Abstract
While the majority of Drosophila species lays eggs onto fermented fruits, females of Drosophila suzukii pierce the skin and lay eggs into ripening fruits using their serrated ovipositors. The changes of oviposition site preference must have accompanied this niche exploitation. In this study, we established an oviposition assay to investigate the effects of commensal microbes deposited by conspecific and heterospecific individuals and showed that the presence of microbes on the oviposition substrate enhances egg laying of Drosophila melanogaster and Drosophila biarmipes, but discourages that of D. suzukii. This result suggests that a drastic change has taken place in the lineage leading to D. suzukii in how females respond to chemical cues produced by microbes. We also found that hardness of the substrate, resembling that of either ripening or damaged and fermenting fruits, affects the response to microbial growth, indicating that mechanosensory stimuli interact with chemosensory-guided decisions to select or avoid oviposition sites.
Keywords: mechanosensory stimulus, decision-making, acetic acid bacteria, Gluconobacter, Acetobacter, spotted-wing Drosophila
1. Introduction
Oviposition site selection is a critical factor in determining the survival rate of offspring in insect species. A nutritionally suitable resource may be heavily used by other insects and the offspring may suffer from intense competition. The females of Drosophila suzukii Matsumura (Diptera: Drosophilidae) have the ability to pierce the skin of ripening fruits and lay eggs into the flesh by using serrated ovipositors [1–3]. Because many other closely related Drosophila species lay eggs onto fermented fruits, this behaviour allows D. suzukii to use a carbohydrate-rich resource before interspecific competition becomes intense [4,5].
The behavioural shift to deposit eggs into ripening fruits must have been accompanied by changes not only in the ovipositor morphology but also in the sensory systems used to evaluate the oviposition substrate. Karageorgi et al. [6] showed that when given the choice between ripe and rotten strawberry fruits, D. suzukii strongly preferred ripe over rotten fruit, whereas Drosophila melanogaster showed an opposite tendency and preferred rotten fruit, consistent with other studies [7,8]. In the same experiment, Drosophila biarmipes, a closely related species of D. suzukii showed no preference between ripe and rotten fruit, indicating that they are at an intermediate evolutionary stage between D. suzukii and D. melanogaster. It has also been shown in the same study that while D. biarmipes and D. melanogaster show similarly strong preferences for soft substrates, D. suzukii lay eggs onto both hard and soft agarose gel substrates, a pattern similar to other studies [4,9]. Therefore, these studies indicate that D. suzukii has widened the range of potential substrates to include those with different degrees of hardness and does not necessarily prefer a harder fruit surface [10–12]. Thus, hardness alone does not account for the strong preference for ripe fruits as an oviposition substrate. Other sensory modifications are also likely to underlie the radical shift to an unexploited resource in D. suzukii after divergence from the D. biarmipes lineage.
The evolutionary changes in the D. suzukii chemosensory system and response to attractants from ripening fruits have been documented [6,13,14], but possible repellents of fermenting fruits have not been investigated in detail. As shown in the previous studies, inoculation of the substrate from D. melanogaster adults significantly reduced the number of eggs laid by D. suzukii [15,16]. The factors causing such aversive behaviour are not known. The deposition of aggregation pheromones is one likely factor [17–19]. In addition, microbial populations on fermenting fruits originating from the surrounding environment as well as individuals that have visited the fruit represent another source of aggregation signals. The presence of non-pathogenic microbes guides a wide array of behavioural decisions in insects, including adult aggregation, feeding decisions and oviposition choice [20–24]. Partnering with commensal microbes provides several benefits for insect hosts including protection from pathogenic microbes, increased access to nutritional resources and improved offspring survival [25]. The response of D. suzukii oviposition to the microbial environment has been largely unstudied but may represent an essential aspect of the new host exploitation in this species.
Assessing the fruit condition and making the decision to select the oviposition site involve an integration of multiple sensory cues. It has been shown that D. suzukii has the ability to make complex decisions between healthy and fermenting fruits depending on the availability of the resource [8]. We hypothesize that one of the unexplored factors that D. suzukii sense could be the commensal microbes on fermenting fruits that have been deposited by conspecific and heterospecific individuals. The avoidance of fruits with chemicals (metabolites) from such microbes may be an effective strategy to access the fruit resource before overripening or fermenting reactions proceed. However, other information such as texture also is likely to be perceived and used to make ultimate decisions. Indeed in D. melanogaster, mechanosensory (texture) and chemosensory (taste) information are integrated to direct feeding and oviposition decisions [26–28]. Therefore, it is an intriguing question as to how different sensory information is processed and integrated in D. suzukii in comparison with D. biarmipes and D. melanogaster, both of which have different decision-making criteria for choosing oviposition sites.
In this study, we investigate the effects of commensal microbes on oviposition site preferences, both independent of and in combination with the effect of the substrate hardness, in D. suzukii, D. biarmipes and D. melanogaster. In our assay, D. suzukii exhibited a strong avoidance of microbes transferred from other flies. This response was distinct from the other two species, suggesting that the behaviour has evolved in the lineage leading to D. suzukii after the split from D. biarmipes. Furthermore, we tested the combinatorial effect of the hardness and the presence or absence of microbes on the oviposition site selection. The mechanical stimuli provided by substrate hardness superseded the influence of microbial chemical signals. We show that this property was conserved among the three species despite differential preference towards hardness and microbial stimuli.
