Summary
Immunotherapy has revolutionised the treatment of oncologic malignancies. Immune checkpoint inhibitors represent a new class of immunotherapy drugs. Although these drugs show promise, they are associated with immune-related adverse reactions. An increasing number of patients who undergo surgery will have had treatment with immune checkpoint inhibitors. In this narrative review article, we discuss their mechanism of action, therapeutic effects, pertinent toxicities, and address specific perioperative considerations for patients treated with immune checkpoint inhibitors.
Keywords: adverse events, anaesthesia, cancer, immunotherapy, complications, immune checkpoint inhibitors, perioperative management, side effects
Editor's key points.
-
•
Immune checkpoint inhibitors are a new type of immunotherapy drug used to treat cancer. They show promising results but are associated with immune-related adverse events.
-
•
The authors review the current literature, discuss complications of these new drugs, and consider the implications for anaesthesiologists.
The use of the immune system to fight cancer is not a novel concept. As early as 1891, surgeon William Coley observed that repeat inoculations with erysipelas, a streptococcal infection, had the potential to induce cancer remission.1 These early observations laid the groundwork to understand the role of the immune system on tumour regression. In 2001, ground-breaking studies by Shankaran and colleagues2 and Dunn and colleagues3 unravelled the mystery of how tumour cells escape eradication and survive in immune-competent hosts. These studies identified immune-mediated mechanisms for tumour survival and inspired Nobel Prize laureate, such as James Allison and Tasuku Honjo, to develop pioneering therapies that weakened tumour defence mechanisms. A wide variety of options are now available and are designed to target specific components of the immune system (Table 1). These novel therapies include immune checkpoint inhibitors, chimeric antigen receptor T cell therapies, cancer vaccines, and non-specific immunotherapies.
Table 1.
Mechanisms of action and clinical indications of approved classes of immunotherapy drugs.
| Immune checkpoint inhibitors (ICI) | Monoclonal antibodies target specific receptor–ligand pathways on T cells | Ipilumamb (Yervoy®)—metastatic melanoma |
|---|---|---|
| Chimeric antigen receptor T cell (CAR-T) therapy | T cells are removed from the patient and genetically modified to express specific chimeric antigen receptors (receptors developed in the laboratory to bind specific proteins on tumour cells). T cells are then cloned and returned to the patient to trigger an immune response. | CAR-T cells—acute lymphoblastic leukaemia and lymphoma |
| Non-specific immunotherapies | No specific targets. Use of cytokines (e.g. interleukin [IL]-2, interferon α & β) to boost immune system or slow angiogenesis. | IL-2 therapy (Proleukin®)—kidney cancer BCG (intravesivular immunotherapy)—bladder cancer |
| Cancer vaccines | Exposure to tumour antigens activates a tumour-specific humoral response. | Sipuleucel-T (Provenge®)—prostate cancer |
Of these therapies, immune checkpoint inhibitors deserve the most consideration because they are indicated across a wide range of malignancies and carry a significant risk of immune-mediated adverse reactions. To date, the safety and the risk of adverse events for patients receiving immune checkpoint inhibitors in the perioperative period has not been adequately investigated.4 As an increasing number of patients on immune checkpoint inhibitors present for surgery, it is essential that anaesthesiologists are familiar with these drugs and their side-effects to reduce the risk of perioperative adverse events. This review introduces anaesthesiologists to emerging concepts in tumour immunology and related drug development. We discuss immune checkpoint inhibitors with attention to their clinical indications, mechanism of actions, and immune-related drug reactions. We conclude with a discussion on the anaesthetic considerations for patients on immune checkpoint inhibitors and provide future directions for research to optimise the perioperative care for cancer patients.
Cancer and the immune system: basic concepts and targets for therapy
Basic immunology
The immune system consists of two components: the innate and the adaptive immune response. The innate response is immediate and represents the first line of host defence. In the innate response, antigen-presenting cells, consisting of macrophages and dendritic cells, engulf pathogens through phagocytosis and present foreign antigens to immune cells through surface receptors, known as major histocompatibility complex class molecules.5 Antigen-presenting cells respond to foreign antigens by releasing cytokines to recruit and activate a complex network of immune cells including natural killer cells, mast cells, eosinophils, basophils, and phagocytic cells (e.g. macrophages, neutrophils) at the site of inflammation to boost the immune defence.
