Abstract
Effective removal of 4-chloro-2-methylphenoxyacetic acid (MCPA), an emerging agrochemical contaminant in water with carcinogenic and mutagenic health effects has been reported using hydrothermally synthesized MIL-101(Cr) metal-organic framework (MOF). The properties of the MOF were ascertained using powdered X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, thermal gravimetric analysis (TGA), field emission scanning electron microscopy (FESEM) and surface area and porosimetry (SAP). The BET surface area and pore volume of the MOF were 1439 m2 g−1 and 0.77 cm3 g−1, respectively. Artificial neural network (ANN) model was significantly employed for the accurate prediction of the experimental adsorption capacity (qe) values with minimal error. A rapid removal of the pollutant (99%) was recorded within short time (approx. 25 min), and the reusability of the MOF (20 mg) was achieved up to six cycles with over 90% removal efficiency. The kinetics, isotherm and thermodynamics of the process were described by the pseudo-second-order, Freundlich and endothermic adsorption, respectively. The adsorption process is spontaneous based on the negative Gibbs free energy values. The significant correlation between the experimental findings and simulation results suggests the great potential of MIL-101(Cr) for the remediation of MCPA from water matrices.
Keywords: metal-organic framework, adsorption, MCPA, reusability, artificial neural network
1. Introduction
Over the last decades, there has been scientific detection and identification of new emerging pollutants that contaminate surface and groundwater resources [1]. These compounds and their metabolites are found to endanger human health as well as destroy aquatic animals [2]. Evidence from previous studies have shown that agrochemicals such as 4-chloro-2-methylphenoxyacetic acid (MCPA) have immensely contributed to water pollution through agricultural runoff, direct spray and leaching [3,4]. MCPA is among the most extensively used herbicides that have been applied to selectively control weeds in farmlands, roads and gardens [5,6]. It has a high water solubility of 825 mg l−1 with a field half-life of 31 days [7]. When excessively applied, their residues may remain in the soil, thereby making it easily available to runoff, and to be leached down to contaminate surface and groundwater [8]. Thus, it is easily transported from a point source to non-point sources over long distance [9]. Some of the physico-chemical properties of the MCPA are highlighted in table 1.
Table 1.
Some properties of the studied herbicide.
| common name | chemical name | molecular formula | structure | solubility (mg l−1) | pKa | log P |
|---|---|---|---|---|---|---|
| MCPA | 4-chloro-2-methylphenoxyacetic acid | C9H9ClO3 |  |
8.25 | 3.13 | 2.8 |
MCPA can easily bioaccumulate and be biomagnified in the tissues of plants and aquatic animals and cause serious health risk to humans [10]. The uncontrolled discharge of this pollutant has significantly contributed to the scarcity of clean water for consumption and other domestic use, particularly in developing countries with high population, agricultural dependent and poor living standard [11]. According to the World Health Organization (WHO), as of 2015, an estimate of 663 million people do not have access to clean water, and by 2050, one in every four persons will consume contaminated water in developing nations [12]. The United States Environmental Protection Agency (USEPA) have listed MCPA as priority pollutant with possible carcinogenic and mutagenic effects [13]. The maximum standard limit for MCPA in drinking water is set by the WHO and USEPA as 0.1 µg l−1 [14]. These shortfalls motivate scientists and researchers to search for practical methods for wastewater treatment.
Over the years, various remediation techniques such as coagulation, flocculation, membrane filtration, precipitation, ion exchange, chemical oxidation, biodegradation and adsorption have been applied to remove pollutants from water [15]. Of these methods, adsorption has been singled out to be the most practical and promising technique due to its low cost, simple operations, high selectivity, environmentally benign, convenient recycling and availability of various adsorbents materials [16]. Some adsorbents such as activated [17,18], biochar [19], clay minerals [8] and goethite [20] have been previously used for MCPA adsorption. However, the ideal adsorbent is expected to possess a relatively high surface area, large pore volume, good water and thermal stability under harsh condition [21]. Thus, the quest for finding the best adsorbent materials seems limitless.
Metal-organic frameworks (MOFs) have been substantial applied in different fields due to their peculiar properties for versatile application. They have been considered as one of the highly porous and advanced materials recently discovered [22,23]. MOFs have quickly attracted the attention of researchers for various applications such as CO2 capture, gas storage, sensing, catalysis, biomedical imaging and wastewater remediation [24,25]. They are highly porous, crystalline materials with ultra-high surface area, consisting of metal clusters and multifunctional organic linkers [25].
Among the several MOFs reported, the Materials Institute Lavoisier (MILs) class are exceptionally promising material that have been applied for the remediation of contaminants such as pharmaceuticals, dyes and heavy metals [26,27] in wastewaters. The MIL-101(Cr) is formed from a combination of chromium (III) oxide octahedral trimmers and dicarboxylate linker, resulting in a high class of hybrid supertetrahedron azeotypic mesoporous MOF [28]. The chemical stability of this MOF improves with increasing inertness of the central metal ions and the thermal stability can be explained by the strength of the metal oxygen bond. This is due to the remarkable inertness of Cr(III) that allows the formation of strong Cr-O bond [29]. This MOF is very rigid with large surface area and pore size. MIL-101(Cr) was recently applied by Mirsoleimani-Azizi et al. [30] for the removal of pesticide (diazinon) in an aqueous medium. High removal efficiency (92.5%) was achieved. The high adsorption capacity of dyes (377–392 mg g−1) was recorded by MIL-101(Cr) with a fast removal efficiency of 99.9% within 30 min [31]. Another study conducted by Gao et al. [32] shows rapid removal of pharmaceuticals by MIL-101(Cr) within 60 min. These applications outperformed most of the previous materials applied for the remediation of contaminants in water.
Adsorption process involves nonlinear relationships between several factors that are difficult to model using conventional methods. In previous studies, only one parameter is varied at a time without modelling the simultaneous interaction between the critical adsorption parameters (contact time, initial concentration, dosage, pH and temperature). Limited works have been carried out to model and predict the interactive adoption behaviour of MCPA onto MIL-101(Cr). Thus, this study introduces the artificial neural network (ANN) model to evaluate and model the adsorption process and interaction between the adsorption parameters. The ANN is used for the prediction of the experimental findings through learning the pattern of the process. ANN can be trained to develop a non-parametric relationship between multiple input parameters that control the adsorption process [33]. It is a model that mimics the human brain as such does not require prior knowledge of the process controlling the adsorption system. Thus, the aim of this study is to evaluate the removal efficiency of MIL-101(Cr) metal-organic framework for the adsorption of MCPA in an aqueous medium. Batch adsorption experiment has been used to study the effect of the parameters, kinetics and isotherm of the process. ANN was used to model and predict the nonlinear relationship of the adsorption process under the said experimental conditions.
2. Materials and methods
2.1. Materials
Chromium nitrate nonahydrate (Cr(NO3)3·9H2O, 99%), 1,4-benzene dicarboxylic acid (H2BDC, 99%), N,N-dimethyl formamide (DMF, 99%), methanol (99.5%), ethanol (99.9%) hydrochloric acid and sodium hydroxide were of analytical grade and supplied by Avantis Laboratory (Ipoh Perak, Malaysia) and were used without further purification. MCPA with 98% purity was purchased from Sigma-Aldrich (St Louis, MO, USA).
