Abstract
An association between a novel pediatric hyperinflammatory condition and SARS-CoV-2 was recently published and termed pediatric inflammatory multisystem syndrome, temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome (in children) (MIS(-C)). We performed a systematic review and describe the epidemiological, clinical, and prognostic characteristics of 953 PIMS-TS/MIS(-C) cases in 68 records. Additionally, we studied the sensitivity of different case definitions that are currently applied. PIMS-TS/MIS(-C) presents at a median age of 8 years. Epidemiological enrichment for males (58.9%) and ethnic minorities (37.0% Black) is present. Apart from obesity (25.3%), comorbidities are rare. PIMS-TS/MIS(-C) is characterized by fever (99.4%), gastrointestinal (85.6%) and cardiocirculatory manifestations (79.3%), and increased inflammatory biomarkers. Nevertheless, 50.3% present respiratory symptoms as well. Over half of patients (56.3%) present with shock. The majority of the patients (73.3%) need intensive care treatment, including extracorporal membrane oxygenation (ECMO) in 3.8%. Despite severe disease, mortality is rather low (1.9%). Of the currently used case definitions, the WHO definition is preferred, as it is more precise, while encompassing most cases.
Conclusion: PIMS-TS/MIS(-C) is a severe, heterogeneous disease with epidemiological enrichment for males, adolescents, and racial and ethnic minorities. However, mortality rate is low and short-term outcome favorable. Long-term follow-up of chronic complications and additional clinical research to elucidate the underlying pathogenesis is crucial.
|
What is Known: • A novel pediatric inflammatory syndrome with multisystem involvement has been described in association with SARS-CoV-2. • To date, the scattered reporting of cases and use of different case definitions provides insufficient insight in the full clinical spectrum, epidemiological and immunological features, and prognosis. | |
|
What is New: • This systematic review illustrates the heterogeneous spectrum of PIMS-TS/MIS(-C) and its epidemiological enrichment for males, adolescents, and racial and ethnic minorities. • Despite its severe presentation, overall short-term outcome is good. • The WHO MIS definition is preferred, as it is more precise, while encompassing most cases. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-021-03993-5.
Keywords: PIMS-TS, MIS-C, COVID-19, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), led to a pandemic health crisis within a few months’ time [1–3]. Severe COVID-19 and associated mortality has been highest in elderly and patients with comorbidities, such as cardiovascular disease, diabetes mellitus, and chronic lung disease [4–6]. Since the outbreak, COVID-19 was generally described as asymptomatic or mild in children, causing few pediatric hospitalizations and minimal mortality [7–10].
Since April 2020, several countries from Europe and North America reported on young patients with a severe multisystem inflammatory syndrome associated with SARS-CoV-2. The initial descriptions exposed important clinical heterogeneity, partially overlapping with features of Kawasaki disease (KD) or toxic shock syndrome (TSS), but nevertheless distinct from these known inflammatory conditions [11, 12]. In contrast with (acute) COVID-19 respiratory disease, a significant proportion of children were reported with severe or fatal disease [11, 13–17]. Since its description, this novel disease is mostly referred to as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS) [18, 19] or multisystem inflammatory syndrome in children (MIS(-C)) [18, 19].
At present, it is pivotal to optimize the characterization and the diagnostic criteria of this inflammatory syndrome related to COVID-19. To date, the scattered case reporting provides insufficient insight in the full clinical, epidemiological, immunological, and prognostic spectrum. Hence, we performed a systematic review, the most extensive to date to our knowledge, to describe the diagnostic criteria and clinical manifestations of this novel pediatric COVID-19-associated phenotype.
Methods
Original studies describing cases meeting the definition of PIMS-TS or MIS(-C) by the Royal College of Paediatrics and Child Health (RCPCH) [20], World Health Organization (WHO) [19], or Centers for Disease Control and Prevention (CDC) [18], were eligible for inclusion (Supplementary information 1). Primary outcome analysis focused on epidemiological, clinical, and outcome parameters.
A search strategy was designed with keywords combining the pediatric population, COVID-19, and hyperinflammatory presentations (Table 1), including articles published from December 31, 2019, to August 13, 2020. Electronic databases were searched (PubMed, Embase), including pre-print (bioRxiv, medRxiv) and COVID-19-specific repositories (Cochrane COVID-19 Study Register and WHO COVID-19 Global Research Database). The reference lists of included studies were considered additional sources.
Table 1.
Search strategy criteria
|
Inclusion criteria 1 Study population: hyperinflammatory syndrome meeting the case definitions of PIMS-TS [19] or MIS(-C) [20, 21] in children (0–21 years of age) with a temporal association with confirmed or probable COVID-19 2 Outcome: clinical, epidemiological, and immunological descriptions; therapeutic management and clinical effect; and prognosis of individuals or cohorts of patients. 3 Types of study designs: RCT, observational studies, case-control studies, cross-sectional studies, case reports, and case series Exclusion criteria 1 Studies on adult patients with SARS-CoV-2 infection and/or SARS-CoV-2 associated hyperinflammatory syndromes 2 Studies on pediatric patients with other coronavirus infections (SARS-CoV-1 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection or other respiratory infections. 3 Studies with incomplete or lacking necessary data 4 Duplicate studies 5 Studies without accessible full-text versions 6 Studies not in English language |
After duplicate removal, two reviewers (LH/RVP) independently applied the inclusion and exclusion criteria, first, by screening titles and abstracts and, second, by examining full texts. LH extracted data using a standardized form, while RVP cross-checked for correctness and completeness. Any disagreement was resolved by FH. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist guided study selection and extraction. Risk for bias [21] and levels of evidence [22] were assessed (LH) with verification (RVP). Prior to conducting the review, the protocol was published (PROSPERO CRD42020189248). Data was analyzed with R v3.6.3 (Supplementary information 4).
Cohort studies and studies reporting single-case data were analyzed separately. To report on most variables, we used the sum of cases of only the records reporting on the variable. As such, the denominator in the proportions varies depending on the publications reporting on a given variable. Rare conditions (e.g., death), however, were calculated on the total group of cases. “Severe course” was defined as the presence of one or more of following conditions: coronary dilatation/aneurysm, shock, death, need for mechanical ventilation, extracorporeal membrane oxygenation (ECMO), renal replacement therapy, inotropes, or PICU admission. Data was extracted from pre-print publications for 4 records [23–26]. During the process of conducting this systematic review, 3 of these manuscripts [27–29] were published in peer-reviewed literature. The data extracted in this review was left unchanged and is thus based on the pre-print publications.
