybrid minimally invasive esophagectomy (HMIE) associated with improved long-term survival compared with open esophagectomy, and what are the potential risk factors in survival?
Findings
In this post hoc follow-up study of a randomized clinical trial involving 207 patients with esophagus cancer, overall survival and disease-free survival were comparable between the HMIE and open esophagectomy procedures. No statistically significant difference in recurrence rate or location was found between groups, and major postoperative overall and pulmonary complications were identified as risk factors associated with decreased overall survival and disease-free survival.
Meaning
Findings from this study suggest that HMIE is associated with improved long-term oncological results compared with open esophagectomy primarily because of decreased postoperative complications.
Abstract
Importance
Available data comparing the long-term results of hybrid minimally invasive esophagectomy (HMIE) with that of open esophagectomy are conflicting, with similar or even better results reported for the minimally invasive esophagectomy group.
Objective
To evaluate the long-term, 5-year outcomes of HMIE vs open esophagectomy, including overall survival (OS), disease-free survival (DFS), and pattern of disease recurrence, and the potential risk factors associated with these outcomes.
Design, Setting, and Participants
This randomized clinical trial is a post hoc follow-up study that analyzes the results of the open-label Multicentre Randomized Controlled Phase III Trial, which enrolled patients from 13 different centers in France and was conducted from October 26, 2009, to April 4, 2012. Eligible patients were 18 to 75 years of age and were diagnosed with resectable cancer of the middle or lower third of the esophagus. After exclusions, patients were randomized to either the HMIE group or the open esophagectomy group. Data analysis was performed on an intention-to-treat basis from November 19, 2019, to December 4, 2020.
Interventions
Hybrid minimally invasive esophagectomy (laparoscopic gastric mobilization with open right thoracotomy) was compared with open esophagectomy.
Main Outcomes and Measures
The primary end points of this follow-up study were 5-year OS and DFS. The secondary end points were the site of disease recurrence and potential risk factors associated with DFS and OS.
Results
A total of 207 patients were randomized, of whom 175 were men (85%), and the median (range) age was 61 (23-78) years. The median follow-up duration was 58.2 (95% CI, 56.5-63.8) months. The 5-year OS was 59% (95% CI, 48%-68%) in the HMIE group and 47% (95% CI, 37%-57%) in the open esophagectomy group (hazard ratio [HR], 0.71; 95% CI, 0.48-1.06). The 5-year DFS was 52% (95% CI, 42%-61%) in the HMIE group vs 44% (95% CI, 34%-53%) in the open esophagectomy group (HR, 0.81; 95% CI, 0.55-1.17). No statistically significant difference in recurrence rate or location was found between groups. In a multivariable analysis, major intraoperative and postoperative complications (HR, 2.21; 95% CI, 1.41-3.45; P < .001) and major pulmonary complications (HR, 1.94; 95% CI, 1.21-3.10; P = .005) were identified as risk factors associated with decreased OS. Similarly, multivariable analysis of DFS identified overall intraoperative and postoperative complications (HR, 1.93; 95% CI, 1.28-2.90; P = .002) and major pulmonary complications (HR, 1.85; 95% CI, 1.19-2.86; P = .006) as risk factors.
Conclusions and Relevance
This study found no difference in long-term survival between the HMIE and open esophagectomy groups. Major postoperative overall complications and pulmonary complications appeared to be independent risk factors in decreased OS and DFS, providing additional evidence that HMIE may be associated with improved oncological results compared with open esophagectomy primarily because of a reduction in postoperative complications.
Trial Registration
ClinicalTrials.gov Identifier: NCT00937456
Introduction
Esophageal cancer is an important and increasing global health problem. In 2012, more than 456 000 new cases of esophageal cancer were diagnosed worldwide, and the incidence of this disease, especially esophageal adenocarcinoma, is expected to increase further.1,2 Although substantial improvements in multimodal therapy have been achieved, the all-stage mortality rate of esophageal cancer is still among the highest, with a reported 5-year survival between 10% and 25%.3,4,5,6 Radical resection is the mainstay of curative treatment for esophageal cancer but is associated with a high rate of postoperative complications, especially pulmonary.
As a strategy to decrease pulmonary morbidity, several studies have demonstrated the potential benefits of minimally invasive techniques in esophageal cancer surgery.7 In the 3-year follow-up study of the TIME (Traditional Invasive vs Minimally Invasive Esophagectomy) trial, Straatman et al8 confirmed the oncological safety of totally minimally invasive esophagectomy, showing that its overall survival (OS) and disease-free survival (DFS) were equivalent to those of open esophagectomy. Similar OS and DFS findings were obtained in the recently published, single-center ROBOT (Robot-Assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer) trial, which compared totally minimally invasive esophagectomy with a robotic thoracic phase vs open esophagectomy.9 Survival results from the ROMIO (Randomized Esophagectomy: Minimally Invasive or Open) trial are forthcoming.10 In 2019, Mariette et al11 published the results of a multicenter, open-label, randomized clinical trial that compared hybrid minimally invasive esophagectomy (HMIE) with open esophagectomy. This study demonstrated the benefit of HMIE over open esophagectomy, with HMIE resulting in decreased major overall intraoperative and postoperative as well as pulmonary complication rates.11 In contrast to the previously mentioned trials, the study by Mariette et al11reported that, after a median follow-up of 48.8 months, nonsignificant prolongations of OS (hazard ratio [HR] for death, 0.67; 95% CI, 0.44-1.01) and DFS (HR for death, 0.76; 95% CI, 0.52-1.11) were observed in the HMIE group. Accordingly, a recent meta-analysis of largely nonrandomized data by Gotlieb-Vedi et al12 and a propensity score–matched multi-institutional comparison by Hölscher et al13 suggested that minimally invasive esophagectomy may be associated with superior long-term survival compared with open esophagectomy. Whether the observed improved survival associated with minimally invasive esophagectomy is long term and whether survival may be mediated by the surgical approach itself or by the lower incidence of major complications as previously described remain unknown.14
In this follow-up study of A Multicentre Randomized Controlled Phase III Trial (MIRO trial),15 we evaluated the long-term, 5-year outcomes of HMIE vs open esophagectomy, including OS, DFS, and pattern of disease recurrence, along with the potential risk factors associated with these outcomes.