2.2. Synthesis of MIL-101(Cr) adsorbent
MIL-101(Cr) was hydrothermally synthesized according to the previous procedure [34] using Cr(NO3)3·9H2O (8 g), H2BDC (3.32 g) and deionized water (100 ml). The mixture was stirred for 30 min using a magnetic stirrer. HF (10 mmol) was gradually added to the mixture and stirred for 15 min. The mixture was placed in a stainless-steel Teflon-lined autoclave, sealed and inserted into a preheated electric oven at 483 K for 8 h. Next, the autoclave was allowed to cool to room temperature and the product was filtered and recovered. The as-synthesized product was further purified using deionized water, DMF and ethanol to remove possible impurities in the pores. The product yield reached as high as 89%. The purified product was finally dried overnight, cooled to room temperature and stored in a desiccator.
2.3. Characterization of MIL-101(Cr) adsorbent
The crystallinity and structural properties of the MIL-101(Cr) were recorded on a Bruker D8 Advance X-ray diffraction (XRD). The thermal stability of the adsorbent was assessed by thermogravimetric analysis (TGA) under N2 atmosphere using Shimadzu TGA-50 Analyser which was heated from 30 to 800°C at a heating rate of 10°C min−1. The functional group of the material was determined using Perkin Elmer FTIR Spectrometer which was scanned from 400 to 4000 cm−1. Field emission scanning electron microscopy (FESEM) was used to determine the morphology using Zeiss Supra 55 VP instrument, while the BET surface area and pore size were analysed using N2 adsorption–desorption with Micromeritics ASAP 2020.
2.4. Batch adsorption studies
Adsorption experiments were carried out by preparing a stock solution of MCPA (1000 mg l−1) by dissolving 100 mg of the analyte in a 1000 ml volumetric flask containing water and was kept in a refrigerator (0°C) prior to use. From the stock, solutions containing different initial concentrations (5–50 mg l−1) were studied by dispersing 20 mg of MIL-101(Cr) adsorbent in 100 ml conical flask. The total volume used for each experiment was 50 ml. The flask was then inserted into a thermostatic incubator shaker (incubator ES 20/60, Biosan) and agitated at 150 r.p.m. for 5–60 min. The sample solution (2 ml) was collected and filtered with a nylon syringe membrane (0.45 µm) at every 5 min interval. The absorbance of the MCPA solutions was measured with a UV-Vis spectrophotometer (Shimadzu, Lamda 25). The effects of pH and temperature were studied by adjusting the initial pH from 2 to 12 using either 0.1 M HCl or 0.1 M NaOH, while the temperature was varied from 25 to 50°C. The effect of dosage was also studied by varying the dose from 5 to 50 mg. All the adsorption data were recorded in triplicate from which the average values were calculated.
The quantity of MCPA adsorbed at equilibrium (qe), percentage removal (%R) and quantity adsorbed at a time interval (qt) were calculated using the following equations:
| 2.1 |
| 2.2 |
| 2.3 |
where Co, Ct and Ce are the initial, time and equilibrium concentration of MCPA (mg g−1), V is the volume of the solution (l) and w is the weight of the adsorbent (g).
2.5. Artificial neural network model
ANN mimics the behaviour of the human brain in processing information and can learn, predict and correlate the pattern of experimental data when subjected to training [35,36]. The technique provides a platform that can determine the impact of some optimized adsorption parameters in the behaviour of a target output. In this study, the multilayer-perceptron feed-forward-neural network (MLP-FF-ANN) with a back-propagation algorithm and log-sigmoid activation function [37] was used to predict the adsorption capacity of MCPA onto MIL-101(Cr) in correlation with the experimental result. The network structure of the MLP-FF-ANN consists of multiple neurons that are organized in layers. The number of hidden neurons was determined on the basis of trial and error, which forms the training process [38]. The dataset was divided into training (60%), testing (20%) and validation (20%). The network was trained by adjusting the weight to learn the data pattern, and the testing subset was used to evaluate the generalization ability of the network, while the validation datasets were used to estimate the network efficiency. Using these models, criteria such as coefficient of determination (R2), adjusted R2 (R2adj), root mean square error (RMSE) and Akaike information criteria (AIC) are considered as the best fit to judge the performance of our adsorption process by regression analysis. The following equations were used:
| 2.4 |
| 2.5 |
| 2.6 |
| 2.7 |
where xi represents the observed data that was determined experimentally, yi is the predicted data, n is the number of observation and p denotes the number of parameters.
2.6. Adsorption isotherms
Adsorption isotherms provide information on the type of interaction mechanism that exists between MIL-101(Cr) and the studied herbicide. It describes the amount of pollutants adsorbed per unit weight of an adsorbent and the residual pollutant concentration in solution at equilibrium. The Langmuir, Freundlich and Temkin isotherms were used to describe the adsorption of MCPA onto MIL-101(Cr). The models are described by the following equations [39].
Langmuir model
| 2.8 |
and
| 2.9 |
where Ce is the concentration at equilibrium (mg g−1), qe is the quantity of MCPA adsorbed at equilibrium (mg g−1), qm and KL are the constants representing adsorption capacity and adsorption energy, respectively. RL depicts the favourability of the adsorption process (RL > 1, unfavourable; 0 < RL < 1, favourable; RL = 1, linear).
Freundlich model
| 2.10 |
where KF is the Freundlich constant of adsorption capacity, n is the adsorption intensity and Ce is the equilibrium concentration of MCPA (mg g−1).
Temkin model
| 2.11 |
where B is the heat of adsorption (J mol−1) and AT is the Temkin equilibrium binding constant corresponding with the maximum binding energy (l g−1).
2.7. Adsorption kinetics model
The adsorption rate, reaction mechanism and equilibrium time are fundamental in determining the effectiveness and efficiency of the adsorbent material as well as the mass transfer which explains the rate-limiting steps [40]. The pseudo-first-order, pseudo-second-order and intraparticle diffusion model were used to ascertain the best fitting for the experimental data.
Lagergren pseudo-first-order model
| 2.12 |
Pseudo-second-order model
| 2.13 |
Intraparticle diffusion model
| 2.14 |
where qt and qe are the amounts of MCPA adsorbed at certain equilibrium and time t (mg g−1), K1 (min−1) is the pseudo-first-order rate constant, K2 (g mg−1 min−1) is the equilibrium rate constant of the pseudo-second-order, and the intraparticle diffusion rate constant is represented as Kp (mg g−1 min−1).
2.8. Thermodynamics studies
The Gibbs free energy change (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) were used to determine the feasibility of the adsorption process based on the temperature changes. This helps to describe whether the adsorption process is spontaneous, exothermic or endothermic. The equations are
| 2.15 |
and
| 2.16 |
where ΔG° is the free energy (J K mol−1), T (K) and R (J K mol−1) are the temperature and universal gas constant for the adsorption, respectively and KC is the equilibrium constant.