Results
Study characteristics
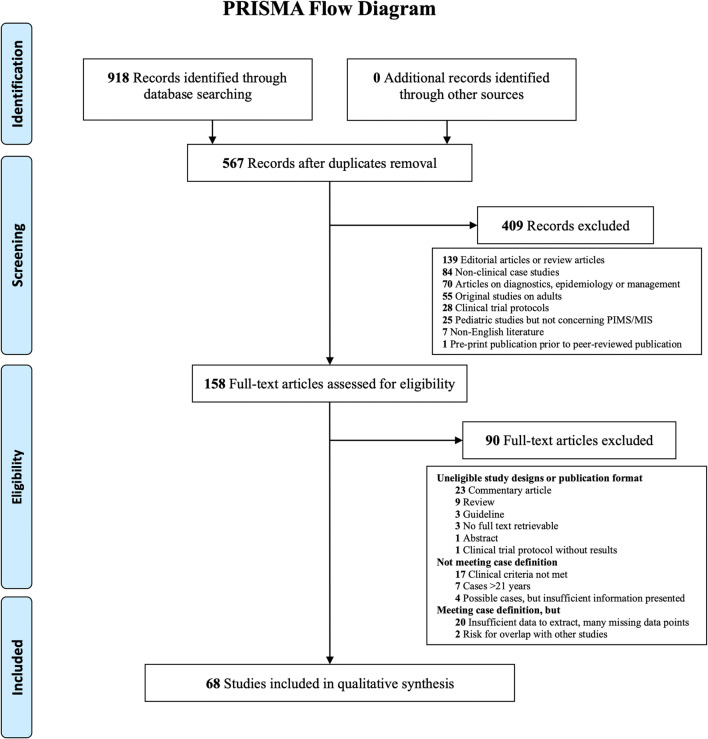
The search strategy yielded 918 records. After removing duplicates, 567 unique publications were screened on title and abstract of which 409 were excluded, mostly because it concerned editorial or review articles (n = 139), non-clinical case studies (n = 84), or articles on PIMS-TS/MIS(-C) diagnostics, epidemiology, or management (n = 70). One hundred and fifty-eight full-text articles were assessed for eligibility. Finally, 68 studies were included (Fig. 1). In general, risk of bias was low (Supplementary information 2), despite short follow-up in all studies.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram
All studies were published after May 9, 2020, and presented observational data from single case reports [24, 30–57] or case series (2–186 cases per publication) [11–17, 23, 25, 26, 58–86] (Fig. 2 - Supplementary 3). Four manuscript published on pre-print servers were included [23–26]. Most studies were non-controlled, although three publications used historical KD [15, 64, 81], MAS [81], or TSS [15] cohorts as a reference population. Limited studies prospectively included control cohorts of non-PIMS-TS/MIS(-C) pediatric COVID-19 [23, 26, 83], KD [26], (adult) COVID-19-associated acute respiratory distress syndrome (ARDS) [25], or convalescent plasma donors [25]. Studies were mostly conducted in the USA (n = 28), the UK (n = 10), or France (n = 6) and India (n = 6).
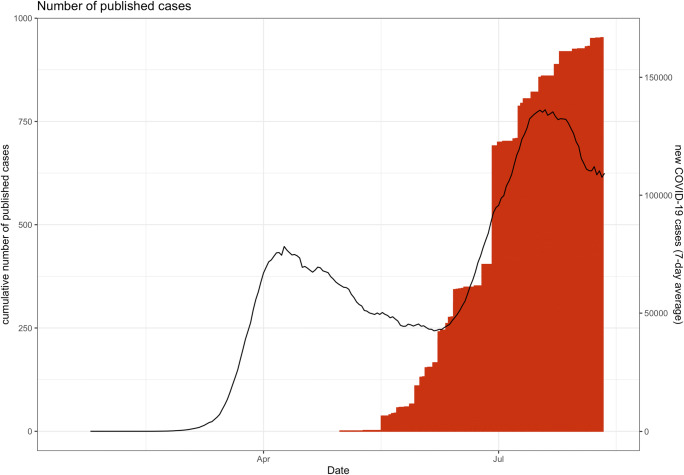
Fig. 2.
Cumulative number of published PIMS-TS/MIS(-C) cases (bars) in relation to total worldwide reported COVID-19 cases, according to data from Johns Hopkins Coronavirus Resource Center (lines; 7-day rolling average)
Demographics
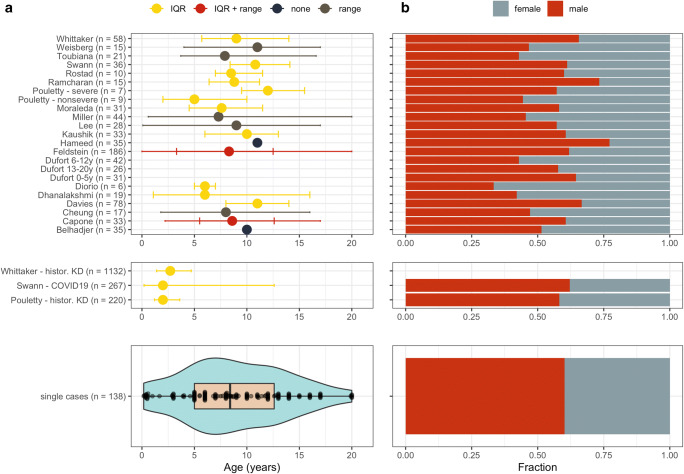
In total, 953 patients with PIMS-TS/MIS(-C) were reported, with individual patient information (single-case data) available for 138 patients (14.5%). Fifty-five patients (5.8%) were reported in duplicate, although the corresponding manuscripts [13, 16, 17, 78, 83] did not provide sufficient information to filter for unique data.
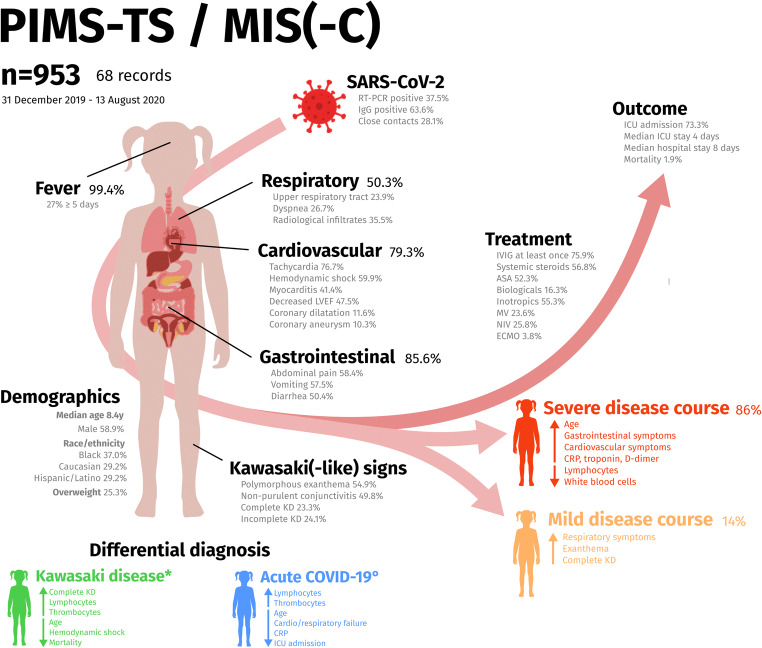
Among single cases, a median age of 8.4 years (IQR 5–12.6) was found (Fig. 3a), corresponding with a median age of at least 8 years noted in 14/20 cohorts (586/716 cohort patients) [13, 14, 16, 17, 23, 25, 26, 59, 63–65, 78, 81, 86]. Remarkably, age was substantially higher compared to non-COVID-19 KD cases (median age 2.0–2.7y) [15, 64] or non-PIMS-TS/MIS(-C) pediatric COVID-19 (median age 2.0 years) [23]. Additionally, a male predominance (561/953; 58.9%; Fig. 3b) was found, comparable to historic KD groups [64] and non-PIMS-TS/MIS(-C) pediatric COVID-19 [23].
Fig. 3.
Demographic characteristics of all included studies. a Age distribution. b Fraction of males and females in each study. IQR, interquartile range. “Control” corresponds to the control populations with Kawasaki disease as described by Pouletty et al. [64] and Whittaker et al. [15] and non-PIMS-TS/MIS(-C) pediatric COVID-19 by Swann et al. [23]. Data from Swann et al. [23], Rostad et al. [26], and Weisberg et al. [25] was extracted from pre-print publications and these references and have subsequently been published in the peer-reviewed literature [27–29]
PIMS-TS/MIS(-C) cases were frequent Black (240/647; 37.0%), followed by patients of Caucasian (189/647; 29.2%) or Asian origin (56/647; 8.7%) [11, 13–15, 17, 23–26, 30, 35, 58, 61, 63, 65–67, 70, 71, 76, 78–81, 83, 86]. However, many mixed/other/unknown origins (144/647; 22.3%) were reported. Hispanic/Latino was reported in 97/332 (29.2%). Overweight (BMI > 25 kg/m2 or >85th percentile for age/sex) was found in 147/581 (25.34%). Other comorbidities were infrequent, and mainly consisted of respiratory diseases, including asthma (39/953; 4.1%) or chronic lung disease (14/953; 1.5%), cardiovascular diseases (12/953; 1.3%) and immunodeficiencies (10/953; 1.0%) [13–15, 17, 23, 25, 55, 59, 60, 63, 64, 66, 68, 70, 81, 82, 86].