Methods
From October 26, 2009, to April 4, 2012, a multicenter, open-label, phase 3, prospective randomized clinical trial was conducted that enrolled patients who were diagnosed with thoracic esophageal cancer and eligible for curative surgical resection (Ivor Lewis procedure) at 13 different centers in France (Hôpital Claude Huriez, Lille University Medical Center, Lille; Hôpital de la Croix-Rousse, Lyon University Medical Center, Lyon; Hôpital Ambroise Paré, Assistance Publique–Hôpitaux de Paris [AP-HP], Boulogne-Billancourt; Bordeaux University Medical Center, Bordeaux; Institut Mutualiste Montsouris, Paris; Centre Hospitalier Universitaire [CHU] Clermont-Ferrand, Clermont-Ferrand; CHU Marseille, Marseille; CHU Strasbourg, Strasbourg; CHU Toulouse, Toulouse; St Louis Hospital, AP-HP, Paris; Louis Mourier Hospital, AP-HP, Colombes; Pontchaillou Hospital, Rennes University Medical Center, Rennes; and Hôpital Caremeau, Nîmes).15 Hybrid minimally invasive esophagectomy (laparoscopic gastric mobilization with open right thoracotomy) was compared with open esophagectomy. A detailed overview of the trial protocol (Supplement 1) has been published.15 The MIRO trial was approved by the Nord-Ouest II Ethics Committee and the Agence Française de Sécurité Sanitaire des Produits de Santé. Written consent was obtained from all participants. The trial was performed according to the principles of the Declaration of Helsinki16 and the guidelines for Good Clinical Practice and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. This follow-up study was conducted from October 8, 2019, to December 4, 2020.
Trial Population, Procedures, Randomization, and Follow-up
Patients between aged 18 and 75 years who were diagnosed with squamous cell carcinoma or adenocarcinoma of the middle or lower third of the esophagus or a junctional (Siewert I) tumor and who were eligible for surgical resection were included in the MIRO trial. A comprehensive description of the inclusion and exclusion criteria is shown in eTable 1 in Supplement 2.
Preoperative workup as well as anesthesiologic, perioperative and postoperative, and follow-up procedures that patients underwent are described in the trial protocol.15 Randomization was performed centrally by means of a stratified-field block randomization (blocks of 4) for each participating center. A randomization list was generated for each center with prepared numbered envelopes. Follow-up was organized according to the French national guidelines.17 A last systematic update of patient records was performed in October 2019.
End Points
The primary end point of the MIRO trial was the rate of major intraoperative or postoperative complications within 30 days after surgery. Complications were registered as surgical or medical and classified according to the Clavien-Dindo classification system.18 Complications were considered major in the case of a Clavien-Dindo grade of II or higher (grade II, complication requiring pharmacological intervention, including total parenteral nutrition and blood transfusion; grade III, complication requiring intervention under local anesthesia or general anesthesia; grade IV, life-threatening complication requiring intensive unit support; and grade V, death of a patient). This 5-grade system includes subgrades in grades III and IV, with the higher grades indicating more life-threatening complications. The most severe complication in a patient was considered for the classification of the primary end point.
The primary end points of this post hoc follow-up study were OS and DFS. For OS, the event studied was death (from any cause). For DFS, the event studied was the first recurrence (locoregional, distant, or mixed) or death. The secondary end points were the site of disease recurrence and potential risk or mediating factors associated with DFS and OS.
Statistical Analysis
Statistical analysis was performed from November 19, 2019, to December 4, 2020, using Stata software, version 13.1 (StataCorp LLC) on an intention-to-treat basis. Continuous variables were described as means with SDs or medians with ranges. Discrete end points were described with frequencies and percentages with 95% CIs. Comparisons were performed with a χ2 or Fisher exact test.
Overall survival was defined as the time between the date of surgery and the date of death or last follow-up. Patients who were still alive were censored on the cutoff date (October 7, 2019). Disease-free survival was defined as the time between the date of surgery and the date of the first event (recurrence or death) or last follow-up. Patients who were still alive and had no recurrence were censored on the cutoff date. Both DFS and OS were analyzed using the Kaplan-Meier method and compared using the log-rank test.
Survival end points were described by their rate at specific time points with a 95% CI. Hazard ratios were calculated using univariable and multivariable Cox proportional hazards regression analyses. Correlations and interactions were tested between the variables. Multivariable models used to identify risk factors for OS and DFS were supplemented with a Harrell concordance index to allow for the evaluation of the overall adequacy of risk prediction procedures with censored survival data. The higher the Harrell C index, the better the predictive capacity of the model. A 2-sided P < .05 was considered to be statistically significant. More details on the statistical analysis are found in the eMethods in Supplement 2.