2.9. Reusability studies
The reusability of MIL-101(Cr) was studied to assess its potential for regeneration. After the adsorption experiments, the supernatant was decanted, filtered and washed with acetone and distilled water several times. The MOF was vacuum dried for 4 h at 80°C and reused as an adsorbent for the removal of MCPA in water. The process was repeated for six consecutive cycles and the removal of the herbicide was calculated for each cycle.
3. Results and discussion
3.1. Characterization of MIL-101(Cr)
The diffraction pattern of the obtained MIL-101(Cr) (figure 1a) indicates peaks that are in agreement with those reported in the previous studies [31,41], confirming a well-formed crystalline structure of the MIL-101(Cr). The FTIR spectra of the MOF is presented in figure 1b. The peak at 567 cm−1 is attributed to the Cr-O bond which depicts the formation of a well-structured material, and the bands at 746 and 1287 cm−1 are assigned to the C-H bond [42]. The peak at 1384 cm−1 depicts the symmetric vibration indicating the presence of dicarboxylate group in the MOF [43]. The peak at 1581 cm−1 denotes stretching vibration [44], and the strong-broad band at approximately 3433 cm−1 shows the presence of O-H group in the material [32]. TGA reveals the thermal stability of the MIL-101(Cr) adsorbent. Three stages of weight loss were observed in figure 1c. The first weight loss is found in the temperature range of 5–200°C. The second weight loss is at approximately 200–308°C, attributed to desorption of adsorbed guest molecules from the pores. The third weight loss (308–600°C) denotes complete decomposition of terephthalic acid in the framework. The MOF was completely decomposed at 800°C, in agreement with an earlier study [41]. The FESEM image of the MIL-101(Cr) (figure 1d) corresponds to an octahedral crystalline structure similar to the previously reported study [42]. The elemental composition at the surface of the MOF as shown in the EDX (figure 1d) contains chromium (38.4%), oxygen (38%) and carbon (23.6%). The surface area and pore size of the MIL-101(Cr) was determined by Brunauer–Emmett–Teller (BET) under N2 adsorption–desorption. The BET surface area of the MOF is approximately 1439 m2 g−1 as detailed in table 2.
Figure 1.
(a) XRD pattern, (b) FTIR spectrum, (c) TGA thermogram and (d) FESEM-EDX spectrum of MIL-101(Cr).
Table 2.
BET surface area of MIL-101(Cr) MOF.
| properties | MIL-101 (Cr) |
|---|---|
| BET surface area (m2 g−1) | 1439 |
| Langmuir surface area (m2 g−1) | 2124 |
| micropore surface area (m2 g−1) | 182 |
| pore size (nm) | 0.773 |
3.2. Artificial neural network prediction model
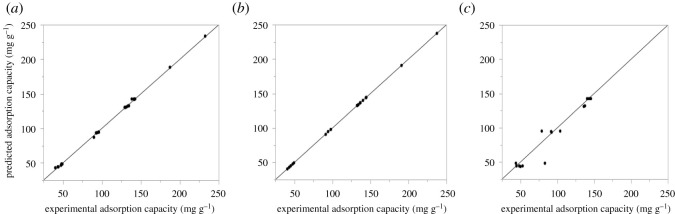
To develop the best ANN model for accurate prediction requires a careful selection and design of the network architecture, input combinations and model uncertainties [45]. A total of 264 experimental datasets obtained through the Design Expert 11 software were used to train, test and validate the ANN model. Several hidden neurons (table 3) were trained based on a trial and error approach to arrive at the best combination for the prediction of MCPA adsorption capacity, qe (mg g−1). The best ANN architecture comprising 5–8–1 topology (figure 2) was obtained during the training process. The input layer of the selected ANN topology comprises five parameters (contact time, initial concentration, adsorbent dosage, pH and temperature), the hidden layer has eight neurons and the output layer makes up one predicted response (adsorption capacity for MCPA, qe (mg g−1)). The 5–8–1 topology gave the most significant predicted values indicating a better correlation between the experimental and observed datasets with R2 0.998, 0.999, 0.981 and RMSE 0.088, 0.024, 0.066 for training, testing and validation, respectively (table 3). The scatter plots for the training, testing and validation subsets that show the correlation between the experimental and predicted values are presented in figure 3. The ANN model was also applied to predict the batch adsorption experimental qe values for each range of experimental conditions in table 4. The result shows a significant correlation between the experimental qe and the predicted qe values with minimal errors recorded. The predicted qe is in agreement with the pseudo-second-order kinetics (qe, experimental and qe, calculated), indicating the potential of ANN as a technique for predicting wastewater remediation. This can be attributed to the ability of the ANN to learn the complexity of a dataset when subjected to training and can also model the nonlinear relationship between the actual and predicted variables.
Table 3.
Optimum ANN architecture for the prediction of MCPA adsorption capacity.
| no. | neurons | training |
testing |
validation |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2adj | RMSE | R2 | R2adj | RMSE | R2 | R2adj | RMSE | ||
| 1 | [3] | 0.985 | 0.920 | 1.723 | 0.989 | 0.983 | 1.541 | 0.977 | 0.962 | 1.750 |
| 2 | [4] | 0.996 | 0.953 | 0.686 | 0.997 | 0.994 | 0.612 | 0.978 | 0.971 | 0.608 |
| 3 | [5] | 0.986 | 0.981 | 1.511 | 0.988 | 0.981 | 1.420 | 0.986 | 0.981 | 1.407 |
| 4 | [6] | 0.998 | 0.985 | 1.095 | 0.999 | 0.997 | 1.201 | 0.977 | 0.970 | 1.540 |
| 5 | [7] | 0.997 | 0.991 | 2.188 | 0.998 | 0.995 | 2.321 | 0.967 | 0.918 | 3.053 |
| 6 | [8] | 0.998 | 0.996 | 0.088 | 0.999 | 0.997 | 0.024 | 0.981 | 0.979 | 0.066 |
| 7 | [9] | 0.998 | 0.994 | 1.620 | 0.990 | 0.988 | 1.511 | 0.965 | 0.964 | 1.657 |
| 8 | [10] | 0.996 | 0.987 | 2.797 | 0.989 | 0.981 | 2.833 | 0.982 | 0.981 | 4.729 |
| 9 | [5 5] | 0.988 | 0.913 | 1.668 | 0.932 | 0.931 | 0.321 | 0.941 | 0.930 | 0.944 |
| 10 | [5 7] | 0.934 | 0.922 | 0.392 | 0.912 | 0.908 | 1.443 | 0.910 | 0.906 | 1.321 |
| 11 | [7 6] | 0.911 | 0.902 | 1.866 | 0.905 | 0.910 | 1.832 | 0.909 | 0.899 | 1.612 |
Figure 2.
Artificial neural network topology.
Figure 3.
Experimental and Predicted ANN scatter plots (a) training, (b) testing and (c) validation.
Table 4.