Clinical presentation
Fever was documented in nearly all patients (922/928; 99.4%) [11–17, 23, 24, 30–85], commonly during at least 5 days (258/928; 27.0%). The majority (598/699; 85.6%) presented gastrointestinal symptoms, mostly abdominal pain (315/539; 58.4%), vomiting (306/532; 57.5%), and diarrhea (268/532; 50.4%) [11–14, 16, 24, 26, 30–37, 39, 40, 43–45, 47, 49–52, 54–77, 79, 80, 82, 84–86]. Cardiovascular manifestations were found in 79.3% of patients (307/387) [11–17, 24, 30–37, 39–41, 44–49, 51–86]. Tachycardia (194/253; 76.7%), hemodynamic shock or hypotension (416/695; 59.9%), myocarditis (128/309; 41.4%) and mild or moderate decreased left ventricular ejection fraction (LVEF between 30 and 55%; 211/522; 40.4%) were frequently observed cardiovascular abnormalities. Severe complications such as LVEF less than 30% (36/506; 7.1%), coronary dilatation (z-score between 2.0 and 2.5; 74/638; 11.6%) or aneurysms (z-score above 2.5; 59/572; 10.3%) were found in a minority of cases. Pericardial effusion was frequently found (114/511; 22.3%). Half of cases (295/587; 50.3%) showed respiratory symptoms, including upper respiratory tract symptoms (95/397; 23.9%), dyspnea (101/378; 26.7%) and (multiple) radiological infiltrates (114/321; 35.5%) [14, 15, 23, 33, 35, 37, 38, 40, 44, 45, 47, 52, 59, 60, 66, 68, 71, 73, 77, 82]. Thirteen cases (1.4%) revealed thrombotic complications [11, 13, 16, 17, 74, 78], including 2 splenic infarctions [16]. (Hemorrhagic) Cerebral strokes during ECMO (n = 5), a recognized complication, contributed substantially to thrombotic complications [11, 16, 17, 74, 75].
A quarter of patients (130/557; 23.3%) fulfilled criteria for complete KD [12, 13, 15, 30, 35, 37, 41, 45, 46, 50, 53, 56, 59, 61, 63, 64, 68, 69, 72, 73, 77, 79, 81, 83, 85, 86] (criteria in Supplementary information 1). A similar proportion (99/411; 24.1%) fulfilled 2 or 3 of the KD criteria in combination with prolonged fever, resembling incomplete KD [11–13, 32, 36, 39, 44, 47, 48, 52, 54, 57, 61–63, 65–69, 77, 79, 81, 83]. Polymorphous exanthema (466/849; 54.9%) and non-purulent conjunctivitis (423/849; 49.8%) occurred the most. Although shock was frequently reported in single cases (103/138; 74.6%), shock only presented in half of single cases with complete KD (11/24; 45.8%) [12, 30, 35, 41, 45, 46, 53, 68, 69, 72, 77].
Biological markers
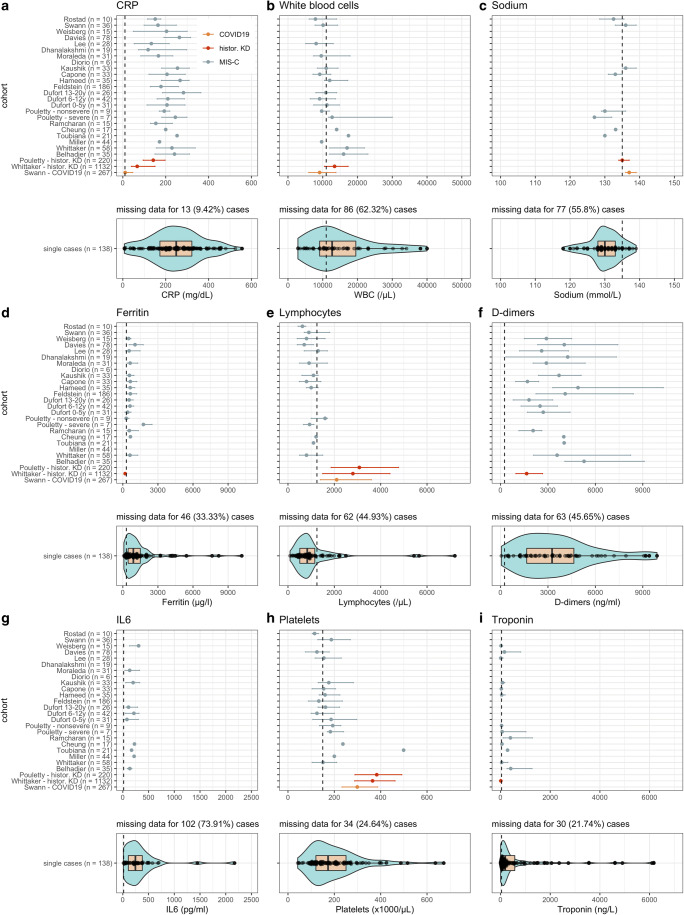
Increased inflammatory markers were frequently documented (Fig. 4) [11, 12, 24, 30–57, 60, 62, 64, 66–73, 75–77, 80, 84], including C-reactive protein (CRP; median 249 mg/l [IQR 173–322] in single cases), ferritin (910 μg/l [457–1521]), and interleukin-6 (244.5 pg/ml [107–379]). Notably, patients exhibited substantially higher inflammation compared to historical KD [15, 64] or non-PIMS-TS/MIS(-C) [23] cohorts.
Fig. 4.
Laboratory tests values and distribution for each study. Error bars correspond to the interquartile range. Dashed vertical line equals the upper limit of normal (CRP, white blood cells, ferritin, d-dimers, IL6, and troponin) or the lower limit of normal (sodium, lymphocytes, and platelets). For studies that report multiple values for the same test, the maximum (CRP, white blood cells, ferritin, d-dimers, IL6, and troponin) or the minimum (sodium, lymphocytes, platelets) was used. “Covid” (red line) equals values corresponding to the COVID-19-related hyperinflammatory syndrome; “control” (gray line) equal values corresponding to the control populations with Kawasaki disease described by Pouletty et al. [64] and Whittaker et al. [15] and (orange line) non-PIMS-TS/MIS(-C) pediatric COVID-19 by Swann et al. [23]. Data from Swann et al. [23], Rostad et al. [26], and Weisberg et al [25]. was extracted from pre-print publications and these references have subsequently been published in the peer-reviewed literature [27–29]
Although white blood cell counts were frequently increased (12,800/μl [9150–20,075]), lymphocytopenia was common (831.5/μl [510–1157.5]) [12, 24, 30–36, 39–41, 43–46, 48–50, 52–57, 60, 62, 67–73, 75, 77, 80, 84], contrasting with historical KD cohorts [15, 64] (median lymphocytes 2800–3080/μl) or non-PIMS-TS/MIS(-C) (median 2100/μl) [23]. Most PIMS-TS/MIS(-C) presented reduced to normal thrombocytes (platelets below 150,000/μl in 44/104; 42.3%) [11, 12, 24, 30, 32–36, 38, 40, 41, 43–46, 48, 50, 52–56, 62, 66–68, 70, 71, 77, 80, 84]. Thrombocytosis (platelets above 450,000/μl), a typical KD sign and a laboratory criterium for incomplete KD [87], occurred in only 5/104 (4.8%).