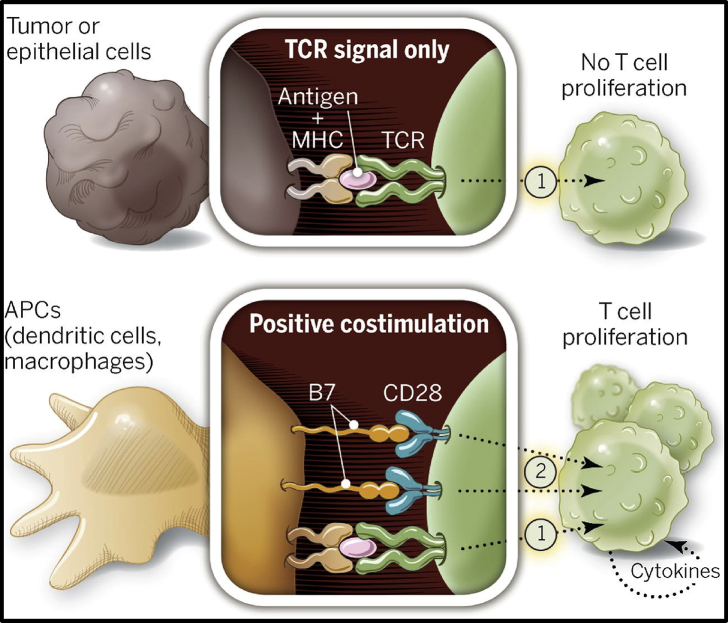
The innate immune system works in synergy with the adaptive immune system to promote eradiation of tumour cells. The adaptive immune system orchestrates a specific and highly coordinated attack through the activation of lymphocytes. When naïve T cells encounter a foreign antigen via specific T-cell receptors (TCRs), T cells become ‘primed’ initiating a signalling cascade for T-cell activation. Naïve T cells require at least two signals to sustain T-cell activation.6,7 The second activation signal occurs when CD28, a costimulatory receptor on the surface of T cells, binds to a B7 ligand, expressed on antigen-presenting cells (Fig. 1).8 Formation of the CD28-B7 receptor–ligand complex stimulates the proliferation and differentiation of T cells into naïve CD4+ and CD8+ T cells.9 Cytokine release from CD4+ and CD8+ T cells promote maturation into T cell subsets (e.g. helper T cells, natural killer T cells, regulatory T cells) and contribute to B-cell activation.
Fig 1.
Signalling cascade from the interactions of tumour cells and antigen-presenting cells with naive T cells. Two signals are required for T-cell activation and proliferation. The first signal comes from T-cell receptor binding to an antigen presented on a major histocompatibility complex on the surface of a tumour cell/antigen-presenting cell. Without a costimulatory receptor, T cells remain in a quiescent state. The second signal occurs with the binding of CD28 on T cells and B7 proteins on tumour/antigen-presenting cells. These two signals initiate T-cell activation and proliferation. APC, antigen-presenting cells; MHC, major histocompatibility complex; TCR, T-cell receptor. From ‘The future of immunotherapy’, P. Sharma and J. Allison, Science, 2015; 348: 56–61 Reprinted with permission.
Although the CD28-B7 receptor–ligand complex plays a pivotal role in T-cell activation, the interaction of structural homologue of CD28 and B7 can produce an inhibitory effect on T-cell function (Table 2). The expression of inhibitory receptors on T cells, such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), exert a suppressive effect on T cell function when these receptors are bound to specific B7 homologue on antigen-presenting cells or tumour cells. Similarly, the presence of PD-L1 or -L2 ligands (B7 ligands) on antigen-presenting cells and tumour cells produces an inhibitory effect when they bind to the PD-1 receptor on activated T cells.
Table 2.
Effect of receptor–ligand interactions on T cell function. CD28 consists of a family of receptors expressed on T cells. B7 proteins consist of a family of ligands, expressed on antigen-presenting cells and tumour cells. The binding of the CD28 receptor and B7 ligand has a stimulatory effect on T cell function. Homologues of CD28 and B7 have an inhibitory effect on T-cell function and are listed above. The cluster of differentiation is a classification system to identify specific surface proteins and is widely used for immunophenotyping. The cluster of differentiation (CD) is designated for each receptor and ligand. CTL-4, T-lymphocyte-associated antigen 4; PD-1, programmed cell death 1.