Comparison between the experimental and predicted MCPA adsorption capacity, qe (mg g−1).
| runs | contact time (min) | initial concentration (mg l−1) | adsorbent dosage (mg) | pH | temperature (°C) | experimental, qe (mg g−1) | predicted, qe (mg g−1) | error |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 20 | 20 | 4 | 30 | 93.620 | 91.878 | 1.742 |
| 2 | 5 | 10 | 30 | 2 | 35 | 44.388 | 44.208 | 0.180 |
| 3 | 15 | 20 | 40 | 4 | 30 | 94.585 | 94.516 | 0.429 |
| 4 | 15 | 20 | 20 | 8 | 30 | 92.173 | 92.610 | 0.437 |
| 5 | 25 | 40 | 20 | 4 | 30 | 188.577 | 188.445 | 0.132 |
| 6 | 25 | 10 | 10 | 2 | 35 | 48.379 | 49.236 | 0.857 |
| 7 | 15 | 20 | 20 | 4 | 45 | 94.585 | 95.661 | 1.076 |
| 8 | 5 | 30 | 10 | 2 | 35 | 130.441 | 131.085 | 0.644 |
| 9 | 5 | 30 | 10 | 6 | 25 | 132.634 | 133.108 | 0.474 |
| 10 | 5 | 30 | 30 | 2 | 25 | 130.441 | 133.754 | 3.313 |
| 11 | 5 | 10 | 30 | 6 | 35 | 44.388 | 43.845 | 0.543 |
| 12 | 25 | 10 | 10 | 6 | 35 | 48.379 | 48.833 | 0.454 |
| 13 | 25 | 30 | 30 | 6 | 35 | 142.568 | 141.699 | 0.869 |
| 14 | 25 | 30 | 10 | 2 | 35 | 141.910 | 141.187 | 0.723 |
| 15 | 5 | 10 | 10 | 2 | 25 | 44.388 | 44.110 | 0.278 |
| 16 | 5 | 30 | 10 | 6 | 35 | 130.441 | 130.853 | 0.412 |
| 17 | 35 | 20 | 20 | 4 | 30 | 95.243 | 93.853 | 1.390 |
| 18 | 5 | 30 | 30 | 6 | 35 | 130.441 | 131.781 | 1.340 |
| 19 | 5 | 30 | 30 | 6 | 25 | 132.634 | 133.854 | 1.220 |
| 20 | 25 | 10 | 30 | 2 | 25 | 48.379 | 50.190 | 1.811 |
| 21 | 25 | 30 | 10 | 6 | 35 | 142.568 | 141.418 | 1.150 |
| 22 | 15 | 20 | 20 | 10 | 30 | 93.620 | 92.955 | 0.665 |
| 23 | 25 | 10 | 30 | 6 | 25 | 48.379 | 49.095 | 0.716 |
| 24 | 25 | 10 | 30 | 6 | 35 | 48.379 | 48.697 | 0.318 |
| 25 | 45 | 20 | 20 | 4 | 30 | 94.585 | 96.674 | 2.089 |
| 26 | 25 | 20 | 10 | 4 | 40 | 95.243 | 94.028 | 1.557 |
| 27 | 5 | 10 | 10 | 6 | 25 | 44.651 | 43.242 | 1.409 |
| 28 | 5 | 10 | 30 | 6 | 25 | 43.028 | 41.792 | 1.236 |
| 29 | 25 | 30 | 30 | 2 | 35 | 142.568 | 141.741 | 0.827 |
| 30 | 5 | 30 | 10 | 2 | 25 | 131.213 | 133.267 | 2.054 |
| 31 | 25 | 30 | 30 | 2 | 25 | 141.910 | 142.031 | 0.121 |
| 32 | 5 | 10 | 10 | 2 | 35 | 44.388 | 44.869 | 0.481 |
| 33 | 5 | 30 | 30 | 2 | 35 | 132.013 | 131.321 | 0.692 |
| 34 | 25 | 30 | 10 | 6 | 25 | 142.568 | 141.679 | 0.889 |
| 35 | 5 | 10 | 30 | 2 | 25 | 43.537 | 42.995 | 0.542 |
| 36 | 25 | 10 | 10 | 6 | 25 | 48.379 | 48.379 | 0.000 |
| 37 | 15 | 20 | 50 | 4 | 30 | 93.620 | 93.620 | 0.000 |
| 38 | 15 | 20 | 20 | 4 | 30 | 93.620 | 93.620 | 0.000 |
| 39 | 25 | 50 | 10 | 4 | 40 | 233.577 | 233.576 | 0.001 |
| 40 | 5 | 10 | 10 | 6 | 35 | 44.218 | 44.542 | 0.324 |
| 41 | 15 | 10 | 10 | 2 | 25 | 46.537 | 46.537 | 0.000 |
| 42 | 25 | 30 | 30 | 6 | 25 | 142.568 | 142.180 | 0.388 |
| 43 | 25 | 30 | 10 | 2 | 25 | 142.568 | 142.568 | 0.000 |
| 44 | 25 | 20 | 10 | 4 | 35 | 48.379 | 48.379 | 0.000 |
3.3. Optimization studies of adsorption parameters
3.3.1. Effect of pH in the adsorption process
The initial pH condition of MCPA solution is a key parameter that determines the adsorption process and capacity. In this study, the effect of pH was investigated by varying the pH at the range of 2–12, herbicide concentration (20 mg l−1), dosage (20 mg) and temperature (40°C) as displayed in figure 4. At pH value higher than the pKa of MCPA (3.13), the herbicides solution will be predominantly in the anionic form (negatively charged) due to deprotonation. On the other hand, the surface of MIL-101(Cr) is positively charged at low pH (4–6) and negatively charged when the pH is high (>6). Hence, adsorption at high pH is very low due to the relatively weak interaction between the adsorbent and the herbicide molecule. Thus, a fast and higher removal of 98.62% is observed at low pH (4–6) because of the attraction of the negatively charged pollutant in the solution with a positive surface of the MOF adsorbent. Therefore, the mechanism of MCPA adsorption onto MIL-101(Cr) can be attributed to the electrostatic interaction. At neutral and alkaline pH (7–12), the surface of MIL-101(Cr) is negatively charged, leading to electrostatic repulsion with a gradual decrease in the adsorption capacity.
Figure 4.
Effect of solution pH for MCPA removal (dosage: 20 mg; concentration, 20 mg l−1; temperature, 40°C; equilibrium time, 25 min; r.p.m.: 150).
3.3.2. Effect of adsorbent dosage
The amount of adsorbent dose that is sufficient for the removal of pollutants in water can be determined by varying the loading. In this study, the dose of MIL-101(Cr) was varied from 5 to 50 mg in 50 ml solution containing 20 mg l−1 of MCPA at 40°C. High recovery efficiency (96.4%) was obtained even at the smallest dosage of 5 mg. As the adsorbent dose increases from 10 to 50 mg, the removal efficiency also increased to 98.8% due to the availability of vacant and active adsorption sites. Figure 5a shows that 20 mg is the optimum dose for the removal of MCPA in water. Thus, 20 mg of MIL-101(Cr) adsorbent was adopted for all the subsequent experiments.
Figure 5.