Results
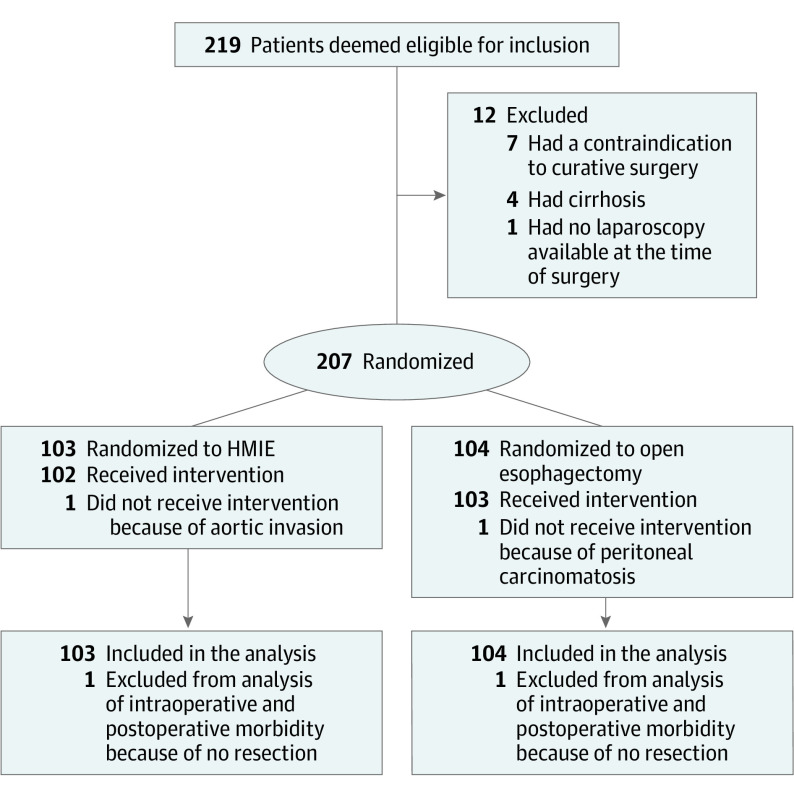
Between October 26, 2009, and April 4, 2012, a total of 219 patients were deemed eligible for inclusion, of which 207 were randomized to either the HMIE (n = 103) or the open esophagectomy (n = 104) procedure (Figure 1). Among the 207 patients, 175 were men (85%) and 32 were women (15%), with a median (range) age of 61 (23-78) years. An overview of demographic data and baseline characteristics is shown in Table 1. Details on the pathological analysis are summarized in eTable 2 in Supplement 2. No significant differences were observed between the groups. The short-term primary and secondary outcomes have been described by Mariette et al11 (eTable 3 in Supplement 2).
Figure 1. CONSORT Diagram of the Study Population.
HMIE indicates hybrid minimally invasive esophagectomy.
Table 1. Demographics and Baseline Characteristics.
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| Total population (n = 207) | Procedure | |||
| HMIE (n = 103) | Open esophagectomy (n = 104) | |||
| Sex | ||||
| Male | 175 (85) | 88 (85) | 87 (84) | .72 |
| Female | 32 (15) | 15 (15) | 17 (16) | |
| Age, median (range), y | 61 (23-78) | 59 (23-75) | 62 (41-78) | .18b |
| BMI, median (IQR) | 25 (16-37) | 26 (16-37) | 25 (18-35) | .64c |
| ASA Physical Status score | ||||
| 1 | 59 (29) | 25 (24) | 34 (33) | .32 |
| 2 | 119 (57) | 61 (59) | 58 (56) | |
| 3 | 29 (14) | 17 (17) | 12 (12) | |
| WHO Performance Status score | ||||
| 0 | 120 (58) | 67 (65) | 53 (51) | .05 |
| 1 | 79 (38) | 31 (30) | 48 (46) | |
| 2 | 8 (4) | 5 (5) | 3 (3) | |
| Tumor histological findings | ||||
| Squamous cell carcinoma | 84 (41) | 46 (45) | 38 (37) | .23 |
| Adenocarcinoma | 123 (59) | 57 (55) | 66 (63) | |
| Location of tumor in esophagus | ||||
| Upper third | 1 (1) | 0 | 1 (1) | >.99d |
| Middle third | 63 (30) | 32 (31) | 31 (30) | |
| Lower third | 143 (69) | 71 (69) | 72 (69) | |
| Clinical tumor classification, No./total No. (%)e | ||||
| cT1 | 37/198 (19) | 18/98 (18) | 19/100 (19) | .91 |
| cT2 | 63/198 (32) | 30/98 (31) | 33/100 (33) | |
| cT3 | 98/198 (49) | 50/98 (51) | 48/100 (48) | |
| Clinical node classification, No./ total No. (%)e | ||||
| cN0 | 89/199 (45) | 41/98 (42) | 48/101 (48) | .72 |
| cN1 | 102/199 (51) | 53/98 (54) | 49/101 (49) | |
| cN2 | 8/199 (4) | 4/98 (4) | 4/101 (4) | |
| Neoadjuvant therapy | ||||
| Chemotherapy | 86 (42) | 41 (40) | 45 (43) | .67 |
| Chemoradiotherapy | 66 (32) | 36 (35) | 30 (29) | |
| None | 55 (27) | 26 (25) | 29 (28) | |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HMIE, hybrid minimally invasive esophagectomy; IQR, interquartile range; WHO, World Health Organization.