Besides inflammatory parameters, coagulation markers were substantially upregulated, including d-dimers (3750 ng/ml [1946–6896]) and fibrinogen (640 mg/dl [504–800]) [11, 12, 31–35, 38, 40, 43–47, 49, 51, 55–57, 60, 62, 66–68, 70, 71, 75–77, 80, 84]. Furthermore, myocardial injury markers such as troponins (188 ng/l [60–614]) and brain natriuretic peptide (BNP) (median 1619 pg/ml [424–3325]) were often elevated [11, 12, 30, 32–36, 39, 40, 46–49, 54–57, 60, 62, 66–71, 75, 77, 80, 84, 87, 88]. Hyponatremia (130 mmol/l [128–133]) [12, 24, 30, 32, 35, 37, 41, 44–46, 48–50, 52, 54, 55, 62, 67, 69, 76, 84] was frequent, contrasting with KD controls (135 mmol/l [134–137]) [64] or non-PIMS-TS/MIS(-C) COVID-19 (137 mmol/l [136–139]) [23].
SARS-CoV-2 testing and diagnostic criteria
Current or recent SARS-CoV-2 infection was assessed with RT-PCR (nasopharyngeal or fecal swab) and/or serological assays (IgG/IgM/IgA) [12–17, 25, 26, 30–34, 36–53, 55, 57–72, 75–82, 84–86]. Two-thirds of patients were IgG-positive (362/569; 63.6%). IgM (substantial variation between 5.7 and 100%) [16, 26, 59, 82] and IgA positivity (25/35; 71.4%) [59] were documented in only four and one cohort(s), respectively. All single patients being IgA-positive (19/138; 13.8%) [36, 62, 70] or IgM-positive (8/138; 5.8%) [12, 34, 46, 47, 69, 72], had detectable IgG as well. Only 338/901 (37.5%) had positive respiratory RT-PCR. Positive fecal RT-PCR was rare (7/268; 2.6%) [16, 39, 40, 45, 58, 59, 62, 64, 79]. Close contacts with COVID-19 were registered in 168/598 (28.1%) [11–13, 17, 24, 34, 36, 40, 44, 47, 48, 53, 59, 61, 63–65, 70, 72, 73, 75, 77, 78, 80–82]. Of single cases, 115/138 (83.3%) had a microbiologically confirmed SARS-CoV-2 infection (PCR and/or serology-positive). Of the 23 cases negative (or missing) for both techniques [11, 12, 24, 35, 50, 54, 56, 62, 73, 77, 79], 15 additionally had no known COVID-19 contact [11, 12, 35, 50, 54, 56, 62, 79].
As an inclusion criterium, all cases in this review corresponded to at least one of the recognized case definitions [18–20]. The RCPCH definition, not requiring proven or probable SARS-CoV-2 infection, was most comprehensive, and subsequently comprised all single cases. Although more stringent concerning clinical manifestations and the relationship with SARS-CoV-2, the WHO definition nevertheless included 97% (18 not assessable), missing only 4 mild cases that did not present multisystem dysfunction (criterium 3). In contrast, the CDC definition comprised only 62% (8 not assessable) of single cases and neglected on 31 cases with severe course, all failing to achieve the CDC criterium concerning the multi-organ (≥2 organ systems) dysfunction. Of single cases, the WHO definition missed 1 out of 5 patients needing ECMO because of insufficient data reported to assess [74], while the CDC definition not included 2/5 ECMO cases [11, 52, 66, 74, 75] and 2/6 deaths [11, 24, 66, 74, 75, 79].
Therapeutic management
Three quarter of patients (662/872; 75.9%) received intravenous immunoglobulins (IVIG) [11–15, 17, 25, 30, 35, 37, 39, 41, 43–48, 50, 52–54, 56–69, 71–73, 75–86]. Only few papers report IVIG dosages, which were mainly immunomodulatory. Multiple IVIG doses were needed in 73/662 (11.0%). Systemic corticosteroids were prescribed in 516/908 (56.8%) [11–15, 17, 23, 25, 31, 33–35, 43, 46, 47, 50, 54, 57–67, 69, 71, 72, 76–86]. Acetylsalicylic acid was reported in 171/327 (52.3%) of which 39/171 (22.8%) received high, anti-inflammatory dosages (80–100mg/kg/day) [11, 12, 30, 35, 37, 39, 44, 45, 47, 48, 50, 52–54, 56, 61, 63–67, 69, 71, 77–79, 81, 85, 86]. Heparin (259/563; 46.0%) was a frequent anti-thrombotic [11, 13, 17, 33, 34, 43, 51, 52, 55, 59, 60, 63, 65, 66, 68, 74, 75, 78, 80, 81, 84, 86].
One hundred and fifty-five cases (155/953; 16.3%) were treated with biopharmaceuticals, including IL-1R antagonist (anakinra) (72/953; 7.6%), interleukin-6 inhibitors (tocilizumab/siltuximab; 64/953; 6.7%), and to a lesser extent, TNFα-inhibitors (infliximab) (22/953; 2.3%) [11, 13, 15, 17, 25, 33, 38, 41–43, 45, 46, 51, 57–60, 63, 64, 66–68, 70, 75, 76, 78–81, 84–86]. Remdesivir (22/953; 2.3%) was rarely prescribed [17, 25, 57, 60, 66, 78, 81, 82].
Inotropics were given to 477/863 (55.3%) [11–17, 23, 26, 31–33, 35, 39, 45–47, 52, 54, 57, 59–72, 75, 77, 78, 80, 81, 83–86]. Mechanical and non-invasive ventilation was initiated in 219/928 (23.6%) and 130/503 (25.8%), respectively [11, 13–17, 23, 30, 31, 33, 39, 41, 46, 47, 52, 54, 58–62, 64–72, 74, 75, 78, 80–86]. A relative high rate of ECMO (36/953; 3.8%) [11, 13–17, 52, 59, 66, 74, 75, 78] was reported.
Prognosis and outcomes
Intensive care admission was common (564/769; 73.3%) with median duration of 4 days (IQR 3.75–8) in single cases and 4–7 days in cohorts [11, 13, 14, 16, 17, 23, 26, 33, 45, 47, 54, 59–67, 70, 71, 79, 81–83, 85, 86]. The median time of hospitalization was 8 days (IQR 7–12) in single cases and 4–12 days in cohorts [13, 17, 30, 54, 55, 59, 63, 65–67, 70, 71, 86]. A majority of single cases (118/138; 86%) experienced severe course. Such patients were substantially older and presented more gastrointestinal and cardiovascular symptoms as compared to mild PIMS-TS/MIS(-C) (Table 2). They presented however less respiratory symptoms, exanthema, or complete KD. Laboratory measurements in patients with severe disease showed lower WBC counts and more lymphopenia, higher CRP, and ferritin (but lower IL-6), and higher platelet counts, d-dimer, and troponin. There were no differences in sex, microbiology, or medical treatment.
Table 2.