(a) Effect of adsorbent dose and (b) temperature on MCPA removal (concentration, 20 mg l−1; pH, 4; equilibrium time, 20 min; r.p.m.: 150).
3.3.3. Effect of temperature and thermodynamics
The temperature at which adsorption takes place plays an important role in the removal process. Thus, the effect of temperature (25–50°C) on the adsorption efficiency was investigated and the results are displayed in figure 5b. The positive correlation between temperature and adsorption efficiency implies that the removal of MCPA increases with an increase in temperature. This is because the increase in the temperature will reduce the viscosity of the solution, which allows easy mobility of the adsorbate molecules [46]. Also, the rise in temperature improves the surface activities and pore capacity of the adsorbent as well as the kinetic energy of the solution. Table 5 shows the thermodynamic parameters. The negative values of the Gibbs free energy (ΔG°) represent a spontaneous adsorption process. The adsorption is endothermic due to the positive enthalpy value.
Table 5.
Thermodynamic parameters for the adsorption of dicamba onto MIL-101(Cr).
| temp (°C) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) |
|---|---|---|---|
| 25 | −127.856 | 28.334 | 428.952 |
| 30 | −130.007 | ||
| 35 | −132.145 | ||
| 40 | −134.290 | ||
| 45 | −136.753 | ||
| 50 | −138.902 |
3.3.4. Adsorption isotherm
In this study, the equilibrium data for the removal of MCPA by MIL-101(Cr) were fitted using the Langmuir, Freundlich and Temkin isotherms. The data obtained from the fitted models are presented in table 6 and figure 6a–c). From the results calculated, the Freundlich isotherm model best fit the adsorption process based on the regression analysis with the highest R2 (0.999), R2adj (0.997); lowest RMSE (0.023); and the least AIC (–48.017) values. The Freundlich model implies an adsorption process on heterogeneous surfaces with binding sites that are not equivalent.
Table 6.
Isotherm parameters for adsorption onto MIL-101(Cr).
| isotherm model | parameters | MIL-101(Cr) MCPA |
|---|---|---|
| Langmuir | qm (mg g−1) | 370.37 |
| KL (l mg−1) | 0.45 | |
| RL | 0.1 | |
| R2 | 0.92 | |
| R2 adj | 0.899 | |
| RMSE | 0.001 | |
| AIC | −80.57 | |
| Freundlich | KF (mg g−1) | 7.524 |
| n | 1.444 | |
| R2 | 0.999 | |
| R2 adj | 0.997 | |
| RMSE | 0.023 | |
| AIC | −48.017 | |
| Temkin | A (mg g−1) | 8.253 |
| bT (kJ mol−1) | 62.105 | |
| R2 | 0.914 | |
| R2 adj | 0.893 | |
| RMSE | 26.597 | |
| AIC | 40.937 |
Figure 6.
(a) Langmuir, (b) Freundlich and (c) Temkin isotherm for the removal of MCPA (dosage: 20 mg; concentration, 20 mg l−1; temperature, 40°C; equilibrium time, 20 min; r.p.m.: 150).
3.3.5. Effect of contact time and adsorption kinetics
The impact of contact time in the removal of MCPA by MIL-101(Cr) using different concentrations (5–50 mg l−1), contact time (5–60 min), pH (pH 4), adsorbent dose and temperature conditions (pH 3, 20 mg and 40°C) is displayed in figure 7. The results obtained show an excellent and rapid removal of the herbicides within short time (5–10 min) due to the good interaction and porous nature of the MOF adsorbent. This is corroborated by the high BET surface area of the adsorbent (1439 m2 g−1). Hence, equilibrium was attained within 25 min. The contact time was extended until 60 min to ensure the better interactions of the MOF with the analyte after the equilibrium was established.
Figure 7.
Effect of contact time on MCPA removal (dosage: 20 mg; concentration, 5–50 mg l−1; temperature, 40°C; equilibrium time, 5–60 min; r.p.m.: 150).
To better understand the mechanism of adsorption such as a chemical reaction and mass transfer, the kinetics data were fitted using the pseudo-first-order, pseudo-second-order and intraparticle diffusion models. The kinetics results for the models are displayed in table 7 and figure 8a, which indicate that the pseudo-second-order best fit experimental data have the highest coefficient of determination (R2 = 0.998), R2adj = 0.996, lowest RMSE (0.005) and least AIC (–108.511). This is because the qe values calculated for the pseudo-second-order kinetic are in agreement with the experimental results. The maximum qe value for MCPA was determined as 233.576 mg g−1 at the equilibrium point, which represents the adsorption capacity of MIL-101(Cr). Thus, the result further explains that the adsorption process is controlled by chemical interaction. The intraparticle diffusion mechanism was also used to describe the interaction and the movement of the molecules inside the particles of the MOF adsorbent. Figure 8b indicates two major stages that represent an external diffusion of the pollutant to the surface of the adsorbent and the transport of the molecules from the surface inside the pore of the MOF. This process describes the rate-limiting step of the adsorption.
Table 7.
Adsorption kinetics parameters for the removal of MCPA.
| pseudo-first order | mg l−1 | qe,exp (mg g−1) | qe,cal (mg g−1) | K1 (min)−1 | R2 | R2adj | RMSE | AIC |
|---|---|---|---|---|---|---|---|---|
| 5 | 24.388 | 9.653 | 0.150 | 0.783 | 0.711 | 0.795 | −0.851 | |
| 10 | 48.379 | 23.203 | 0.170 | 0.735 | 0.647 | 0.802 | −0.767 | |
| 20 | 95.243 | 66.980 | 0.225 | 0.919 | 0.892 | 0.558 | −4.396 | |
| 30 | 142.568 | 84.208 | 0.224 | 0.821 | 0.761 | 0.749 | −1.499 | |
| 40 | 188.576 | 169.236 | 0.238 | 0.883 | 0.844 | 0.529 | −4.917 | |
| 50 | 233.576 | 170.187 | 0.253 | 0.833 | 0.824 | 0.644 | −2.959 |
| pseudo-second-order MCPA | qe,exp (mg g−1) | qe cal (g mg−1) | K2 (g mg−1 min−1) | R2 | R2adj | RMSE | AIC | |
|---|---|---|---|---|---|---|---|---|
| 5 | 24.388 | 24.390 | 0.135 | 0.996 | 0.995 | 0.053 | −71.372 | |
| 10 | 48.379 | 48.309 | 0.059 | 0.995 | 0.994 | 0.026 | −81.626 | |
| 20 | 95.243 | 96.153 | 0.019 | 0.998 | 0.996 | 0.005 | −108.511 | |
| 30 | 142.568 | 142.857 | 0.020 | 0.996 | 0.995 | 0.009 | −119.225 | |
| 40 | 188.576 | 188.679 | 0.010 | 0.994 | 0.993 | 0.007 | −126.536 | |
| 50 | 233.576 | 232.558 | 0.010 | 0994 | 0.993 | 0.006 | −131.463 |
| intraparticle diffusion MCPA |
Kp (mg−1 g−1 min1/2) C |
R2 | R2adj | RMSE | AIC | |||
|---|---|---|---|---|---|---|---|---|
| 5 | 2.006 | 8.658 | 0.573 | 0.431 | 7.904 | 22.120 | ||
| 10 | 3.956 | 17.01 | 0.575 | 0.433 | 15.536 | 28.877 | ||
| 20 | 8.110 | 30.922 | 0.632 | 0.509 | 28.263 | 34.861 | ||
| 30 | 11.756 | 50.189 | 0.578 | 0.438 | 45.828 | 39.695 | ||
| 40 | 16.026 | 58.755 | 0.650 | 0.533 | 53.666 | 41.274 | ||
| 50 | 19.538 | 79.755 | 0.600 | 0.466 | 72.833 | 44.328 | ||
Figure 8.