P value calculated with unpaired, 2-tailed t test.
P value calculated with Mann-Whitney nonparametric test.
P value calculated with Fisher exact test.
The cTNM (clinical tumor, node, metastasis) classification was based on the American Joint Committee on Cancer AJCC Cancer Staging Manual, 6th edition.
Long-term OS, DFS, and Recurrence Location Outcomes by Procedure
The median follow-up duration was 58.2 (95% CI, 56.5-63.8) months in the entire study population, 59.4 (95% CI, 57.7-89.7) months in the HMIE group, and 56.6 (95% CI, 48.2-69.0) months in the open esophagectomy group. Of the 207 patients who were randomized, 98 (47%) died during follow-up, of whom 44 (43%) were in the HMIE group and 54 (52%) were in the open esophagectomy group.
The global median OS was 95 months (95% CI, 43 months to limit not reached), with the median OS being 96 months (95% CI, 59 months to limit not reached) in the HMIE group and 46 months (95% CI, 30 months to limit not reached) in the open esophagectomy group. The 5-year OS was 59% (95% CI, 48%-68%) in the HMIE group and 47% (95% CI, 37%-57%) in the open esophagectomy group. The survival rate in the HMIE group was higher compared with the open esophagectomy group, although this difference did not reach statistical significance (HR, 0.71; 95% CI, 0.48-1.06; log-rank P = .09) (Figure 2A).
Figure 2. Kaplan-Meier Analysis of Overall Survival and Disease-Free Survival.
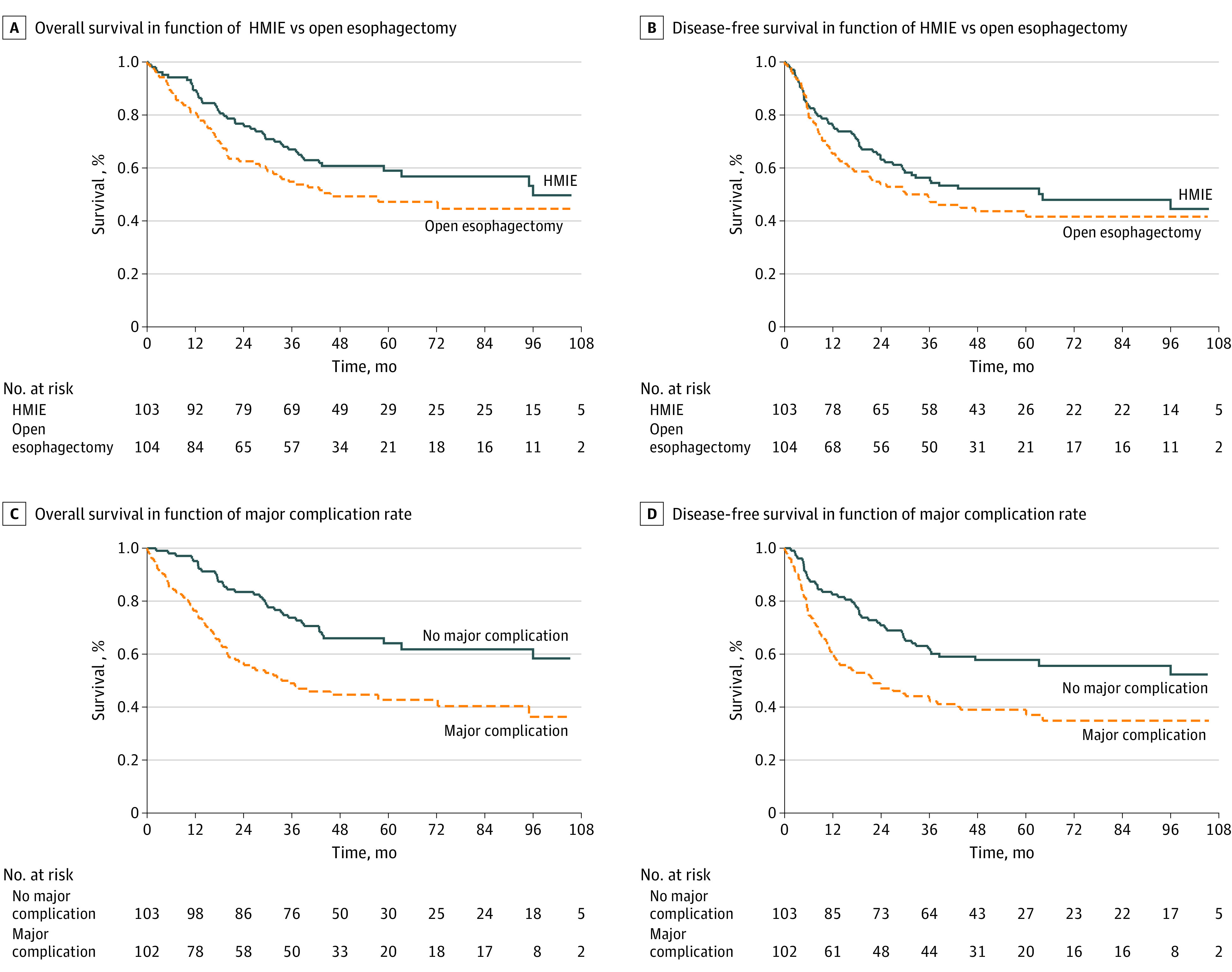
HMIE indicates hybrid minimally invasive esophagectomy.