| Receptor (T cell) | Ligands (antigen-presenting cell or tumour cell) | Effect of receptor–ligand binding on T cell function |
|---|---|---|
| CD28 | B7 | Stimulatory |
| CTLA-4 (CD152) | B7-2 (CD86) | Inhibitory |
| PD-1 (CD279) | PD-L1 (CD274 or B7-H1) | Inhibitory |
| PD-1 (CD279) | PD-L2 (CD273 or B7-DC) | Inhibitory |
Host cells achieve immune homeostasis and self-tolerance by maintaining a balance between stimulatory and inhibitory signals from these receptor–ligand interactions. For this reason, these receptor–ligand interactions are often described as ‘immune checkpoints’ because the binding of TCRs to stimulatory and inhibitory ligands acts as an on–off switch for the immune system.10,11 Tumour cells have the capacity to evade immune surveillance by learning mechanisms to limit the immune response and protect themselves from T cell attack.12 The disruption of the ligand–receptor interaction remains the most sophisticated mechanisms for tumour cells to escape cell death.13 Tumour cells circumvent immune checkpoints through the upregulation of inhibitory proteins to escape recognition and elimination by the immune system. By examining specific immune checkpoint signalling pathways, we can better understand how immune checkpoint inhibitors exert their effect on tumour cells.
Targets in cancer immunology
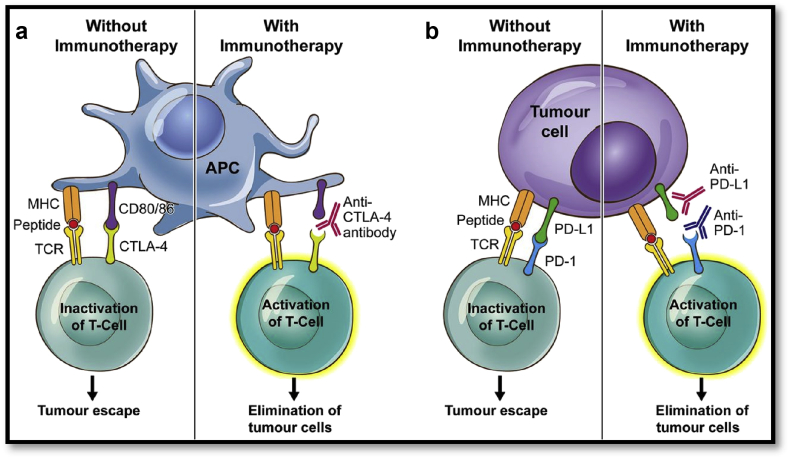
The two major immune checkpoint pathways targeted in anti-cancer therapy are: the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoint pathways. CTLA-4 (CD152) was the first TCR signalling pathway studied extensively. CTLA-4 (CD152) is expressed on activated T cells. It is structurally similar to the costimulatory receptor CD28, giving the molecule the ability to bind to B7 ligands on APCs residing in the lymph nodes or spleen (Fig. 2a).14 The main differences between CD28 and CTLA-4 (CD152) receptors is that CTLA-4 (CD152) has a greater binding affinity to B7 ligands and its overexpression inhibits T-cell activation.10 Tumour antigens induce the overexpression of CTLA-4 (CD152) on the surface of T cells, thereby suppressing T-cell activation. When immune checkpoint inhibitors interfere with the binding of the CTLA-4 receptor to B7 ligand, T cells are activated and eradicate tumour cells (Fig. 2a).
Fig 2.
Ligand–receptor interactions between tumour cells and activated T cells and targets for anti-PD-1 and anti-CTLA-4 therapy. (a) The interaction of the CTLA-4 receptor on T cells with the CD-80 ligand (B-7 homologue) on an antigen-presenting cells promotes tumour escape. The binding of an anti-CTLA-4 antibody promotes T-cell activation and elimination of tumour cells. (b) The interaction of PD-1 receptor on T cells with PD-L1 ligand on tumour cells promotes T-cell anergy and tumour escape. In the presence of an anti-PD-1 or anti-PD-L1 antibody, T cells become activated and initiate tumour cell death. CTL-4, T-lymphocyte-associated antigen 4; PD-1, programmed cell death 1; APC, antigen-presenting cells; MHC, major histocompatibility complex; TCR, T-cell receptor. From ‘CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition’, E. Buchbinder and A. Desai, American Journal of Clinical Oncology, 2016; 39: 98–106. Reprinted with permission.