(a) Pseudo-second-order kinetics and (b) intraparticle diffusion model kinetics for MCPA removal (dosage: 20 mg; 40°C; equilibrium time: 25 min; r.p.m.: 150).
3.3.6. Reusability studies
The feasibility of MIL-101(Cr) towards the repeated removal of MCPA was studied in view of its regeneration possibility (figure 9). The MOF maintained a steady and high removal efficiency after the third cycle (approx. 98.6%) which indicates better removal capability when compared with the other materials in table 8. A slight decline in the percentage removal (3, 5, 9%) is noticed in the fourth, fifth and sixth cycles, respectively. Nevertheless, the MOF retains approximately 90% removal efficiency after the sixth cycle.
Figure 9.

Regeneration and reusability potential of MIL-101(Cr) adsorbent.
Table 8.
Adsorbents reported for the removal of MCPA from water.
| adsorbent | surface area, (m2 g−1) | concentrations (mg l−1) | (%) R | Qe (mg g−1) | equilibrium time (min) | reuse | ref. |
|---|---|---|---|---|---|---|---|
| activated | 592 | 50 | 70 | 417 | 210 | — | [47] |
| carbon | |||||||
| bentonite | 20 | 1 | 80–95 | 1440 | — | [48] | |
| biochar | 1.1 | 100 | 90 | 28 | 360 | — | [49] |
| metal | nil | 50 | 84 | 42 | 240 | nil | [50] |
| hydroxide | |||||||
| NH2@COF | 335.7 | 97.3 | 11 | [51] | |||
| vinylCOF | 345.4 | 94.3 | 2 | ||||
| MIL-101(Cr) | 1439 | 50 | 98.62 | 234 | 20 | 5 | this work |
3.3.7. Comparison of different adsorbents for the removal of 4-chloro-2-methylphenoxyacetic acid
Different adsorbents that have been applied for the removal of MCPA in water are summarized in table 8. The superiority of the MIL-101(Cr) adsorbent is readily seen, especially in terms of high surface area, adsorption capacity, % removal efficiency (98.6%), fast equilibration time (approx. 25 min) and prospects for regeneration (approx. 90%) after the sixth cycle.
3.3.8. Possible adsorption mechanisms
Electrostatic interaction is an important mechanism that determines the adsorptive removal of contaminants in water. The positively charged surface of the MOF can easily interact with the negatively charged adsorbate molecules. The solution pH determines the net surface charge of the adsorbent. The high adsorption capacity of MCPA onto MIL-101(Cr) at low pH is attributed to the electrostatic attraction between the MCPA anions and the positively charged surface of the MOF. As the pH increases, the surface charge gradually decreases causing electrostatic repulsion, thus retarding the adsorption process. The fast and feasible adsorption is also attributed to the chemisorption process which is in agreement with the pseudo-second-order kinetics model. The better fitting with the Freundlich isotherm describes a heterogeneous adsorption surface and an exponential distribution of active sites and energies for better adsorption of MCPA onto the MOF.
4. Conclusion
An in-depth assessment of the removal of MCPA using the adsorbent MIL-101(Cr) was conducted by batch experiments. The experimental qe values for each range of experimental conditions were also predicted by the ANN model with significant correlations and minimal errors. Fast adsorption equilibrium was reached within approximately 25 min using a small dose of the adsorbent material (20 mg). The adsorption process follows the pseudo-second-order kinetics model (R2 > 0.998, RMSE 0.005). The maximum qe value of the model is 233.576 mg g−1. The intraparticle diffusion model indicated a fast phase, signifying an external diffusion of the MCPA molecules from the solution to the surface of the MOF as the rate-determining step, and the slower phase was followed signifying the adsorption of the MCPA molecules to the internal pores of the MOF, until equilibrium is attained. The adsorption process best fit the Freundlich isotherm (R2, 0.999; RMSE, 0.023 and AIC, –48.017). In comparison with other previously reported adsorbent materials, MIL-101(Cr) performed well in the removal of MCPA in terms of fast equilibration, removal efficiency, high adsorption capacity and reusability. It is worth noting that the adsorption process and dataset used for the ANN prediction were based on controlled laboratory conditions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
This article does not present research with ethical considerations.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
B.S. designed the work and proofread the manuscript; H.A.I. and Z.U.Z. carried out the experiment and statistical modelling; K.J., N.S.S. and A.M. co-supervised the project and made a valid contribution to the manuscript.
Competing interests
The authors declare no competing interest.
Funding
This work was funded by the Universiti Teknologi PETRONAS under the YUTP research grant with cost centre 015LCO-211 and UTM CRG Collaborative Research grant no. 015MD0 044.