Among the 207 patients who were randomized, 111 (54%) had recurrent disease or died, of whom 52 (50%) were in the HMIE group and 59 (57%) were in the open esophagectomy group. The global median DFS was 38.5 months (95% CI, 27 months to limit not reached), and DFS was not significantly improved in the HMIE group. The median DFS was 64.2 months (95% CI, 29 months to limit not reached) in the HMIE group and 30.1 months (95% CI, 17 months to limit not reached) in the open esophagectomy group. The 5-year DFS was 52% (95% CI, 42%-61%) in the HMIE group vs 44% (95% CI, 34%-53%) in the open esophagectomy group. The HR for the effect of HMIE on DFS was 0.81 (95% CI, 0.55-1.17; log-rank P = .26) (Figure 2B).
Disease recurrence was recorded in 87 (42%) of 207 patients. In the HMIE group (n = 103), 41 patients (40%) were diagnosed with recurrent disease, with 12 having locoregional recurrence, 23 having distant metastases, and 6 having a mixed recurrence pattern. In the open esophagectomy group (n = 104), recurrence was noted in 46 patients (44%), with 12 having locoregional recurrence, 24 having distant metastases, and 10 patients having mixed recurrence. No significant difference in recurrence rate or location was found between the 2 groups.
Long-term OS, DFS, and Recurrence Location Outcomes by Major Overall and Pulmonary Complication Rate
Of the 205 patients for whom complication rate data were available, 102 (50%) had a major complication. Of these 102 patients, 59 (58%) died during follow-up vs 43 (42%) who were still alive at the censoring date (χ2 P = .002) (Figure 2C). Among 49 patients (24%) with pulmonary complications, 29 (59%) died during follow-up compared with 20 (41%) who were still alive at the censoring date (χ2 P = .047).
When comparing DFS between patients with or without major complications (Clavien-Dindo grade ≥II), 64 of 111 patients (58%) with major complications had recurrent disease or died vs 45 patients (40%) without major complications (χ2 P = .006). The 5-year DFS was 39% (95% CI, 29%-48%) in patients with major complications and 58% (95% CI, 47%-67%) in patients without major complications. The HR for recurrent disease or death was 1.88 (95% CI, 1.28-2.74; log-rank P = .001) (Figure 2D).
Of the 111 patients who had recurrent disease or who died, 32 (29%) had major postoperative pulmonary complications. Of the 49 patients with major postoperative pulmonary complications, 32 (65%) presented with recurrent disease or died vs 17 (18%) who were disease free (χ2 P = .05). The 5-year DFS was 36% (95% CI, 23%-50%) in patients with major pulmonary complications and 52% (95% CI, 44%-60%) in patients without major pulmonary complications. The HR for recurrent disease or death was 1.73 (95% CI, 1.15-2.62; log-rank P = .008).
Of the 87 patients with recurrent disease, 48 (55%) had a major complication, with 11 having locoregional recurrent disease, 26 having distant metastases, and 11 having a mixed recurrence pattern. Disease recurrence was observed in 39 patients (45%) without major complications, with 13 having locoregional recurrence, 21 having distant metastases, and 5 having mixed recurrence. No significant difference in recurrence rate or location was found between patients with and those without major complications.
Recurrent disease was noted in 24 patients (28%) who had major pulmonary complications, with 4 having locoregional recurrence, 14 having distant metastases, and 6 having mixed recurrence. In 63 patients (72%) with recurrent disease, no major pulmonary complications were recorded, with 20 having locoregional recurrence, 33 having distant metastases, and 10 having a mixed recurrence pattern. No significant difference in recurrence rate or location was observed.
Risk Factors Associated With OS and DFS
In a multivariable analysis, besides tumor T stage, major overall intraoperative and postoperative complications (Clavien-Dindo grade≥II) (HR, 2.21; 95% CI, 1.41-3.45; P < .001) (Table 2) and major pulmonary complications (HR, 1.94; 95% CI, 1.21-3.10; P = .005) (Table 3) were identified as risk factors of impaired OS. Multivariable analysis of DFS also identified overall intraoperative and postoperative complications (HR, 1.93; 95% CI, 1.28-2.90; P = .002) (Table 2) and major pulmonary complications (HR, 1.85; 95% CI, 1.19-2.86; P = .006) (Table 3) as adverse risk factors. These multivariable analyses of OS and DFS were adjusted on the treatment groups, age, T and N stages, and neoadjuvant treatment, which were candidates from the univariable analysis. For the multivariable analyses of OS, American Society of Anesthesiologists Physical Status score and sex were included, and for DFS multivariable analyses, American Society of Anesthesiologists score, sex, and age were included to achieve the best discriminate capacity of the model (higher Harrell C index). Additional results of the interaction analyses for OS and DFS as well as results of the multivariable analyses with the interaction analyses introduced in the model are discussed in the eResults and eTables 4 to 7 in Supplement 2.