2. Material and methods
2.1. Fly strains
The following strains were used to compare the ovipositon site preference: D. suzukii strain Hilo collected in Hilo, Island of Hawai‘i, USA in 2017, D. biarmipes strain MYS118, collected in Mysore, India, in 1981, and D. melanogaster strain Canton S BL#9515. All the strains were maintained at 25 ± 1°C under the 12 h light: 12 h dark light cycle. All flies were fed with standard corn meal food mixed with yeast, glucose and agar.
2.2. Oviposition assay to test the preference for substrates with microbial growth
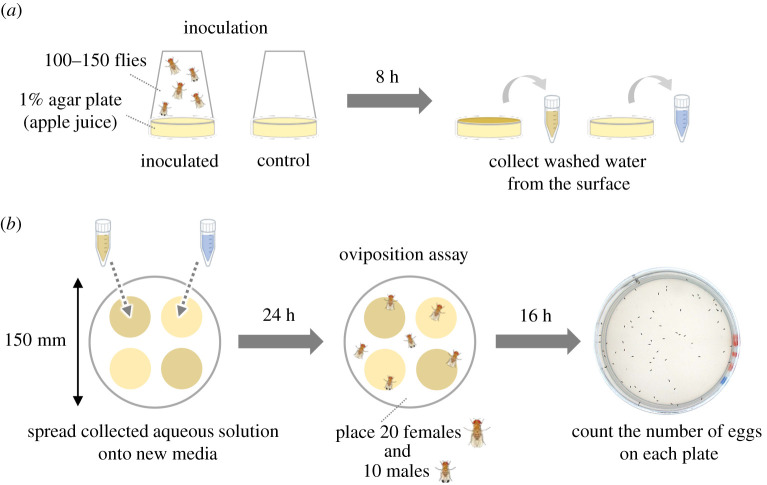
The procedure is illustrated in figure 1. Inoculation was conducted by using D. melanogaster (3–7 days after eclosion), D. biarmipes (3–7 days after eclosion) or D. suzukii (7–14 days after eclosion). One hundred to 150 flies were placed into the inoculation chamber without anaesthesia and left for 8 h. An inoculation chamber consists of a plastic cup (100 ml, Tri-Corner Beakers) and a petri dish (57 mm diameter × 16 mm height, IWAKI 1010-060) filled with 5 ml 1% agar (Drosophila agar type II, Apex) in apple juice (SUNPACK, JAN code: 4571247510950) diluted to 50%. No flies were placed into the control inoculation chamber. After inoculation, the surface of the substrate was washed with 1 ml distilled water by pipetting 10 times. Wash solutions (100 µl) from inoculated or control plates (figure 1a) were spread onto a new agar plate (40 mm diameter × 13 mm height, Azunol 1-8549-01) and incubated for 24 h at 25 ± 1°C. Microbial colonies were visible on the media spread with aqueous solution from the inoculated media after 24 h incubation.
Figure 1.
Experimental scheme of the oviposition assay to quantify response to microbes deposited by flies on the surface of media. (a) Washed water collected from the surface of inoculated and control plates. (b) Oviposition assay using media inoculated with solutions from (a) for 24 h.
The oviposition assay was conducted with a petri dish chamber (150 mm diameter × 20 mm height, IWAKI 3030-150) containing four 40 mm diameter petri dishes with two types of media placed alternatively (figure 1b). Twenty females and 10 males were placed into the chamber without anaesthesia within 3 h before the dark cycle and kept for 16 h in the dark condition. The assay was conducted under the condition of 25 ± 1°C and 50 ± 5% relative humidity. The photo image of each petri dish with substrate was taken by a camera (Olympus DP73) with transmitted light from the bottom. The number of eggs on each substrate was counted.
The preference index (PI) for the substrate with microbial growth was calculated by using the following formula:
where Ninoculated and Ncontrol are the total numbers of eggs on the substrates with microbial growth and the control plates, respectively.
To confirm that the PI measurements for substrates inoculated with microbial colonies reflect the activity of microbes, collected solutions from the inoculated media were filter sterilized using a syringe filter (0.22 µm Millex®-GV Filter Unit). After washing the surface of the inoculated medium by repeatedly pipetting 1.2 ml distilled water 10 times, the aqueous solution was filtered and used in the oviposition assay as described earlier.
2.3. Oviposition preference assay for substrate hardness, with and without microbes
Inoculant from D. melanogaster was collected from three inoculation chambers, pooled and divided into 24 (8 × 3 species) 40 mm diameter petri dishes with medium. Plates without any solution were used for the assays that did not test microbial inoculation. The remaining steps were the same as in §2.2. The PI for the soft substrate was calculated by using the following formula:
where N1% agar and N3% agar are the total numbers of eggs on the 1% and 3% agar media, respectively.
2.4. 16S-rRNA gene sequencing of microbial colonies used for the oviposition assays
To collect the microbes tested for the oviposition assays, the surface of the inoculated substrate was washed with distilled water as described earlier. The solution was diluted to 200 µl total volume and spread onto a petri dish (90 mm diameter × 16 mm height, IWAKI SH90-15) filled with 10 ml apple juice agar as described earlier. The media were incubated for 24 to 40 h at 25 ± 1°C, and single colonies were selected randomly for DNA extraction. Each colony was picked with a 10 µl pipette tip, suspended in 20 µl of sterile water and incubated for 15 min at 95°C after adding 20 µl 100 mM NaOH. Then, 4.4 µl of 1 M Tris–HCl pH 7.0 was added to each sample and used as template DNA.