References
- 1.Starling MCVM, Amorim CC, Leão MMD. 2019. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 372, 17–36. ( 10.1016/j.jhazmat.2018.04.043) [DOI] [PubMed] [Google Scholar]
- 2.Sophia AC, Lima EC. 2018. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 150, 1–17. ( 10.1016/j.ecoenv.2017.12.026) [DOI] [PubMed] [Google Scholar]
- 3.Le TDH, Scharmüller A, Kattwinkel M, Kühne R, Schüürmann G, Schäfer RB.. 2017. Contribution of waste water treatment plants to pesticide toxicity in agriculture catchments. Ecotoxicol. Environ. Saf. 145, 135–141. ( 10.1016/j.ecoenv.2017.07.027) [DOI] [PubMed] [Google Scholar]
- 4.Kelly J, Morrison G, Skillen N, Manesiotis P, Robertson PKJ. 2019. An investigation of the role of pH in the rapid photocatalytic degradation of MCPA and its primary intermediate by low-power UV LED irradiation. Chem. Eng. J. 359, 112–118. ( 10.1016/j.cej.2018.11.142) [DOI] [Google Scholar]
- 5.Turek M, Pawłowska B, Różycka-Sokołowska E, Biczak R, Skalik J, Owsianik K, Marciniak B, Bałczewski P. 2020. Ecotoxicity of ammonium chlorophenoxyacetate derivatives towards aquatic organisms: unexpected enhanced toxicity upon oxygen by sulfur replacement. J. Hazard. Mater. 382, 121086 ( 10.1016/j.jhazmat.2019.121086) [DOI] [PubMed] [Google Scholar]
- 6.Muter O, Berzins A, Strikauska S, Pugajeva I, Bartkevics V, Dobele G, Truu J, Truu M, Steiner C. 2014. The effects of woodchip- and straw-derived biochars on the persistence of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) in soils. Ecotoxicol. Environ. Saf. 109, 93–100. ( 10.1016/j.ecoenv.2014.08.012) [DOI] [PubMed] [Google Scholar]
- 7.López-piñeiro A, Peña D, Albarrán A, Sánchez-llerena J, Becerra D. 2013. Behavior of MCPA in four intensive cropping soils amended with fresh, composted, and aged olive mill waste. J. Contam. Hydrol. 152, 137–146. ( 10.1016/j.jconhyd.2013.07.003) [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Yun Y, Jiang L, Wu C. 2018. Influence of dissolved organic matter on sorption and desorption of MCPA in ferralsol. Sci. Total Environ . 616–617, 1449–1456. ( 10.1016/j.scitotenv.2017.10.169) [DOI] [PubMed] [Google Scholar]
- 9.Awad AM, Shaikh SMR, Jalab R, Gulied MH, Nasser MS, Benamor A, Adham S. 2019. Adsorption of organic pollutants by natural and modified clays: a comprehensive review. Sep. Purif. Technol. 228, 115719 ( 10.1016/j.seppur.2019.115719) [DOI] [Google Scholar]
- 10.Wei C, Feng D, Xia Y. 2016. Fast adsorption and removal of 2-methyl-4-chlorophenoxy acetic acid from aqueous solution with amine functionalized zirconium metal-organic framework. RSC Adv. 6, 96 339–96 346. ( 10.1039/c6ra18520g) [DOI] [Google Scholar]
- 11.Solís RR, Rivas FJ, Martínez-Piernas A, Agüera A. 2016. Ozonation, photocatalysis and photocatalytic ozonation of diuron: intermediates identification. Chem. Eng. J. 292, 72–81. ( 10.1016/j.cej.2016.02.005) [DOI] [Google Scholar]
- 12.World Health Organization. 2015. Water sanitation hygiene, water supply and sanitation monitoring and evidence, key facts from JMP 2015 report. See https://www.who.int/water_sanitation_health/monitoring/jmp-2015-key-facts/en/. [Google Scholar]
- 13.Spaltro A, Pila M, Simonetti S, Álvarez-Torrellas S, Rodríguez JG, Ruiz D, Compañy AD, Juan A, Allegretti P. 2018. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: experimental and computational studies. J. Contam. Hydrol. 218, 84–93. ( 10.1016/j.jconhyd.2018.10.003) [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Cao M, Wan Y, Wang H, Liu J, Pan F, He W, Huang H, He Z. 2020. Spatial variation of 2,4-D and MCPA in tap water and groundwater from China and their fate in source, treated, and tap water from Wuhan, Central China. Sci. Total Environ. 727, 138691 ( 10.1016/j.scitotenv.2020.138691) [DOI] [PubMed] [Google Scholar]
- 15.Mantasha I, Saleh HAM, Qasem KMA, Shahid M, Mehtab M, Ahmad M. 2020. Efficient and selective adsorption and separation of methylene blue (MB) from mixture of dyes in aqueous environment employing a Cu(II) based metal organic framework. Inorganica Chim. Acta 511, 119787 ( 10.1016/j.ica.2020.119787) [DOI] [Google Scholar]
- 16.Anastopoulos I, Pashalidis I, Orfanos AG, Manariotis ID, Tatarchuk T, Sellaoui L, Bonilla-Petriciolet A, Mittal A, Núñez-Delgado A. 2020. Removal of caffeine, nicotine and amoxicillin from (waste)waters by various adsorbents. A review. J. Environ. Manage. 261, 131–139. ( 10.1016/j.jenvman.2020.110236) [DOI] [PubMed] [Google Scholar]
- 17.Derylo-marczewska A, Blachnio M, Marczewski AW, Swiatkowski A. 2017. Adsorption of chlorophenoxy pesticides on activated carbon with gradually removed external particle layers. Chem. Eng. J. 308, 408–418. ( 10.1016/j.cej.2016.09.082) [DOI] [Google Scholar]
- 18.Loklindt N, Nikbakht M, Krisztian P, Mu J. 2019. Synergy of combined adsorption and electrochemical degradation of aqueous organics by granular activated carbon particulate electrodes. Sep. Purif. Technol. 208, 51–58. ( 10.1016/j.seppur.2018.05.023) [DOI] [Google Scholar]
- 19.Cederlund H, Börjesson E, Lundberg D, Stenström J. 2016. Adsorption of pesticides with different chemical properties to a wood biochar treated with heat and iron. Water Air Soil Pollut. 203, 1–12. ( 10.1007/s11270-016-2894-z) [DOI] [Google Scholar]
- 20.Iglesias A, López R, Gondar D, Antelo J, Fiol S, Arce F. 2010. Adsorption of MCPA on goethite and humic acid-coated goethite. J. Hazard. Mater. 183, 664–668. ( 10.1016/j.jhazmat.2010.07.077) [DOI] [PubMed] [Google Scholar]
- 21.Gusain R, Kumar N, Ray SS. 2020. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 405, 213111 ( 10.1016/j.ccr.2019.213111) [DOI] [Google Scholar]
- 22.Liu X, Valentine HL, Pan W, Cao Y, Yan B. 2016. 2D metal–organic frameworks: syntheses, structures, and electrochemical properties. Inorganica Chim. Acta 447, 162–167. ( 10.1016/j.ica.2016.03.048) [DOI] [Google Scholar]
- 23.Batten SR, Champness NR. 2017. Coordination polymers and metal-organic frameworks: materials by design. Phil. Trans. R. Soc. A 375, 20160032 ( 10.1098/rsta.2016.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaka S, Kumar R, Deep A, Kurade MB, Ji SW, Jeon BH. 2019. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 380, 330–352. ( 10.1016/j.ccr.2018.10.003) [DOI] [Google Scholar]
- 25.Dunne LJ, Manos G. 2018. Statistical mechanics of binary mixture adsorption in metal-organic frameworks in the osmotic ensemble. Phil. Trans. R. Soc. A 376, 20170151 ( 10.1098/rsta.2017.0151) [DOI] [PubMed] [Google Scholar]
- 26.Samokhvalov A 2018. Aluminum metal–organic frameworks for sorption in solution: a review. Coord. Chem. Rev. 374, 236–253. ( 10.1016/j.ccr.2018.06.011) [DOI] [Google Scholar]