Table 2. Multivariable Analysis for Overall Survival (OS) and Disease-Free Survival (DFS), Including Major Intraoperative and Postoperative Complications.
| Variable | OS (Harrell concordance index = 0.702) | DFS (Harrell concordance index = 0.686) | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Procedure | ||||
| Open esophagectomy | 1 [Reference] | .35 | 1 [Reference] | .48 |
| HMIE | 0.81 (0.35-1.26) | 0.86 (0.57-1.31) | ||
| Major complications | ||||
| CD grade <II | 1 [Reference] | <.001 | 1 [Reference] | .002 |
| CD grade ≥II | 2.21 (1.41-3.45) | 1.93 (1.28-2.90) | ||
| Age, y | ||||
| ≤60 | 1 [Reference] | .10 | 1 [Reference] | .22 |
| >60 | 1.45 (0.93-2.25) | 1.29 (0.86-1.93) | ||
| Sex | ||||
| Male | 1 [Reference] | .99 | 1 [Reference] | .79 |
| Female | 1 (0.55-1.81) | 0.93 (0.53-1.63) | ||
| ASA Physical Status score | ||||
| 1 | 1 [Reference] | .67 | 1 [Reference] | .81 |
| 2 | 1.25 (0.76-2.05) | 1.17 (0.73-1.87) | ||
| 3 | 1.09 (0.53-2.25) | 1.12 (0.59-2.15) | ||
| Neoadjuvant treatment | ||||
| No | 1 [Reference] | .89 | 1 [Reference] | .51 |
| Yes | 0.95 (0.46-1.94) | 0.8 (0.41-1.55) | ||
| T stage | ||||
| T0-T1 | 1 [Reference] | .008 | 1 [Reference] | .001 |
| T2 | 3.12 (1.19-8.16) | 4.13 (1.61-10.56) | ||
| T3 | 4.70 (1.74-12.70) | 6.16 (2.32-16.32) | ||
| N stage | ||||
| N0 | 1 [Reference] | .29 | 1 [Reference] | .16 |
| N1-N3 | 1.34 (0.78-2.32) | 1.45 (0.86-2.42) | ||
Abbreviations: ASA, American Society of Anesthesiologists; CD, Clavien-Dindo classification system; HMIE, hybrid minimally invasive esophagectomy; HR, hazard ratio.
Table 3. Multivariable Analysis for Overall Survival (OS) and Disease-Free Survival (DFS), Including Major Pulmonary Complications.
| Variable | OS (Harrell concordance index = 0.6864) | DFS (Harrell concordance index = 0.68) | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Procedure group | .16 | .32 | ||
| Open esophagectomy | 1 [Reference] | 1 [Reference] | ||
| HMIE | 0.73 (0.47-1.14) | 0.81 (0.54-1.22) | ||
| Major pulmonary complications | ||||
| No | 1 [Reference] | .005 | 1 [Reference] | .006 |
| Yes | 1.94 (1.21-3.10) | 1.85 (1.19-2.86) | ||
| Age, y | ||||
| ≤60 | 1 [Reference] | .09 | 1 [Reference] | .20 |
| >60 | 1.46 (0.94-2.26) | 1.30 (0.87-1.95) | ||
| Sex | ||||
| Male | 1 [Reference] | .82 | 1 [Reference] | .91 |
| Female | 1.07 (0.59-1.93) | 0.97 (0.55-1.70) | ||
| ASA Physical Status score | ||||
| 1 | 1 [Reference] | .42 | 1 [Reference] | .61 |
| 2 | 1.34 (0.81-2.20) | 1.26 (0.79-2.01) | ||
| 3 | 0.99 (0.47-2.06) | 1.09 (0.57-2.09) | ||
| Neoadjuvant treatment | ||||
| No | 1 [Reference] | .65 | 1 [Reference] | .42 |
| Yes | 0.84 (0.40-1.76) | 0.76 (0.38-1.49) | ||
| T stage | ||||
| T0-T1 | 1 [Reference] | .006 | 1 [Reference] | <.001 |
| T2 | 3.24 (1.22-8.59) | 4.22 (1.64-10.88) | ||
| T3 | 5.18 (1.86-14.45) | 6.57 (2.44-17.71) | ||
| N stage | ||||
| N0 | 1 [Reference] | .31 | 1 [Reference] | .20 |
| N1-N3 | 1.34 (0.76-2.34) | 1.41 (0.83-2.37) | ||
Abbreviations: ASA, American Society of Anesthesiologists; HMIE, hybrid minimally invasive esophagectomy; HR, hazard ratio.
Discussion
This 5-year follow-up report of the multicenter, open-label MIRO randomized clinical trial found no difference in the long-term oncological outcomes between HMIE and open esophagectomy. The 5-year OS and DFS rates were higher in the HMIE than in the open esophagectomy group, but the difference did not reach statistical significance.11 The incidence of disease recurrence and the site of recurrence were not statistically different between the HMIE and open esophagectomy groups. When comparing the oncological outcomes between patients with and patients without major postoperative overall and pulmonary complications, OS and DFS were statistically significantly improved in patients without major complications. In a multivariable analysis, these results were confirmed; the HMIE procedure itself was not independently associated with increased OS or DFS. However, major overall intraoperative and postoperative complications as well as major pulmonary complications were identified as independent adverse risk factors associated with OS and DFS.