Colony PCR was performed with 16S-rRNA universal primers 8F (AGAGTTTGATCMTGGCTCAG) [29,30] and 1492R (GGYTACCTTGTTACGACTT) [31,32] in a 30 µl reaction using Ex Taq (TaKaRa). Amplification condition for the PCR included an initial denaturation step of 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 53 or 55°C for 30 s and 72°C for 60 s, and a final extension step of 72°C for 5 min. Reaction products were checked for size and purity on 1% agarose gel and were sequenced after purification by using either BrilliantDye Terminator Cycle Sequencing Kit v. 2.1 (Nimagen) and a 3130 xl DNA Analyzer (Thermo Fisher Science) or BigDye Terminator v. 3.1 Cycle Sequencing Kit (Thermo Fisher Science) and a 3170xl DNA Analyzer (Thermo Fisher Science). Sequences were aligned by using MEGA7 [33] and trimmed from the nucleotide positions 61 to 628 of the Escherichia coli reference sequence (CP023349.1:226,883-228,438). The genus level identity of each sequence was assigned by the highest score entries in the NCBI database, ‘16S ribosomal RNA (Bacteria and Archaea type strains)' (as of 28 May 2020) by local BLAST (BLAST + 2.10.0).
3. Results
The oviposition site preference of D. suzukii for ripening fruits relies on shifts in mechanosensation as well as chemosensation [6]. Recent work has shown that consistent with their preference towards ripening fruits over fermenting fruits, D. suzukii females tend to lay more eggs on non-inoculated media compared with media inoculated by D. melanogaster [15]. Our study focused on determining whether microbial presence and the hardness of the oviposition substrate form the basis of D. suzukii oviposition decisions.
3.1. Oviposition site preference against the presence of microbes
Oviposition can be influenced by pheromones or microbial presence. To distinguish between these two possibilities, we first established a method to test only the contribution of microbial growth to oviposition site preference. A water wash was used to collect substances deposited by adult flies, and the inoculum was applied to sterile media (figure 1a). Many of the known pheromones used for Drosophila chemical communication are hydrophobic hydrocarbons, wax esters and wax alcohols [34] and are thus not soluble in water and unlikely to be transferred in the water wash. After incubation, microbial colonies were visible on the inoculated media. Media that had been exposed to water wash from control chambers did not have visible colonies.
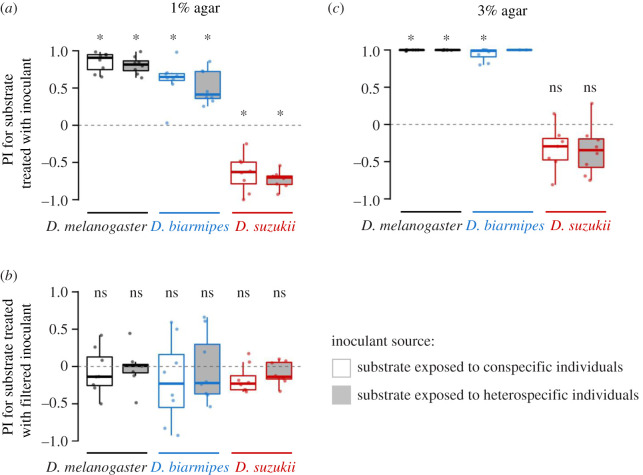
The results from the oviposition assay on soft medium (1% agar) indicated that D. suzukii avoided oviposition substrates with microbial colonies (figure 2a, electronic supplementary material, table S1). Given a choice between substrates with aqueous solutions from inoculated and non-inoculated media, the D. suzukii PI was significantly less than 0, indicating that the microbial growth discouraged oviposition. By contrast, D. melanogaster preferred ovipositing on substrates with the microbial growth (figure 2a), indicating that the presence of microbes positively influenced the choice of oviposition site for this species. To trace the evolutionary trajectory of this preference, we also conducted the same experiments using D. biarmipes, a closely related species to D. suzukii. Remarkably, as with D. melanogaster, the microbes positively influenced the oviposition site choice of D. biarmipes (figure 2a), indicating that the preference for ovipositing at sites with commensal microbes is the ancestral state among these species and that D. biarmipes still retain this characteristic. These results were consistent when using microbes from conspecific and heterospecific inoculation (figure 2a). Thus, the drastic change from the tendency to lay more eggs to fewer eggs on the substrate with microbial growth is predicted to have occurred in the lineage leading to D. suzukii after the separation from the D. biarmipes lineage.
Figure 2.
Comparisons of the preference indices (PIs) of D. melanogaster, D. biarmipes and D. suzukii for oviposition substrates treated with inoculant from conspecific (open boxplots) or heterospecific (filled boxplots in grey) flies. (a) The PIs assayed on soft substrate (1% agar medium) with and without inoculant treatment (microbial growth). (b) The PIs assayed on 1% agar medium for substrates treated with sterile filtered solutions of inoculant. (c) The PIs assayed on hard oviposition substrate (3% agar medium) with and without inoculant treatment (microbial growth). Control substrates were treated with solutions from non-exposed (non-inoculated) substrate in all assays. Species used for heterospecific inoculations were conducted using D. suzukii for D. melanogaster assay, and D. melanogaster for D. biarmipes and D. suzukii assays. Results from assays with fewer than 10 eggs on either substrate were excluded from the analysis. Box signifies the upper and lower quartiles and horizontal bar indicates median. Upper and lower whiskers represent maximum and minimum 1.5 × interquartile range, respectively. The difference from PI = 0 (no preferences) was tested by Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons (six tests). *p < 0.05, ns p ≥ 0.05.