Characteristics of patients with mild versus severe PIMS-TS/MIS(-C) of reported single cases (n = 138). Severe course was defined as presence of coronary dilatation/aneurysm, shock, death, need for mechanical ventilation, extracorporal membrane oxygenation (ECMO), renal replacement therapy, inotropes, or PICU admission. Abbreviations used: ECMO, extracorporal membrane oxygenation; IL, interleukin; IVIG, intravenous immunoglobulins; KD, Kawasaki disease; RRT, renal replacement therapy; WBC, white blood cells
| PIMS-TS/MIS(-C) | ||
|---|---|---|
| Variable | Severe course | Mild course |
| Count, n (%) | 118 (100) | 20 (100) |
| Age (year), median (IQR) | 9 (6–12.7) | 6.5 (3–9.75) |
| Male, n (%) | 70 (59) | 13 (65) |
| Symptoms, n (%) | ||
| Respiratory | 56 (47) | 12 (60) |
| Gastrointestinal | 103 (87) | 10 (50) |
| Cardiovascular | 117 (99) | 7 (35) |
| Shock | 103 (87) | NA |
| Coronary dilatation | 13 (11) | NA |
| Aneurysm formation | 12 (10) | NA |
| Neurological | 38 (32) | 9 (45) |
| Dermatological | 59 (50) | 14 (70) |
| Fever ≥ 5 days | 64 (54) | 12 (60) |
| Complete KD | 16 (14) | 8 (40) |
| Incomplete KD | 29 (25) | 4 (20) |
| SARS-CoV-2, n (%) | ||
| RT-PCR-positive | 54 (45) | 9 (45) |
| Serology-positive | 65 (55) | 11 (55) |
| Critical care interventions, n (%) | ||
| PICU admission | 64 (54) | NA |
| Inotropics | 77 (65) | NA |
| Mechanical ventilation | 35 (30) | NA |
| ECMO | 5 (4) | NA |
| RRT | 2 (2) | NA |
| Medical treatment, n (%) | ||
| IVIG once | 90 (76) | 14 (70) |
| IVIG multiple | 9 (8) | 2 (10) |
| Systemic corticoids | 49 (42) | 9 (45) |
| Anakinra | 8 (7) | 1 (5) |
| Tocilizumab | 22 (19) | 3 (15) |
| Infliximab | 9 (8) | 1 (5) |
| Laboratory markers, median (IQR) | ||
| WBC (/μl) | 12,735 (8602.5–20,075) | 14,950 (10025–18,827.5) |
| Lymphocytes (/μl) | 800 (510–1190) | 920 (700–1030) |
| Platelets (/μl) | 181,000 (123,000–254,000) | 121,000 (104000–151,000) |
| CRP (mg/l) | 251 (183–328) | 197 (132.25–258.7) |
| Ferritin (ng/ml) | 966.5 (482.5–1569.25) | 752.5 (343–1286.5) |
| IL-6 (pg/ml) | 2197.15 (23.125–9927.5) | 3689 (0.75–12,193.75) |
| Sodium (mmol/l) | 130 (128–133) | 132 (129.35–133) |
| d-Dimer (ng/ml) | 3917 (2105.75–8218) | 2818 (633.5–4379) |
| Troponin (ng/l) | 2020.5 (111–9660) | 193.5 (19–10,949.2) |
| Outcome, n (%) | ||
| Death | 6 (5) | NA |
Eighteen deaths were described (18/953; 1.9%) [11, 13–17, 24, 66, 74, 75, 78, 79, 82]. Of deaths with reported ages, 2/12 (16.7%) patients were less than 1 year old [14, 79], 6/12 (50%) were aged 5–12 [13, 14, 17, 66, 74, 75], and 4/12 (33.3%) were older than 13 years [11, 13]. The majority was male (8/11; 72.3%) and Black (5/8; 62.5%), although race/ethnicity was underreported. All reported deaths but one [79] presented with shock and/or myocardial dysfunction, needing inotropics, and/or mechanical circulatory support [11, 13–17, 24, 66, 74, 75]. ECMO was initiated in 10/15 (66.7%) of fatal cases, of which 5 died of (hemorrhagic) cerebral infarction [11, 16, 17, 74, 75]. Comorbidities among fatal cases were obesity (n = 4) [11, 13], acute leukemia (n = 1) [82], glucose-6-phosphate dehydrogenase deficiency (n = 1) [24], asthma (n = 1) [13], and multiple neurological conditions (n = 1) [13]. Residual cardiac dysfunction, often reported as decreased LVEF at discharge or follow-up, was present in 21/287 (7.3%) [17, 59, 63–65, 75, 86]. Two patients showed persistent neurological damage after PIMS-TS/MIS(-C) [76]. No other residual morbidity was reported.
Discussion
Overall, children with COVID-19 exhibit mild or asymptomatic disease. Only limited reports of complicated or fatal COVID-19 in children are published [7, 10, 89, 90]. Although some immunological hypotheses are presented [91–94], hitherto, obvious elucidation on why children display a milder COVID-19 phenotype is lacking.
At the end of April 2020, while over 3 million SARS-CoV-2 infections were reported worldwide, a relative sudden emerge of children presenting a severe hyperinflammatory disorder with multisystem involvement, prompted an international alert. Noteworthy, during the first 4 months after the initial reports, more than 950 individual cases with PIMS-TS/MIS(-C) have been reported in scientific literature, and, subsequently, systematically reviewed herein. Currently, several countries are still struggling with widespread SARS-CoV-2, requiring continuous and evidence-based updates on the COVID-19 spectrum, in particular concerning complicated disease courses. In this context, we performed the most extensive PIMS-TS/MIS(-C) systematic review to appropriately characterize its presentation and prognosis.
The findings in this review confirm the heterogeneous clinical spectrum. The majority of PIMS-TS/MIS(-C) patients present gastrointestinal symptoms. Despite SARS-CoV-2 displaying respiratory tract tropism [95], a large proportion of cases does not exhibit respiratory symptoms, as typically seen in adults. Eventually, in 50.3% respiratory manifestations are noted, although critical illness might have contributed to secondary respiratory failure and ventilator-associated pneumonia. Considering the high rate of ICU admission (73.3%), we conclude that a relevant proportion of critical cases does not exhibit initial respiratory manifestations. In contrast with typical adult COVID-19, PIMS-TS/MIS(-C) predominantly affects cardiovascular, gastrointestinal, and/or neurological organ systems and only occasionally the respiratory system. Cardiovascular manifestations, including severe circulatory failure and myocardial involvement requiring intensive care, burdens PIMS-TS/MIS(-C) substantially, and was dominantly present in all deceased patients. Nevertheless, the majority of patients (98.1%) survived the acute phase of PIMS-TS/MIS(-C). Noteworthy, an overrepresentation of males and minorities (Black, Hispanic/Latino), as well as the paucity of reports from Asian countries, is observed in this review. Apart from obesity, significant co-morbidities are missing, also among fatal cases. So far, underlying factors such as genetic predisposition, prior infections, or immunizations contributing to PIMS-TS/MIS(-C) vulnerability are unclear.
Comparing with historical KD cohorts [15, 64] or non-PIMS-TS/MIS(-C) COVID-19 children [23], PIMS-TS/MIS(-C) patients are substantially older, and represent more systemic inflammation (higher WBC counts and drastically increased CRP and IL-6), more lymphocytopenia and thrombocytopenia, and higher markers of myocardial injury (troponin and NT-pro-BNP) and coagulopathy (d-dimers). Of the PIMS-TS/MIS(-C) cases fulfilling complete KD criteria, half presented with shock, contrasting with non-COVID-19-associated KD shock syndrome with an incidence rate of only 3.3–7% of KD cases [96, 97]. Moreover, coronary dilatation (11.6%) and aneurysm formation (10.3%) are more prevalent than in appropriately treated KD (~5%), as well as mortality rates, typically less than 0.1% in KD (1.9% in PIMS-TS/MIS(-C)) [98]. Despite some overlapping features, this review confirms that PIMS-TS/MIS(-C) is a distinct entity from KD, KD shock syndrome, or (acute) COVID-19 in children. Epidemiological enrichment for adolescents is present, but clinicians should remain vigilant with other age categories as similar disease also presents in series of infants [33, 37, 48, 53, 79], and recently, even in a 36-year-old [99]. Despite the international recognition of this novel disease entity, none of the clinical variables presented in this review seems to be neither sensitive nor specific for PIMS-TS/MIS(-C). Thus, it remains challenging to recognize this heterogeneous disease in daily clinical practice. Prompt recognition is pivotal to insure a good individual prognosis. Knowledge of the disease spectrum (summarized in Fig. 5) and the combination of a detailed medical history, clinical examination, and routine laboratory markers in a child presenting with prolonged fever should allow an experienced clinician to differentiate against diseases with overlapping presentations. In either case, its frequent association with end-organ damage requires the accessibility of a pediatric intensive care unit.