- 27.Opeyemi K 2020. Temperature-controlled activation and characterization of iron-based metal-organic frameworks. Inorganica Chim. Acta 507, 119563 ( 10.1016/j.ica.2020.119563) [DOI] [Google Scholar]
- 28.Maksimchuk NV, Zalomaeva OV, Skobelev IY, Kovalenko KA, Fedin VP, Kholdeeva OA. 2012. Metal-organic frameworks of the MIL-101 family as heterogeneous single-site catalysts. Proc. R. Soc. A 468, 2017–2034. ( 10.1098/rspa.2012.0072) [DOI] [Google Scholar]
- 29.Kang IJ, Khan NA, Haque E, Hwa S. 2011. Chemical and thermal stability of isotypic metal –organic frameworks: effect of metal ions. Eur. J. 4, 6437–6442. ( 10.1002/chem.201100316) [DOI] [PubMed] [Google Scholar]
- 30.Mirsoleimani-Azizi SM, Setoodeh P, Samimi F, Shadmehr J, Hamedi N, Rahimpour MR. 2018. Diazinon removal from aqueous media by mesoporous MIL-101(Cr) in a continuous fixed-bed system. J. Environ. Chem. Eng. 6, 4653–4664. ( 10.1016/j.jece.2018.06.067) [DOI] [Google Scholar]
- 31.Karmakar S, Roy D, Janiak C, De S.. 2019. Insights into multi-component adsorption of reactive dyes on MIL-101-Cr metal organic framework: experimental and modeling approach. Sep. Purif. Technol. 215, 259–275. ( 10.1016/j.seppur.2019.01.013) [DOI] [Google Scholar]
- 32.Gao Y, Liu K, Kang R, Xia J, Yu G, Deng S. 2018. A comparative study of rigid and flexible MOFs for the adsorption of pharmaceuticals: kinetics, isotherms and mechanisms. J. Hazard. Mater. 359, 248–257. ( 10.1016/j.jhazmat.2018.07.054) [DOI] [PubMed] [Google Scholar]
- 33.Gadekar MR, Ahammed MM. 2019. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manage. 231, 241–248. ( 10.1016/j.jenvman.2018.10.017) [DOI] [PubMed] [Google Scholar]
- 34.Férey G., Mellot-Draznieks C., Serre C., Millange F., Dutour J., Surblé S., Margiolaki I.. 2005. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042. ( 10.1126/science.1117808) [DOI] [PubMed] [Google Scholar]
- 35.Altowayti WAH, Algaifi HA, Bakar SA, Shahir S. 2019. The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: characteristics and modelling studies. Ecotoxicol. Environ. Saf. 172, 176–185. ( 10.1016/j.ecoenv.2019.01.067) [DOI] [PubMed] [Google Scholar]
- 36.Isiyaka HA, Juahir H, Phil- P. 2019. Water quality modelling using artificial neural network and multivariate statistical techniques. Model Earth Sys. Environ. 5, 583–593. ( 10.1007/s40808-018-0551-9) [DOI] [Google Scholar]
- 37.Banerjee P, Sau S, Das P, Mukhopadhayay A. 2015. Optimization and modelling of synthetic azo dye wastewater treatment using graphene oxide nanoplatelets: characterization toxicity evaluation and optimization using artificial neural network. Ecotoxicol. Environ. Saf. 119, 47–57. ( 10.1016/j.ecoenv.2015.04.022) [DOI] [PubMed] [Google Scholar]
- 38.Yusuf M, Song K, Li L. 2020. Fixed bed column and artificial neural network model to predict heavy metals adsorption dynamic on surfactant decorated graphene. Colloids Surfaces A 585, 225–240. ( 10.1016/j.colsurfa.2019.124076) [DOI] [Google Scholar]
- 39.Zango ZU, Jumbri K, Sambudi NS, Hanif Abu Bakar NH, Fathihah Abdullah NA, Basheer C, Saad B. 2019. Removal of anthracene in water by MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe) metal-organic frameworks. RSC Adv. 9, 41 490–41 501. ( 10.1039/c9ra08660a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgado N, Capparelli A, Navarro A, Marino D. 2019. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manage. 236, 301–308. ( 10.1016/j.jenvman.2019.01.116) [DOI] [PubMed] [Google Scholar]
- 41.Jafar S, Sedigheh Z, Maryam T. 2019. Synthesis of a chromium terephthalate metal organic framework and use as nanoporous adsorbent for removal of diazinon organophosphorus insecticide from aqueous media. J. Dispers. Sci. Technol. 40, 1–18. ( 10.1080/01932691.2018.1516149) [DOI] [Google Scholar]
- 42.Masood SA, Marzieh Shafiei-Alavijeh NHMHT, Ebrahim G, Alimorad RSF. 2019. Facile and high-yield synthesis of improved MIL-101 (Cr) metal-organic framework with exceptional CO2 and H2S uptake; the impact of excess ligand-cluster. Microporous Mesoporous Mater. 279, 153–164. ( 10.1016/j.micromeso.2018.12.033) [DOI] [Google Scholar]
- 43.Niknam E, Panahi F, Daneshgar F, Bahrami F. 2018. Metal–organic framework MIL-101(Cr) as an efficient heterogeneous catalyst for clean synthesis of benzoazoles. ACS Omega 3, 17 135–17 144. ( 10.1021/acsomega.8b02309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Ning L, Zheng S, Tao M, Shi Y, He Y. 2013. Adsorption of carbon dioxide by MIL-101 (Cr): regeneration conditions. Sci. Rep. 2, 1–6. ( 10.1038/srep02916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uddin Z, Yao L, Lian Q, Islam F, Zappi ME, Dianchen D. 2020. The use of artificial neural network (ANN) for modeling adsorption of sunset yellow onto neodymium modified ordered mesoporous carbon. Chemosphere 256, 127081 ( 10.1016/j.chemosphere.2020.127081) [DOI] [PubMed] [Google Scholar]
- 46.Shahnaz T, Sharma V, Subbiah S, Narayanasamy S. 2020. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 36, 101283 ( 10.1016/j.jwpe.2020.101283) [DOI] [Google Scholar]
- 47.Pandiarajan A, Kamaraj R, Vasudevan S. 2018. OPAC (orange peel activated carbon) derived from waste orange peel for the adsorption of chlorophenoxyacetic acid herbicides from water: adsorption isotherm, kinetic modelling and thermodynamic studies. Bioresour. Technol. 261, 329–341. ( 10.1016/j.biortech.2018.04.005) [DOI] [PubMed] [Google Scholar]
- 48.Durán E, Bueno S, Hermosín MC, Cox L, Gámiz B. 2019. Optimizing a low added value bentonite as adsorbent material to remove pesticides from water. Sci. Total Environ. 672, 743–751. ( 10.1016/j.scitotenv.2019.04.014) [DOI] [PubMed] [Google Scholar]
- 49.Essandoh M, Wolgemuth D, Pittman CU, Mohan D, Mlsna T. 2017. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 174, 49–57. ( 10.1016/j.chemosphere.2017.01.105) [DOI] [PubMed] [Google Scholar]
- 50.Kamaraj R, Davidson DJ, Sozhan G, Vasudevan S. 2014. An in situ electrosynthesis of metal hydroxides and their application for adsorption of 4-chloro-2-methylphenoxyacetic acid (MCPA) from aqueous solution. J. Environ. Chem. Eng. 2, 2068–2077. ( 10.1016/j.jece.2014.08.027) [DOI] [Google Scholar]
- 51.Ji WH, Guo YS, Wang X, Lu XF, Guo DS. 2019. Amino-modified covalent organic framework as solid phase extraction absorbent for determination of carboxylic acid pesticides in environmental water samples. J. Chromatogr. A 1595, 11–18. ( 10.1016/j.chroma.2019.02.048) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.