To confirm that the presence of microbes in the water wash is the primary factor in guiding oviposition, we passed the collected aqueous solution through a 0.22 µm filter to remove microbes and large food particles while keeping nutrients, metabolites and other small molecules found in faeces. In all species, filter sterilization of the inoculant eliminated both positive and negative oviposition preferences (figure 2b, electronic supplementary material, table S2). Therefore, microbes that can be removed by a 0.22 µm filter are likely to be the main factor affecting oviposition site preferences.
To identify the main bacterial species that were present in the water washes of inoculated media, we sampled microbial colonies from the medium after 24–40 h of growth and performed PCR amplification of the 16S-rRNA gene sequence. The bacterial species classified at the genus level and the frequencies estimated from the sampled colonies are shown in electronic supplementary material, figure S1 and electronic supplementary material, tables S3–S5. The bacteria used for our oviposition preference assay were mostly from the Acetobacter and Gluconobacter genera.
3.2. Combinatorial effect of the presence of microbes and the hardness of the oviposition substrate
In addition to chemosensory signals, another factor that is known to affect Drosophila oviposition site preference is the hardness of the substrate. Choice assays using agarose media with different degrees of hardness have shown that D. suzukii females exhibit a much weaker preference towards softer substrates, resembling damaged and fermented fruits, compared with D. biarmipes and D. melanogaster [6]. To investigate the combinatorial effect of hardness and microbial growth, we conducted choice assays using hard oviposition substrate (3% agar medium) with and without the presence of microbes (figure 2c, electronic supplementary material, table S6).
When substrates were hard, D. melanogaster and D. biarmipes showed a PI close to 1, which is indicative of even stronger preferences for ovipositing on media with microbial growth than when using 1% agar media (figure 2a). Interestingly, the aversion to substrates with microbial growth exhibited by D. suzukii was reduced when the harder 3% media were used. No significant preference or aversion was detected for microbial growth when the substrates in the oviposition chamber were all hard (figure 2c). From the outcome of this combinatorial assay, it was clear that the hardness of the substrate modifies the preferences against microbes.
Next, we investigated whether the choice between soft (1%) and hard (3%) agar media was affected by the presence of microbes (figure 3a). Our experimental results using 1% and 3% agar media without microbes showed that D. suzukii had no strong preference towards either substrate, in contrast to the strong preference exhibited by the other two species (figure 3b, electronic supplementary material, table S7), which was consistent with the previous study [4,6,9]. Interestingly, whether the microbes were present or not did not affect the PI between soft and hard substrates in D. melanogaster and D. suzukii. The preference towards the softer substrate became significantly weaker when microbes were present than when they were absent in D. biarmipes, but only slightly. These results indicate that rather than the presence or absence of microbial growth, the hardness of the substrate is the dominant factor determining the oviposition site selection in D. melanogaster and D. biarmipes. In D. suzukii, the lack of preference to lay more eggs on either the soft or hard substrate was persistent and unaltered by the microbial growth.
Figure 3.
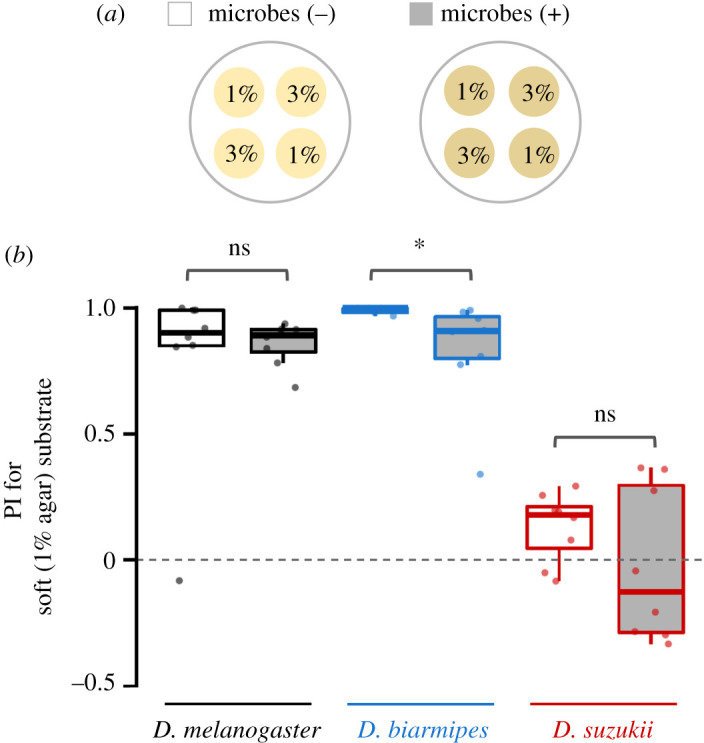
Preference indices (PIs) for the soft substrate with and without microbes. (a) The substrate placement in the chambers for the oviposition assay. ‘1%' and ‘3%' indicate soft (1% agar medium) and hard (3% agar medium) oviposition substrates, respectively. The microbe (+) chambers have been treated with inoculant collected from substrate surface exposed to D. melanogaster; microbial (−) chambers were treated with inoculant from non-exposed surfaces. (b) The preference indices (PIs) for soft oviposition substrate in the absence (open boxplots) and presence (filled boxplots in grey) of microbes. Results from assays with fewer than 10 eggs on either substrate were excluded from the analysis. Box signifies the upper and lower quartiles and horizontal bar indicates median. Upper and lower whiskers represent maximum and minimum 1.5 × interquartile range, respectively. Statistical significance was tested by permutation test with Bonferroni correction for multiple comparisons (six tests). *p < 0.05, ns p ≥ 0.05.