Fig. 5.
Summary figure presenting the findings of this systematic review on the clinical spectrum of PIMS-TS/MIS(-C). Comparison of the clinical picture is made, based on relevant differences with control populations such as published on Kawasaki disease (KD) by Pouletty et al. [64] and Whittaker et al. [15] (*), and non-PIMS-TS/MIS(-C) pediatric COVID-19 by Swann et al. [23] (°). For each variable, the percentage denoting the fraction of included cases is displayed. PIMS-TS/MIS(-C) disease severity is assessed as described in the “Methods” section. Arrows pointing upwards mean that a higher proportion of cases display one of the mentioned symptoms or that higher values for the laboratory markers are found. Arrows pointing down denote lower values or frequencies
Severe COVID-19 might be related to host immune overdrive and unbounded cytokine release [100, 101]. In contrast with adult COVID-19, respiratory symptoms are less common in PIMS-TS/MIS(-C), and primary respiratory failure does not seem a dominant cause for ICU admission. Moreover, the clinical presentation of PIMS-TS/MIS(-C) is mainly characterized by systemic vasculitis, multisystem involvement, and hypercoagulation. However, although abnormal coagulation parameters are frequently reported, thrombotic or embolic events were rare, in contrast with adult COVID-19 [102]. To date, the molecular pathophysiological mechanisms in PIMS-TS/MIS(-C) are insufficiently studied, although publications measuring serological and inflammatory responses have shown some initial insights [28, 103, 104]. Further research efforts are however required as understanding of the involved pathways might contribute to appropriate therapeutics that interfere with these dysregulated immune responses.
With a seroconversion rate of two-thirds, this review confirms the probable association with recent SARS-CoV-2 infection and possibility of antibody-driven pathogenesis in PIMS-TS/MIS(-C). In this review, a proportion of patients are, however, included without microbiological evidence for (past) SARS-CoV-2 infection, which is an important caveat in the current case definitions. The true incidence of PIMS-TS/MIS(-C) remains moreover unknown and a notification bias might be present. In absence of comprehensive pediatric surveillance studies, the proportion of SARS-CoV-2-infected children subsequently suffering from PIMS-TS/MIS(-C) can only be estimated. A better understanding of affected age groups and associated risk factors is thus necessary.
The appropriate use of case definitions should prospectively be assessed. This review currently favors the WHO MIS definition. In contrast with the RCPCH definition, both CDC and WHO case descriptions are more precise (e.g., requiring a proven association with SARS-CoV-2 and multisystem involvement), while the WHO definition comprised 97% of cases, and CDC only 62%.
Ultimately, this review has some limitations. In particular, we were unable to collect individual data of all patients. We did not contact authors of included studies for insights in their data as we believed this would significantly delay the reporting of this pressing data. Due to the nature of included studies, these reports are moreover enriched for severe disease course. Furthermore, only 7 studies contain a control population, of which 3 use historical data [15, 64, 81], 1 uses an adult control population [25] and 2 others report on 15 or less control cases [26, 83]. Additionally, the association with COVID-19 could have triggered a reporting bias, which might result in overdiagnosis of PIMS-TS/MIS(-C). This phenomenon could have affected the in-depth analysis of the case definitions as well, as the “true” false positivity rate remains unknown. To partly overcome this issue, we excluded cases with insufficient data in our sensitivity analysis. As this review was conducted while PIMS-TS/MIS(-C) has only been described since a few months, inevitably, delayed complications or long-term effects were not yet assessed.
Because the relatively small number in the single-case cohort and many lacking data in larger cohorts, formal statistical testing was not conducted. As such, the findings of this review should be interpreted as descriptive and exploratory. Due to the retrospective nature of included studies, and not all studies reporting all variables, we were unable to collect sufficient data for prediction modeling for disease course or treatment response. To date, there is lack of randomized controlled trials concerning PIMS-TS/MIS(-C) and additional prospective cohort studies including control populations are needed. As a surrogate, systematic reviewing of observational data might contribute to the expertise required and identify gaps in knowledge. Updating the dataset of this review, might consecutively provide answers to these ongoing needs.
Conclusions
A novel hyperinflammatory condition with severe multisystem involvement has been described in children and adolescents during the COVID-19 pandemic (PIMS-TS/MIS(-C)). This review systematically assesses this novel syndrome and, as such, illustrates an epidemiological enrichment for males, adolescents, and racial minorities; a clinical heterogeneous presentation with frequent gastrointestinal manifestations and circulatory failure including myocardial injury; and lastly, an overall good prognosis with absence of short-term complications despite frequent critical care interventions. Further epidemiological, clinical, immunological, and genetic research is needed, as well as long-term follow-up studies of PIMS-TS/MIS(-C) patients.
Supplementary Information
(DOCX 52 kb)
Acknowledgements
This research effort was conducted as part of the pediatric COVID<19 research consortium of the Ghent University Hospital.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- ECMO
Extracorporal membrane oxygenation
- IVIG
Intravenous immunoglobulins
- KD
Kawasaki disease
- KDSS
Kawasaki disease shock syndrome
- LVEF
Left ventricular ejection fraction
- MAS
Macrophage activation syndrome
- MIS(-C)
Multisystem inflammatory syndrome (in children)
- PIMS-TS
Pediatric inflammatory multisystem syndrome temporally associated with COVID-19
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TSS
Toxic shock syndrome
Author’s contributions
LH contributed to study conception and design and drafted the review protocol. LH independently carried out the review selection process, extracted data from the included records, and assessed risk of bias and level of evidence. LH drafted the initial manuscript, and has read, contributed to and approved the final version of the manuscript. RVP contributed to study conception and design, independently carried out the review selection process. RVP cross-checked extracted data, verified risk of bias and level of evidence, and carried out the data analysis. RVP has read, contributed to and approved the final version of the manuscript.FH supervised the full study conception, design, data extraction, data analysis and interpretation, and manuscript drafting. FH resolved any disagreement in the record selection process. FH has read, contributed to and approved the final version of the manuscript.
Funding
LH was funded by the VIB Grand Challenge Programs. RVP was funded by a predoctoral fellowship from the Research Foundation Flanders (FWO). FH is supported by Ghent University research grant (BOF-UGent), VIB Grand Challenges Programs and Jeffrey Modell Foundation.
Data availability/Code availability
All code, additional supporting data, and the full data analysis have been made publicly accessible (url provided in Supplementary information)
Code availability
N/A
Declarations
Ethics approval/consent to participate/consent for publication
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hoste Levi and Van Paemel Ruben contributed equally to this work.
Contributor Information
Levi Hoste, Email: levi.hoste@ugent.be.
Ruben Van Paemel, Email: ruben.vanpaemel@ugent.be.
Filomeen Haerynck, Email: filomeen.haerynck@ugent.be.