In a similar mechanism to CTLA-4, the PD-1 (CD279) receptor, another structural homologue of the CD28 receptor, blocks T-cell proliferation and induces immunological tolerance.15 PD-1 (CD279) binds to PD-L1 (CD274) ligand, PD-L2 (CD273) ligand, or both, which belong to the family of B7 proteins (Fig. 2b). PD-1 (CD279) receptor is expressed broadly on peripheral CD4+ and CD8+ T cells, B cells, and myeloid cells.14,16 The ligands PD-L1 (CD274) and PD-L2 (CD273) are not exclusively expressed on antigen-presenting cells and tumour cells, but can also be found in low levels on the surface of non-haematopoietic cells (e.g. endothelial cells, pancreatic islet cells, testes, eye, and cardiomyocytes).16,17 The differential expression of CTLA-4 (CD152) and PD-1 (CD279) receptors on multiple cell lines have clinical importance and may predict clinical responses to immune checkpoint inhibitors.
Immune checkpoint inhibitors: a targeted therapy
The manipulation of immune checkpoints through monoclonal antibody blockade of the checkpoint ligands, receptors, or both has been one of the most successful anti-cancer therapies to date. Early experimental models in mice demonstrated that the use of anti-CTLA-4 monoclonal antibodies not only induced T-cell activation, but also led to tumour regression in specific tumour types.18,19 The effects of CTLA-4 blockade were explored in numerous animal models for melanoma, breast cancer, lymphoma, and prostate cancer, but were most significant for melanoma.20, 21, 22, 23 In 2011, ipilimumab, a human monoclonal CTLA-4 antibody, was the first immune checkpoint inhibitor approved for use in patients with metastatic or unresectable melanoma. Clinical data confirmed that 1 and 2 yr survival rates were equal to 46% and 24% for ipilimumab and 25% and 14% for the control group, respectively.14 The clinical success of ipilimumab in melanoma encouraged the development of new immune checkpoint inhibitors (Table 3). Tremelimumab, an anti-CTLA-4 antibody, was developed after ipilimumab and was tested in clinical trials, but failed to receive Food and Drug Administration (FDA) approval as a monotherapy, placing ipilimumab as the only anti-CTLA-4 antibody to demonstrate overall and progression-free survival for melanoma. Although ipilimumab has improved survival rates for melanoma, its clinical application to other malignancies has been limited. Clinical trials in prostate cancer, small cell lung cancer, and non-small cell lung cancer (NSCLC) have failed to demonstrate an improvement in survival outcomes.24,25
Table 3.
Immune checkpoint inhibitors and their clinical indications. Immune checkpoint inhibitors are classified into three major classes: anti-CTLA-4 monoclonal antibodies, anti-PD-1 monoclonal antibodies and anti-PD-L1 monoclonal antibodies. Each immune checkpoint inhibitor was FDA-approved for specific clinical indications. Tremelimumab is the only immune checkpoint inhibitor that has failed to receive FDA approval. CTL-4, T-lymphocyte-associated antigen 4; FDA, Food and Drug Administration; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; PD-1, programmed cell death 1; RCC, renal cell carcinoma.
| Anti-CTLA-4 monoclonal antibody (mAb) | Ipilimumab (Yervoy®) | Unresectable or metastatic melanoma |
| Tremelimumab | No clinical indication | |
| Anti-PD-1 monoclonal antibody (mAb) | Pembrolizumab (Keytruda®) | Hodgkin's lymphoma |
| Metastatic non-squamous NSCLC | ||
| Locally advanced or metastatic urothelial carcinoma | ||
| Nivolumab (Opdivo®) | Unresectable or metastatic melanoma | |
| Non-squamous and metastatic squamous NSCLC | ||
| Advanced RCC | ||
| Hodgkin's lymphoma | ||
| Locally advanced or metastatic urothelial carcinoma | ||
| Metastatic colorectal cancer | ||
| HCC | ||
| Squamous cell cancer of head and neck | ||
| Anti-PD-L1 mAb | Atezolizumab (Tecentriq®) | Locally advanced or metastatic urothelial carcinoma Metastatic NSCLC |
| Avelumab (Bevancio®) | Metastatic Merkel Cell carcinoma Locally advanced or metastatic urothelial carcinoma | |
| Durvalumab (Imfinzi®) | Unresectable stage III NSCLC Locally advanced or metastatic urothelial carcinoma | |
| Anti-CTLA-4 mAb + Anti-PD-1 mAb | Ipilimumab + Nivolumab (Yervoy® + Opdivo®) | Unresectable or metastatic melanoma |
| RCC | ||
| Metastatic squamous cell carcinoma of the head and neck | ||
| Metastatic NSCLC |
Given its limitations, researchers have redirected their interest to other inhibitory surface proteins. Antibodies targeting the PD-1/PD-L1 pathway represent the second generation of immune checkpoint inhibitors. The development of PD-1 inhibitors, pembrolizumab and nivolumab, have extended survival for patients diagnosed with a wide range of malignancies, ranging from NSCLC to colorectal cancer. In contrast, the spectrum of malignancies responsive to PD-L1 inhibitors is narrow, and treatment has been limited to urothelial carcinoma, NSCLC, and metastatic Merkel cell carcinoma. A complete list of FDA-approved immune checkpoint inhibitors and current clinical indications are featured in Table 3.