4. Discussion
4.1. Commensal microbes deposited by flies affect oviposition site preferences in D. suzukii, D. biarmipes and D. melanogaster, and the preference of D. suzukii is distinct from that of the other species
Fruit flies like many other insects coexist with a community of gut microbes, the composition of which can vary to a large extent due to various field and laboratory conditions [35–38]. To elucidate whether chemicals emitted from gut microbes function as intraspecific or interspecific behavioural cues, we examined the influence of fly-deposited microbes on oviposition behaviour.
Our results show that egg-laying decisions in Drosophila are strongly influenced by the presence of microbial growth, suggesting that they are sensitive to microbe-derived cues. When given a choice using soft media, D. suzukii avoided media inoculated with commensal microbes, in contrast to D. melanogaster and D. biarmipes, both of which showed strong oviposition preferences towards microbe-rich media (figure 2). The significant change in oviposition site preference must have occurred in the D. suzukii lineage after the split from D. biarmipes consistent with the timing of the host shift to ripening fruits. Therefore, the change in microbial preference may have been associated with the new niche exploitation in this lineage.
4.2. Acetic acid bacteria differentially affect oviposition behaviour among Drosophila species
The bacterial species used for oviposition preference assays consisted mainly of Acetobacter and Gluconobacter, both members of the Acetobacteraceae family commonly found in the guts of laboratory-raised and wild fruit fly species [36] including D. suzukii [39,40]. These acetic acid bacteria provide benefits for host flies by accelerating growth and offering protection from pathogenic bacteria [41,42]. Some previous studies on wine grapes have indicated that D. suzukii is capable of vectoring acetic acid bacteria that contribute to the fermentation process of the fruits [43,44]. Nevertheless, the colonies grown on the media are not likely to represent the actual composition of fly-associated microbiota in the wild since growth is restricted by diet and the type of media used (agar in apple juice). Flies from natural populations exhibit a more diverse microbiome [37,45]. In addition, our characterization of the microbiome focused only on bacterial species. It is likely that yeast, which is a common symbiont for drosophilids [46], is also a part of the inoculum and contributes to oviposition preference [47].
Drosophila melanogaster, D. biarmipes and D. suzukii exhibited different proportions of Acetobacter and Gluconobacter (electronic supplementary material, figure S1 and electronic supplementary material, tables S3–S5). However, there were no differences in the responses of the three Drosophila species to conspecific or heterospecific inoculants, indicating that both Acetobacter and Gluconobacter have similar effects on the oviposition site choice (figure 2). While D. suzukii showed a clear aversion for ovipositing on inoculated soft media (figure 2a), the response of females to Gluconobacter volatiles may be context dependent. A previous study showed that females starved for 24 h exhibit clear attraction to Gluconobacter in an olfactometer bioassay [48]. Taken together with our observation that D. suzukii avoids egg laying in the presence of Gluconobacter colonies, it is clear that reproductive and feeding site preferences can be clearly decoupled in this species. Microbial cues that are attractive for feeding may be aversive for oviposition.
4.3. Chemical cues mediating the differential preference against microbes await further investigation
In studies searching for oviposition deterrents for the pest management of fruit crops, at least two chemicals, geosmin and octenol (1-octen-3-ol), both of which are components of volatile metabolites from microorganisms present in rotting fruits, induced aversive responses in D. suzukii [49]. However, because these chemicals are known repellents in D. melanogaster as well [50,51], the aversion to these microbial compounds is not likely to underlie the D. suzukii specific shift in oviposition site.
A study using D. melanogaster indicated that female oviposition is guided by sucrose, a gustatory cue used to sense fermentation by lactic acid-producing Enterococci bacteria [52]. Interestingly, the olfactory system was shown to be dispensable for ovipositional attraction to these microbes. In contrast, the inhibition of synaptic transmission in sweet sensing gustatory neurons, Gr5a and Gr64a neurons, impaired the oviposition preference towards fermentation sources. Whether sucrose sensing also mediates the avoidance of acetic acid bacteria in D. suzukii would be an intriguing question to pursue. Nevertheless, Silva-Soares et al. [4] showed that D. suzukii and D. biarmipes have similar oviposition preferences towards sites with a low protein (yeast) to carbohydrate (sucrose) ratio, suggesting that a differential response to sucrose is not likely to explain the contrasting response to acetic acid bacteria products. The volatiles emanating from microorganisms may also be playing a substantial role in making decisions. Thus, the microbe-derived chemical cues that govern oviposition response await further investigation.
One feature of the experimental design that may impact oviposition decisions is the ventilation of the behavioural chamber. The arena housing the oviposition chambers of our study was not ventilated. The lack of ventilation may obscure the choice within a chamber due to a buildup of odorants or bias the preferences because of an unnaturally concentrated cue. However, neither effect appeared to be a substantial factor in our experiments since there were both instances where a clear choice or no choice was made (figure 2a,c and figure 3b). It also appeared to be not a critical factor in a previous study investigating the effects of acids on positional responses and oviposition preferences using D. melanogaster [53]. Nevertheless, it should be noted that we cannot totally exclude the possibility that the lack of ventilation may have caused some subtle biases in preference.