References
- 1.WHO (2020) Novel coronavirus – China (2020, Jan 12). http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 23 Jul 2020
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;2:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 9.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG, Overcoming COVID-19 Investigators. CDC COVID-19 Response Team Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H, New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M, for the PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA - J Am Med Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hameed S, Elbaaly H, Reid CEL et al (2020) Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology:202543. 10.1148/radiol.2020202543 [DOI] [PMC free article] [PubMed]
- 17.Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, Gillen JK, Perez MM, Soshnick SH, Conway EE, Jr, Bercow A, Seiden HS, Pass RH, Ushay HM, Ofori-Amanfo G, Medar SS. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19), 2020. https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 8 Jul 2020
- 19.World Health Organization (WHO) (2020) Multisystem inflammatory syndrome in children and adolescents with COVID-19. 1–3
- 20.Royal College of Health Paediatrics and Child (RCPCH) (2020) Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-inflammatory syndrome-20200501.pdf. Accessed 8 Jul 2020
- 21.Wells G, Shea B, O’Connell C, et al The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 23 Jul 2020
- 22.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S–4S. doi: 10.1378/chest.95.2_Supplement.2S. [DOI] [PubMed] [Google Scholar]
- 23.Swann OV, Holden KA, Turtle L et al (2020) Clinical characteristics of children and young people hospitalised with covid-19 in the United Kingdom: prospective multicentre observational cohort study. medRxiv. 10.1101/2020.07.14.20153320 [DOI] [PMC free article] [PubMed]
- 24.Al-Aamri MA, Al-Khars FT, Alkhwaitem SJ et al (2020) A Saudi G6PD deficient girl died with pediatric multisystem inflammatory syndrome-COVID-19. medRxiv. 10.1101/2020.07.08.20137497
- 25.Weisberg SP, Connors T, Zhu Y, et al. Antibody responses to SARS-CoV2 are distinct in children with MIS-C compared to adults with COVID-19. Medrxiv. 2020;2020(07):12.20151068. doi: 10.1101/2020.07.12.20151068. [DOI] [Google Scholar]
- 26.Rostad CA, Chahroudi A, Mantus G et al (2020) Serology in children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19. medRxiv. 10.1101/2020.07.10.20150755
- 27.Rostad CA, Chahroudi A, Mantus G, Lapp SA, Teherani M, Macoy L, Tarquinio KM, Basu RK, Kao C, Linam WM, Zimmerman MG, Shi PY, Menachery VD, Oster ME, Edupuganti S, Anderson EJ, Suthar MS, Wrammert J, Jaggi P. Quantitative SARS-CoV-2 serology in children with multisystem inflammatory syndrome (MIS-C) Pediatrics. 2020;146:e2020018242. doi: 10.1542/peds.2020-018242. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, Szabo PA, Wells SB, Dogra P, Gray J, Idzikowski E, Stelitano D, Bovier FT, Davis-Porada J, Matsumoto R, Poon MML, Chait M, Mathieu C, Horvat B, Decimo D, Hudson KE, Zotti FD, Bitan ZC, la Carpia F, Ferrara SA, Mace E, Milner J, Moscona A, Hod E, Porotto M, Farber DL. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, Seth S, Egan C, Hardwick HE, Halpin S, Girvan M, Donohue C, Pritchard M, Patel LB, Ladhani S, Sigfrid L, Sinha IP, Olliaro PL, Nguyen-van-Tam JS, Horby PW, Merson L, Carson G, Dunning J, Openshaw PJM, Baillie JK, Harrison EM, Docherty AB, Semple MG. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki disease in a child with Covid-19. Indian Pediatr. 2020;57:680–681. doi: 10.1007/s13312-020-1900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnapp A, Abulhija H, Maly A, Armoni-Weiss G, Levin Y, Faitatziadou SM, Molho-Pessach V. Introductory histopathologic findings may shed light on COVID19 pediatric hyperinflammatory shock syndrome. J Eur Acad Dermatol Venereol. 2020;34:e665–e667. doi: 10.1111/jdv.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu JS, Lahoud-Rahme M, Schaffer D, Cohen A, Samuels-Kalow M. Kawasaki disease features and myocarditis in a patient with COVID-19. Pediatr Cardiol. 2020;41:1–3. doi: 10.1007/s00246-020-02393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Gonzalez M, Sanchez-Codez M, Gutierrez-Rosa I, Castellano-Martinez A, Rodriguez-Benitez AR-CP (2020) New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol Young:1–4. 10.1017/S1047951120001857 [DOI] [PMC free article] [PubMed]
- 34.Giannattasio A, Maglione M, Zenzeri L, Mauro A, di Mita O, Iodice RM, Tipo V. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID-19 infection: A novel management for a new disease? J Med Virol. 2020;93:112–114. doi: 10.1002/jmv.26189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta K, Finch S. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in South Dakota. S D Med. 2020;73:246–251. [PubMed] [Google Scholar]
- 36.Bapst T, Romano F, Müller M, Rohr M (2020) Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID-19: erythema multiforme. BMJ Case Rep 13. 10.1136/bcr-2020-236986 [DOI] [PMC free article] [PubMed]
- 37.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 38.Haigh K, Syrimi Z, Irvine S, et al. Hyperinflammation with COVID-19: The key to patient deterioration? Clin Infect Pract. 2020;7-8:7–8. doi: 10.1016/j.clinpr.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vari D, Miller J, Rellosa N, et al. Severe cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: Retrospective diagnosis of a puzzling presentation. A case report. Prog Pediatr Cardiol. 2020;58:101270. doi: 10.1016/j.ppedcard.2020.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen DC, Haydar H, Pace ER, Zhang XS, Dobbs KR. Pediatric case of severe COVID-19 with shock and multisystem inflammation. Cureus. 2020;12:e8915. doi: 10.7759/cureus.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr. 2020;57:681–683. doi: 10.1007/s13312-020-1901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puyó V, Moreno L, Díaz de Heredia D, et al. Tocilizumab in a child with acute lymphoblastic leukaemia and COVID-19-related cytokine release syndrome. An Pediatr (Barc) 2020;93:132–133. doi: 10.1016/j.anpedi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klocperk A, Parackova Z, Dissou J, Malcova H, Pavlicek P, Vymazal T, Dolezalova P, Sediva A. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID-19. Front Immunol. 2020;11:1665. doi: 10.3389/fimmu.2020.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cazzaniga M, Baselli LA, Cimaz R, Guez SS, Pinzani R, Dellepiane RM. SARS-COV-2 Infection and Kawasaki disease: case report of a hitherto unrecognized association. Front Pediatr. 2020;8:398. doi: 10.3389/fped.2020.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahrami A, Vafapour M, Moazzami B, Rezaei N (2020) Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. 10.1111/jpc.15048 [DOI] [PMC free article] [PubMed]
- 46.Cogan E, Foulon P, Cappeliez O, Dolle N, Vanfraechem G, de Backer D. Multisystem inflammatory syndrome with complete Kawasaki disease features associated with SARS-CoV-2 infection in a young adult. A Case Report. Front Med. 2020;7:428. doi: 10.3389/fmed.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regev T, Antebi M, Eytan D, Shachor-Meyouhas Y, Ilivitzki A, Aviel YB, Ben-Ari J. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J. 2020;39:E206–E207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 48.Raut S, Roychowdhoury S, Bhakta S, Sarkar M, Nandi M (2020) Incomplete Kawasaki disease as presentation of COVID-19 infection in an infant: a case report. J Trop Pediatr. 10.1093/tropej/fmaa047 [DOI] [PMC free article] [PubMed]
- 49.Bhansali S, Minocha P, Phoon C, Henry G, Chakravarti S, Ramirez M, Bhatla P. Cardiac involvement in a pediatric patient with COVID-19: looking beyond the nonspecific global cardiac injury. Echocardiography. 2020;37:1488–1491. doi: 10.1111/echo.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta A, Gill A, Sharma M, Garg M (2020) Multi-system inflammatory syndrome in a child mimicking Kawasaki disease. J Trop Pediatr. 10.1093/tropej/fmaa060 [DOI] [PMC free article] [PubMed]
- 51.Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, Lai J. Pediatric Crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020;71:153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, Tilford B, Chopra T, Arora H, Ang J, Asmar B. COVID-19 associated pediatric multi-system inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acharyya BC, Acharyya S, Das D. Novel coronavirus mimicking Kawasaki disease in an infant. Indian Pediatr. 2020;57:753–754. doi: 10.1007/s13312-020-1924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020;87:745–747. doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trogen B, Gonzalez FJ, Shust GF (2020) COVID-19-associated myocarditis in an adolescent. Pediatr Infect Dis J:E204–E205. 10.1097/INF.0000000000002788 [DOI] [PubMed]