Because no significant difference in the rate of R1 resections or lymph node yield could be found between the HMIE and the open esophagectomy groups in the MIRO trial report by Mariette et al,11 the hypothesis was that the potential advantage in long-term oncological outcome in the HMIE group may be secondary to a decrease in the postoperative overall and pulmonary complication rate. The present study was designed to evaluate this hypothesis. In a recent meta-analysis of largely nonrandomized trials, Gottlieb-Vedi et al12 reported significantly improved 3- and 5-year OS and DFS for minimally invasive esophagectomy compared with open esophagectomy that was potentially mediated by a reduction in postoperative complication rate. Several previous studies have demonstrated the association between postoperative complications and impaired OS and DFS for open esophagectomy.14,19,20,21 Two other retrospective studies, in contrast, reported that OS and DFS were not associated with the occurrence of postoperative complications.22,23
To our knowledge, this follow-up study of the MIRO trial is the first to directly identify overall postoperative complication as an adverse risk and potential mediating factor associated with OS and DFS in a randomized clinical trial that compared minimally invasive with open esophagectomy. In view of this association, these results provide additional evidence that minimally invasive esophagectomy could offer improved long-term oncological outcomes compared with open esophagectomy. Multiple theories have been proposed to explain the adverse association between postoperative complications and long-term oncological outcomes. In a retrospective analysis of 2439 patients, Markar et al14 demonstrated that anastomotic leakage after esophageal surgery was associated with an increased rate of locoregional recurrence and impaired OS. Based on the findings of in vitro studies, animal models, and retrospective studies in patients with anastomotic leakage after colorectal surgery, it is possible that spillage of viable tumor cells from within the bowel lumen, with upregulation of inflammatory cytokines associated with anastomotic leakage, was responsible for this outcome.14,24,25,26,27 In a recent multicenter cohort study, anastomotic leakage was also associated with impaired long-term survival, whereas overall complications were not identified as a risk factor.28 In the current study, however, major pulmonary complications were independently associated with decreased OS and DFS, which suggests that anastomotic leakage can only partially account for the deleterious effect of overall postoperative complications on survival.
In addition, no difference in recurrence location was found between patients in the HMIE group and those in the open esophagectomy group or between patients who had major postoperative overall and pulmonary complications and those who did not have such complications. Several authors have hypothesized the generalized immunosuppressive effect of postoperative complications as another explanation for the association between postoperative complications and survival.20,29,30 The occurrence of postoperative complications is presumed to induce a higher degree of systemic inflammatory response, with increased levels of proinflammatory cytokines (interleukin [IL] 1β, IL-6, IL-10, and tumor necrosis factor). This response, in turn, results in the additional proliferation of surviving tumor cells, with an increase in their metastatic potential and an increased resistance to chemotherapy.31,32
Another hypothesis, based on several studies in the field of colorectal surgery, is the potential influence of changes in the microbiome on disease recurrence.32 Several studies have documented the importance of a homeostatic microbiome in local and systemic immune system functioning.33,34 The presence of major postoperative complications, accompanied by additional antibiotic therapy, nutritional deficiencies, and exposure to a specific subset of pathogens within a hospital environment, could result in a shift from the normal microbiome to a pathobiome, which in turn could adversely affect the immune system, thus promoting metastatic circumstances.32 Moreover, given that gut microbiome is also involved in the modulation of chemotherapy and immunotherapy, a complication-induced disruption of this microbiome could adversely affect the efficacy of adjuvant chemotherapy or immunotherapy.33,34,35,36 In a discussion of the study by Khuri et al,37 this question was raised: whether the association between postoperative complications and decreased survival was the direct immunosuppressive consequence of the postoperative complications or whether the postoperative complications were merely an expression of another unidentified factor that could affect long-term oncological outcomes. Furthermore, the extent to which postoperative complications led to a delay or cancellation of adjuvant or perioperative chemotherapy was not analyzed in the current follow-up study, but it could be a topic of interest for future studies.
Strengths and Limitations
This trial has some strengths. It used precise definition of postoperative complications and strict follow-up of patients, along with the rigorous collection of data on disease recurrence.
This trial has some limitations. Although it does benefit from well-designed methods, the original trial protocol was not designed for analysis of long-term results and/or the potential factors associated with impaired OS and DFS. As such, we believe that the insufficient statistical power in this context could be the main reason that the HMIE procedure was not directly associated with improved survival in this study.
Conclusions
Hybrid minimally invasive esophagectomy has long-term oncological results that are similar to those of open esophagectomy. Major postoperative overall and pulmonary complications are independent risk factors associated with impaired OS and DFS. This finding provides additional proof that minimally invasive esophagectomy could be associated with improved long-term oncological outcomes compared with open esophagectomy because of a decrease in postoperative complications.
Trial protocol
eMethods.