4.4. Oviposition site hardness supersedes the D. suzukii aversion to microbial presence
Integration of different types of stimuli is essential for critical decision-making processes such as the selection of egg deposition sites, a choice that has large influences on the early life performances of the offspring. In D. melanogaster, neural circuits governing oviposition site combine information from different modalities [53,54]. Recently, several studies [27,28] elucidated an underlying molecular mechanism for integrating mechanosensory and chemosensory information to make egg-laying decisions in D. melanogaster. Our results reveal that two different classes of sensory cues, substrate hardness and the presence of microbes, are integrated in D. suzukii oviposition decisions in a manner that is distinct from D. biarmipes and D. melanogaster (figures 2 and 3). The avoidance of microbes displayed by D. suzukii was evident only in the context of a soft substrate (figure 2a) but not a hard one (figure 2c). These results suggest that mechanical cues from surface hardness take precedence over decisions guided by microbial cues. By contrast, the preference exhibited by both D. melanogaster and D. biarmipes towards microbe-inoculated surfaces strengthened when hard substrates were used (figure 2c), indicating a similar integration of mechanical and microbial chemical cues. Conversely, microbial presence did not affect the choice between hard and soft substrates in all the three species (figure 3).
These results indicate that mechanical and chemical stimuli are not processed additively in these species. The surface hardness modifies the response to microbial cues but not vice versa. Interestingly, previous studies showed that in female D. melanogaster, the presence of chemicals, sucrose and/or fruit juice ingredient obviates the preference for ovipositing on softer surfaces [27,28]. The discrepancy between the direction of interference between mechanical and chemical stimuli suggests that the hierarchy of cues used in oviposition may depend on the nature of the chemical stimulus.
4.5. The integration of mechanical cues and microbial stimuli is conserved in oviposition choice and reflect differences in ecology
The hardness of the substrate assayed using 1% and 3% agar media is intended to mimic damaged fermenting fruits and intact ripening fruits, respectively. However, the agar media used in our assay have uniform texture. This feature may not completely reflect the condition of the real ripening fruits with partially damaged skin in the field. Indeed, it has been shown that egg-laying decisions of flies depend on whether the fruit is injured or not [2,8]. Nevertheless, despite this caveat, our findings in this study can still be interpreted conceptually in the context of ecology of D. suzukii.
In the early fruiting season when all the fruits are hard or likely to have only a small amount of commensal microbial cues left by other flies, D. suzukii females may lay eggs onto any available fruits. This scenario is consistent with the results of our assays using only hard substrate (figure 2c) or only non-inoculated substrates (figure 3). During the ripening period, when fruits become softer and partially damaged, the females may choose fruits with less abundant fermentation cues presumably to avoid competition with other species. This prediction is consistent with our results using only soft substrate (figure 2a). In late fruiting season when the majority of the fruits are on the ground, damaged and rotten, the females may readily lay eggs onto suboptimal fermenting fruits, the situation resembling our assays using only inoculated substrates (figure 3). These explanations are consistent with the study by Kienzle et al. [8], which showed that D. suzukii exhibit stronger preferences towards ovipositing in healthy fruits when healthy and fermenting fruits are both abundant compared with when the former are less abundant. The context-dependent optimization through seasonal change in host fruit condition might explain the evolutionary background of our findings where substrate hardness takes precedence over microbial presence in the decision to oviposit in this species.
Although surface hardness interacts with the response to commensal microbe cues in D. biarmipes and D. melanogaster as in D. suzukii, there may be some qualitative differences in ecological context between these species. Drosophila biarmipes and D. melanogaster show a strong preference towards soft substrates inoculated with microbes, and their preferences for microbes are enhanced when the substrate is hard (figure 2). In the field, it may be the case that flies are more likely to use hard fruits in the presence of a microbial signature, which may be indicative of an immediate onset of fermentation when the skins become partially damaged as the fruits ripen. In contrast to D. suzukii, both D. biarmipes and D. melanogaster tend to prefer soft substrates even when all the substrates in the vicinity have microbial growth (figure 3), indicating that mechanical cues supersede microbial presence in oviposition site selection. Therefore, D. suzukii may have rapidly evolved to adjust the manner in which mechanical and chemical stimuli are integrated to maximize the offspring performance by an egg-laying strategy that is different from other closely related species.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
We are grateful to K. Nakayama and N. Yoneishi for excellent technical assistance, the UHM Microbial Genetics and Analytical Laboratory for use of facilities, Eurofins Genomics K.K. for Sanger sequencing, and members of the Takahashi lab and Yew lab for helpful discussions.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
A.S., J.Y.Y. and A.T. conceived the research and designed the experiments. A.S. performed the experiments. A.S. and K.M.T. analysed the data. A.S. and A.T. drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by JSPS KAKENHI (grant no. JP19H03276) awarded to A.T., Department of Defense United States Army Research Office (grant no. W911NF1610216) and the National Institutes of Health (grant no. 1P20GM125508) awarded to J.Y.Y.