- 56.Yozgat CY, Uzuner S, Duramaz BB et al (2020) Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol Ther:e13770. 10.1111/dth.13770 [DOI] [PMC free article] [PubMed]
- 57.Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020;38:2492.e5–2492.e6. doi: 10.1016/j.ajem.2020.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574.e2. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 60.Joshi K, Kaplan D, Bakar A, Jennings JF, Hayes DA, Mahajan S, Misra N, Mitchell E, Sweberg TM, Taylor MD, Capone CA. Cardiac dysfunction and shock in pediatric patients with COVID-19. JACC Case Rep. 2020;2:1267–1270. doi: 10.1016/j.jaccas.2020.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, Leruez-Ville M, Quartier P, Léger PL, Geslain G, Semaan N, Moulin F, Bendavid M, Jean S, Poncelet G, Renolleau S, Oualha M. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA - J Am Med Assoc. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, Bensaid P, Pichard S, Kouider H, Morelle G, Craiu I, Pondarre C, Deho A, Maroni A, Oualha M, Amoura Z, Haroche J, Chommeloux J, Bajolle F, Beyler C, Bonacorsi S, Carcelain G, Koné-Paut I, Bader-Meunier B, Faye A, Meinzer U, Galeotti C, Melki I. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramcharan T, Nolan O, Lai CY et al (2020) Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol:1–11. 10.1007/s00246-020-02391-2 [DOI] [PMC free article] [PubMed]
- 66.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, Sordillo EM, Tosi M, Trachtman R, Paniz-Mondolfi A. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. 2020;93:424–433. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC, Topjian A, John ARO. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, Sanders JE. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020;38:2246.e3–2246.e6. doi: 10.1016/j.ajem.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blondiaux E, Parisot P, Redheuil A et al (2020) Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology 202288. 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed]
- 70.Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. 2020;4:e21–e23. doi: 10.1016/S2352-4642(20)30164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foong Ng K, Kothari T, Bandi S, et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. 2020;92:2880–2886. doi: 10.1002/jmv.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, Montin D. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 73.Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Ben Saïd P, Mahé E. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. 2020;34:e539–e541. doi: 10.1111/jdv.16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schupper A, Yaeger K, Morgenstern P. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogo T, Mathur K, Purswani M. Systemic inflammation with cardiac involvement in pediatric patients with evidence of COVID-19 in a community hospital in the Bronx, NY. J Pediatric Infect Dis Soc. 2020;9:502–503. doi: 10.1093/jpids/piaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdel-Mannan O, Eyre M, Löbel U et al (2020) Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol:E1–E6. 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed]
- 77.Ferrero P, Piazza I, Bonino C, Ciuffreda M. Patterns of myocardial involvement in children during COVID-19 pandemic: early experience from northern Italy. Ann Pediatr Cardiol. 2020;13:230–233. doi: 10.4103/apc.APC_77_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, Johnson M, Griffiths B, du Pré P, Mohammad Z, Deep A, Playfor S, Singh D, Inwald D, Jardine M, Ross O, Shetty N, Worrall M, Sinha R, Koul A, Whittaker E, Vyas H, Scholefield BR, Ramnarayan P. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vergnano S, Alders N, Armstrong C, Bamber AR, Bandi S, Evans JA, Hajiani N, Kenny J, Kucera F, Tometzki A, Uzun O, Wilkinson N, Ramanan AV. Severe refractory Kawasaki disease in seven infants in the COVID-19 era. Lancet Rheumatol. 2020;2:e520. doi: 10.1016/S2665-9913(20)30231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. 2020;2020:8875987–8875984. doi: 10.1155/2020/8875987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, Lo MS, Platt CD, Chou J, Hoyt KJ, Baker AL, Banzon TM, Chang MH, Cohen E, de Ferranti SD, Dionne A, Habiballah S, Halyabar O, Hausmann JS, Hazen MM, Janssen E, Meidan E, Nelson RW, Nguyen AA, Sundel RP, Dedeoglu F, Nigrovic PA, Newburger JW, Son MBF. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moraleda C, Serna-Pascual M, Soriano-Arandes A, Simó S, Epalza C, Santos M, Grasa C, Rodríguez M, Soto B, Gallego N, Ruiz Y, Urretavizcaya-Martínez M, Pareja M, Sanz-Santaeufemia FJ, Fumadó V, Lanaspa M, Jordan I, Prieto L, Belda S, Toral-Vázquez B, Rincón E, Gil-Villanueva N, Méndez-Echevarría A, Castillo-Serrano A, Rivière JG, Soler-Palacín P, Rojo P, Tagarro A, EPICO-AEP Working Group (2020) Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin Infect Dis. 10.1093/cid/ciaa1042
- 83.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, Lee JH, Jasen C, Balamuth F, Barrett DM, Banwell BL, Bernt KM, Blatz AM, Chiotos K, Fisher BT, Fitzgerald JC, Gerber JS, Gollomp K, Gray C, Grupp SA, Harris RM, Kilbaugh TJ, John ARO, Lambert M, Liebling EJ, Paessler ME, Petrosa W, Phillips C, Reilly AF, Romberg ND, Seif A, Sesok-Pizzini DA, Sullivan KE, Vardaro J, Behrens EM, Teachey DT, Bassiri H. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolfler A, Mannarino S, Giacomet V, Camporesi A, Zuccotti G. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health. 2020;4:e26–e27. doi: 10.1016/S2352-4642(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dhanalakshmi K, Balasubramanian S, Madhusudan M, et al. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020;57:1010–1014. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, Schleien C, Northwell Health COVID-19 Research Consortium. Epstein S, Johnson JC, Kessel A, Misra N, Mitchell E, Palumbo N, Rajan S, Rocker J, Williamson K, Davidson KW. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, Tilford B, Chopra T, Arora H, Ang J, Asmar B. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Souza TH, Nadal JA, Nogueira RJN, et al. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020;55:1892–1899. doi: 10.1002/ppul.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, Naqvi R, Petershack M, Moreira A. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. doi: 10.1016/j.eclinm.2020.100433.eCollection2020Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carsetti R, Quintarelli C, Quinti I, Piano Mortari E, Zumla A, Ippolito G, Locatelli F. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Health. 2020;4:414–416. doi: 10.1016/S2352-4642(20)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 93.Ruggiero A, Attinà G, Chiaretti A. Additional hypotheses about why COVID-19 is milder in children than adults. Acta Paediatr. 2020;109:1690. doi: 10.1111/apa.15328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fischer A. Resistance of children to Covid-19. How? Mucosal Immunol. 2020;13:563–565. doi: 10.1038/s41385-020-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, Watson VE, Best BM, Burns JC. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dominguez SR, Friedman K, Seewald R, Anderson MS, Willis L, Glodé MP. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics. 2008;122:e786–e790. doi: 10.1542/peds.2008-1275. [DOI] [PubMed] [Google Scholar]
- 98.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 99.Sokolovsky S, Soni P, Hoffman T, Kahn P, Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2020;39:253.e1–253.e2. doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S, Cousin N, Durand A, el Kalioubie A, Favory R, Girardie P, Houard M, Jaillette E, Jourdain M, Ledoux G, Mathieu D, Moreau AS, Niles C, Nseir S, Onimus T, Préau S, Robriquet L, Rouzé A, Simonnet A, Six S, Toussaint A, Dupont A, Bauters A, Zawadzki C, Paris C, Trillot N, Wibaut B, Hochart A, Marichez C, Dalibard V, Vanderziepe S, Bourgeois L, Gaul A, Jospin A, Stepina N, Pradines B, Tournoys A, Brousseau T, Rémy M, Hutt A. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 103.Gruber C, Patel R, Trachman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 52 kb)
Data Availability Statement
All code, additional supporting data, and the full data analysis have been made publicly accessible (url provided in Supplementary information)
N/A