eResults. Correlations and Interactions Between Variables
eTable 1. Overview of Inclusion and Exclusion Criteria of the MIRO Trial
eTable 2. Pathological Analysis of Resected Patients
eTable 3. Short-Term Primary and Secondary End Points
eTable 4. Interaction Analysis of Variables for OS
eTable 5. Multivariable Analysis for OS With the Interaction Analysis Between the Treatment Group and the Grade of Major Complications Introduced in the Model
eTable 6. Interaction Analysis of Variables for DFS
eTable 7. Multivariable Analysis for DFS With the Interaction Analysis Between the Treatment Group and the Grade of Major Complications Introduced in the Model
Data sharing statement
References
- 1.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112(8):1247-1255. doi: 10.1038/ajg.2017.155 [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118-128. doi: 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 3.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416-2422. doi: 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, et al. ; CROSS Group . Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074-2084. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 6.Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107-118. doi: 10.1097/CEJ.0000000000000249 [DOI] [PubMed] [Google Scholar]
- 7.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887-1892. doi: 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 8.Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232-236. doi: 10.1097/SLA.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 9.van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thorcolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg. 2019;269(4):621-630. doi: 10.1097/SLA.0000000000003031 [DOI] [PubMed] [Google Scholar]
- 10.Brierley RC, Gaunt D, Metcalfe C, et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer-the Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: protocol for a randomised controlled trial (RCT). BMJ Open. 2019;9(11):e030907. doi: 10.1136/bmjopen-2019-030907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. ; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group . Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152-162. doi: 10.1056/NEJMoa1805101 [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb-Vedi E, Kauppila JH, Malietzis G, Nilsson M, Markar SR, Lagergren J. Long-term survival in esophageal cancer after minimally invasive compared to open esophagectomy: a systematic review and meta-analysis. Ann Surg. 2019;270(6):1005-1017. doi: 10.1097/SLA.0000000000003252 [DOI] [PubMed] [Google Scholar]
- 13.Hölscher AH, DeMeester TR, Schmidt H, Berlth F, Bollschweiler E. Propensity score-matched comparison between open and minimal invasive hybrid esophagectomy for esophageal adenocarcinoma. Langenbecks Arch Surg. 2020;405(4):521-532. doi: 10.1007/s00423-020-01882-3 [DOI] [PubMed] [Google Scholar]
- 14.Markar S, Gronnier C, Duhamel A, et al. ; FREGAT (French Eso-Gastric Tumors) Working Group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie) . The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972-980. doi: 10.1097/SLA.0000000000001011 [DOI] [PubMed] [Google Scholar]
- 15.Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer. 2011;11:310. doi: 10.1186/1471-2407-11-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Lledo G, Mariette C, Raoul JLet al. Cancer de l’œsophage (Dernière mise à jour le 23/09/2016). Thésaurus National de Cancérologie Digestive. Accessed January 25, 2020. https://www.snfge.org/content/1-cancer-de-loesophage
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutegård M, Lagergren P, Rouvelas I, Mason R, Lagergren J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol. 2012;38(7):555-561. doi: 10.1016/j.ejso.2012.02.177 [DOI] [PubMed] [Google Scholar]
- 20.Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016;16(1):32. doi: 10.1186/s12893-016-0149-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Schaaf M, Derogar M, Johar A, et al. Reoperation after oesophageal cancer surgery in relation to long-term survival: a population-based cohort study. BMJ Open. 2014;4(3):e004648. doi: 10.1136/bmjopen-2013-004648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Annoville T, D’Journo XB, Trousse D, et al. Respiratory complications after oesophagectomy for cancer do not affect disease-free survival. Eur J Cardiothorac Surg. 2012;41(5):e66-e73. doi: 10.1093/ejcts/ezs080 [DOI] [PubMed] [Google Scholar]
- 23.Ancona E, Cagol M, Epifani M, et al. Surgical complications do not affect longterm survival after esophagectomy for carcinoma of the thoracic esophagus and cardia. J Am Coll Surg. 2006;203(5):661-669. doi: 10.1016/j.jamcollsurg.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 24.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71(9):659-663. doi: 10.1002/bjs.1800710902 [DOI] [PubMed] [Google Scholar]
- 25.Symes MO, Fermor B, Umpleby HC, Tribe CR, Williamson RC. Cells exfoliated from colorectal cancers can proliferate in immune deprived mice. Br J Cancer. 1984;50(3):423-425. doi: 10.1038/bjc.1984.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fermor B, Umpleby HC, Lever JV, Symes MO, Williamson RC. Proliferative and metastatic potential of exfoliated colorectal cancer cells. J Natl Cancer Inst. 1986;76(2):347-349. [PubMed] [Google Scholar]
- 27.Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240(2):255-259. doi: 10.1097/01.sla.0000133186.81222.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fransen LFC, Berkelmans GHK, Asti E, et al. ; EsoBenchmark Collaborative . The effect of postoperative complications after minimally invasive esophagectomy on long-term survival: an international multicenter cohort study. Ann Surg. Published online October 14, 2020. doi: 10.1097/SLA.0000000000003772 [DOI] [PubMed] [Google Scholar]
- 29.Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg. 2013;100(5):711-718. doi: 10.1002/bjs.9060 [DOI] [PubMed] [Google Scholar]
- 30.Cho JY, Han HS, Yoon YS, Hwang DW, Jung K, Kim YK. Postoperative complications influence prognosis and recurrence patterns in periampullary cancer. World J Surg. 2013;37(9):2234-2241. doi: 10.1007/s00268-013-2106-6 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhang Y, Hu DM, Gong TP, Xu R, Gao J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: a systematic review and meta-analysis of 64 follow-up studies. Asian J Surg. 2020;43(7):719-729. doi: 10.1016/j.asjsur.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 32.Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg. 2018;105(2):e131-e141. doi: 10.1002/bjs.10760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967-970. doi: 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971-976. doi: 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-1089. doi: 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079-1084. doi: 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ; Participants in the VA National Surgical Quality Improvement Program . Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326-341. doi: 10.1097/01.sla.0000179621.33268.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods.
eResults. Correlations and Interactions Between Variables
eTable 1. Overview of Inclusion and Exclusion Criteria of the MIRO Trial
eTable 2. Pathological Analysis of Resected Patients
eTable 3. Short-Term Primary and Secondary End Points
eTable 4. Interaction Analysis of Variables for OS
eTable 5. Multivariable Analysis for OS With the Interaction Analysis Between the Treatment Group and the Grade of Major Complications Introduced in the Model
eTable 6. Interaction Analysis of Variables for DFS
eTable 7. Multivariable Analysis for DFS With the Interaction Analysis Between the Treatment Group and the Grade of Major Complications Introduced in the Model
Data sharing statement