References
- 1.Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O'Neal SD, Zalom FG. 2011. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2, G1–G7. ( 10.1603/IPM10010) [DOI] [Google Scholar]
- 2.Atallah J, Teixeira L, Salazar R, Zaragoza G, Kopp A. 2014. The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. Lond. B 281, 20132840 ( 10.1098/rspb.2013.2840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muto L, Kamimura Y, Tanaka KM, Takahashi A. 2018. An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila. Proc. R. Soc. B 285, 20181635 ( 10.1098/rspb.2018.1635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva-Soares NF, Nogueira-Alves A, Beldade P, Mirth CK. 2017. Adaptation to new nutritional environments: larval performance, foraging decisions, and adult oviposition choices in Drosophila suzukii. BMC Ecol. 17, 21 ( 10.1186/s12898-017-0131-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young Y, Buckiewicz N, Long TAF. 2018. Nutritional geometry and fitness consequences in Drosophila suzukii, the spotted-wing Drosophila. Ecol. Evol. 8, 2842–2851. ( 10.1002/ece3.3849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karageorgi M, Bräcker LB, Lebreton S, Minervino C, Cavey M, Siju KP, Grunwald Kadow IC, Gompel N, Prud'homme B. 2017. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 27, 847–853. ( 10.1016/j.cub.2017.01.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA, Yorgey BM. 2011. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 67, 1358–1367. ( 10.1002/ps.2225) [DOI] [PubMed] [Google Scholar]
- 8.Kienzle R, Groß LB, Caughman S, Rohlfs M. 2020. Resource use by individual Drosophila suzukii reveals a flexible preference for oviposition into healthy fruits. Sci. Rep. 10, 3132 ( 10.1038/s41598-020-59595-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Zhou Z-D, Mao F, Fan X-Y, Liu G-Y, Huang J, Qiao X-M. 2020. Identification of potential mechanosensitive ion channels involved in texture discrimination during Drosophila suzukii egg-laying behaviour. Insect Mol. Biol. 29, 444–451. ( 10.1111/imb.12654) [DOI] [PubMed] [Google Scholar]
- 10.Burrack HJ, Fernandez GE, Spivey T, Kraus DA. 2013. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 69, 1173–1180. ( 10.1002/ps.3489) [DOI] [PubMed] [Google Scholar]
- 11.Kinjo H, Kunimi Y, Ban T, Nakai M. 2013. Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J. Econ. Entomol. 106, 1767–1771. ( 10.1603/ec12505) [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Dalton DT, Swoboda-Bhattarai KA, Bruck DJ, Burrack HJ, Strik BC, Woltz JM, Walton VM. 2016. Characterization and manipulation of fruit susceptibility to Drosophila suzukii. J. Pest Sci. 89, 771–780. ( 10.1007/s10340-015-0692-9) [DOI] [Google Scholar]
- 13.Keesey IW, Knaden M, Hansson BS. 2015. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 41, 121–128. ( 10.1007/s10886-015-0544-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revadi S, et al. 2015. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 40, 54–64. ( 10.1111/phen.12088) [DOI] [Google Scholar]
- 15.Shaw B, Brain P, Wijnen H, Fountain MT. 2018. Reducing Drosophila suzukii emergence through inter-species competition. Pest Manag. Sci. 74, 1466–1471. ( 10.1002/ps.4836) [DOI] [PubMed] [Google Scholar]
- 16.Kidera H, Takahashi KH. 2020. Chemical cues from competitors change the oviposition preference of Drosophila suzukii. Entomol. Exp. Appl. 168, 304–310. ( 10.1111/eea.12889) [DOI] [Google Scholar]
- 17.Lin C-C, Prokop-Prigge KA, Preti G, Potter CJ. 2015. Food odors trigger Drosophila males to deposit a pheromone that guides aggregation and female oviposition decisions. Elife 4, 1–26. ( 10.7554/elife.08688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P, Atkinson R, Jones DNM, Smith DP. 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45, 193–200. ( 10.1016/j.neuron.2004.12.031) [DOI] [PubMed] [Google Scholar]
- 19.Tait G, et al. 2020. Reproductive site selection: evidence of an oviposition cue in a highly adaptive Dipteran, Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 49, 355–363. ( 10.1093/ee/nvaa005) [DOI] [PubMed] [Google Scholar]
- 20.Lewis Z, Lizé A. 2015. Insect behaviour and the microbiome. Curr. Opin. Insect Sci. 9, 86–90. ( 10.1016/j.cois.2015.03.003) [DOI] [PubMed] [Google Scholar]
- 21.Fischer CN, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA. 2017. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 6, e18855 ( 10.7554/eLife.18855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong AC-N, Wang Q-P, Morimoto J, Senior AM, Lihoreau M, Neely GG, Simpson SJ, Ponton F. 2017. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 27, 2397–2404. ( 10.1016/j.cub.2017.07.022) [DOI] [PubMed] [Google Scholar]
- 23.Leitão-Gonçalves R, et al. 2017. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 15, e2000862 ( 10.1371/journal.pbio.2000862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jose PA, Ben-Yosef M, Jurkevitch E, Yuval B. 2019. Symbiotic bacteria affect oviposition behavior in the olive fruit fly Bactrocera oleae. J. Insect Physiol. 117, 103917 ( 10.1016/j.jinsphys.2019.103917) [DOI] [PubMed] [Google Scholar]
- 25.Douglas AE 2015. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 60, 17–34. ( 10.1146/annurev-ento-010814-020822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong YT, Oh SM, Shim J, Seo JT, Kwon JY, Moon SJ. 2016. Mechanosensory neurons control sweet sensing in Drosophila. Nat. Commun. 7, 12872 ( 10.1038/ncomms12872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu SF, Ja YL, Zhang YJ, Yang CH. 2019. Sweet neurons inhibit texture discrimination by signaling TMC-expressing mechanosensitive neurons in Drosophila. Elife 8, 1–24. ( 10.7554/eLife.46165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yu J, Guo X, Wei J, Liu T, Zhang W. 2020. Parallel mechanosensory pathways direct oviposition decision-making in Drosophila. Curr. Biol. 30, 3075–3088. ( 10.1016/j.cub.2020.05.076) [DOI] [PubMed] [Google Scholar]
- 29.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. ( 10.1128/jb.173.2.697-703.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner S, Pryer KM, Miao VP, Palmer JD. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 46, 327–338. ( 10.1111/j.1550-7408.1999.tb04612.x) [DOI] [PubMed] [Google Scholar]
- 31.Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC. 1989. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17, 7843–7853. ( 10.1093/nar/17.19.7843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loy A, Lehner A, Lee N, Adamczyk J, Meier H, Ernst J, Schleifer K-H, Wagner M. 2002. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 68, 5064–5081. ( 10.1128/aem.68.10.5064-5081.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. ( 10.1093/molbev/msw054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yew JY, Chung H. 2017. Drosophila as a holistic model for insect pheromone signaling and processing. Curr. Opin. Insect Sci. 24, 15–20. ( 10.1016/j.cois.2017.09.003) [DOI] [PubMed] [Google Scholar]
- 35.Bing X, Gerlach J, Loeb G, Buchon N. 2018. Nutrient-dependent impact of microbes on Drosophila suzukii development. MBio 9, e02199-17 ( 10.1128/mBio.02199-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broderick NA, Lemaitre B. 2012. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3, 307–321. ( 10.4161/gmic.19896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandler JA, Lang J, Bhatnagar S, Eisen JA, Kopp A. 2011. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 7, e1002272 ( 10.1371/journal.pgen.1002272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong AC-N, Chaston JM, Douglas AE. 2013. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 7, 1922–1932. ( 10.1038/ismej.2013.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandler JA, James PM, Jospin G, Lang JM. 2014. The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2, e474 ( 10.7717/peerj.474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Sañudo I, Simonato M, Squartini A, Mori N, Marri L, Mazzon L. 2018. Metagenomic analysis reveals changes of the Drosophila suzukii microbiota in the newly colonized regions. Insect Sci. 25, 833–846. ( 10.1111/1744-7917.12458) [DOI] [PubMed] [Google Scholar]
- 41.Crotti E, et al. 2010. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 76, 6963–6970. ( 10.1128/AEM.01336-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin SC, Kim S-H, You H, Kim B, Kim AC, Lee K-A, Yoon J-H, Ryu J-H, Lee W-J. 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334, 670–674. ( 10.1126/science.1212782) [DOI] [PubMed] [Google Scholar]
- 43.Ioriatti C, Walton V, Dalton D, Anfora G, Grassi A, Maistri S, Mazzoni V. 2015. Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J. Econ. Entomol. 108, 1148–1155. ( 10.1093/jee/tov042) [DOI] [PubMed] [Google Scholar]
- 44.Ioriatti C, Guzzon R, Anfora G, Ghidoni F, Mazzoni V, Villegas TR, Dalton DT, Walton VM. 2018. Drosophila suzukii (Diptera: Drosophilidae) contributes to the development of sour rot in grape. J. Econ. Entomol. 111, 283–292. ( 10.1093/jee/tox292) [DOI] [PubMed] [Google Scholar]
- 45.Staubach F, Baines JF, Künzel S, Bik EM, Petrov DA. 2013. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS ONE 8, e70749 ( 10.1371/journal.pone.0070749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanini I 2018. Yeast-insect associations: it takes guts. Yeast 35, 315–330. ( 10.1002/yea.3309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellutti N, Gallmetzer A, Innerebner G, Schmidt S, Zelger R, Koschier EH. 2018. Dietary yeast affects preference and performance in Drosophila suzukii. J. Pest Sci. 91, 651–660. ( 10.1007/s10340-017-0932-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzetto F, Gonella E, Crotti E, Vacchini V, Syrpas M, Pontini M, Mangelinckx S, Daffonchio D, Alma A. 2016. Olfactory attraction of Drosophila suzukii by symbiotic acetic acid bacteria. J. Pest Sci. 89, 783–792. ( 10.1007/s10340-016-0754-7) [DOI] [Google Scholar]
- 49.Wallingford AK, Hesler SP, Cha DH, Loeb GM. 2016. Behavioral response of spotted-wing drosophila, Drosophila suzukii Matsumura, to aversive odors and a potential oviposition deterrent in the field. Pest Manag. Sci. 72, 701–706. ( 10.1002/ps.4040) [DOI] [PubMed] [Google Scholar]
- 50.Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. 2012. Spatial representation of odorant valence in an insect brain. Cell Rep. 1, 392–399. ( 10.1016/j.celrep.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 51.Stensmyr MC, et al. 2012. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357. ( 10.1016/j.cell.2012.09.046) [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Zhang K, Li Y, Su W, Hu K, Jin S. 2017. Enterococci mediate the oviposition preference of Drosophila melanogaster through sucrose catabolism. Sci. Rep. 7, 13420 ( 10.1038/s41598-017-13705-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph RM, Devineni AV, King IFG, Heberlein U. 2009. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl Acad. Sci. USA 106, 11 352–11 357. ( 10.1073/pnas.0901419106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. 2008. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683. ( 10.1126/science.1151842) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.