Although the list of clinical indications is expansive, the clinical response to immune checkpoint inhibitors is variable and differences in clinical response limit their broad applicability. Several factors predict long-term clinical benefit including gene overexpression and the presence of mutations in tumour cells. As a case in point, poorly differentiated non-small cell lung tumours overexpress PD-L1, an inhibitory ligand. PD-L1 expression is defined by a tumour proportion score, which represents the percentage of partial or complete staining of PD-L1 proteins in a tumour sample.26 A tumour proportion score ≥50% indicates a high level of PD-L1 expression, and these tumours respond well to anti-PD-L1 inhibitors in advanced NSCLC.27, 28, 29 When pembrolizumab, an anti-PD-L1 inhibitor, was compared with a common chemotherapy regimen with carboplatin plus pemetrexed (an anti-folate anti-neoplastic agent) in patients with a tumour proportion score ≥50%, the progression-free survival was 10.3 months (95% confidence interval [CI], 6.7 to not reached) compared with 6.0 months (95% CI, 4.2–6.2) in the chemotherapy group.26 Another predictor of clinical response is the tumour mutational burden, which measures the number of mutations in a tumour. Melanoma, NSCLC, and bladder cancer represent some of the highest frequency of somatic mutations among tumours and respond well to a range of immune checkpoint inhibitors.30 Researchers argue that tumours with a high tumour mutational burden generate a higher number of foreign antigens, provoking a more robust immune response in the presence of immune checkpoint inhibitors.31, 32, 33 By performing gene sequencing, medical oncologists can obtain the genetic profile of a tumour and can select an immune checkpoint inhibitor based on the genetic characteristics of the tumour rather than the tumour type. Genetic sequencing has been successfully used in the management of NSCLC patients and has been incorporated in practice guidelines. The current guidelines recommend testing for common somatic mutations of EGFR, ALK, ROS1, BRAF, and KRAS genes and the immunohistochemistry testing for the expression of PD-L1 expression before initiating PD-L1 inhibitors in NSCLC patients.34 As a comprehensive list of mutations develop, similar guidelines are emerging as more genetic mutations are identified.
When medical oncologists initiate therapy with an immune checkpoint inhibitor, they typically administer these drugs as an intravenous infusion. The dosing intervals and duration of treatment therapy varies both by drug and disease. The CTLA-4 inhibitor, ipilimumab, is administered every 3 weeks for a total of four doses based on FDA-approved scheduling. PD-1/PD-L1 inhibitor treatment regimen can vary from every other week to every 2–3 weeks. The duration of PD-1/PD-L1 therapy is dependent on the side-effects, response to treatment, or both. Throughout the treatment course, medical oncologists follow patients closely to identify and manage immune-related adverse reactions. Medical oncologists classify the severity of these reactions into five major categories based on the Common Terminology Criteria for Adverse Events. Grade 1 reactions are generally mild and only require observation. Grade 3 or 4 reactions are often severe conditions, life-threatening conditions, or both, requiring intervention. If adverse reactions are life-threatening, medical oncologist may postpone treatment or make the decision to permanently discontinue therapy.
Immune-related reactions and anaesthetic considerations
Immune-related adverse reactions can involve any organ system. The most commonly affected systems are the gastrointestinal tract, skin, and endocrine glands, but severe reactions are associated with less commonly affected organ systems such as pulmonary, cardiac, and neurologic systems.35 The incidence of any grade immune-related adverse reactions has been reported as low as 15% to as high as 90%.36, 37, 38 The incidence also varies among immune checkpoint inhibitors. For anti-CTLA-4 therapy, the incidence of immune related adverse reactions is about 72% (across all grades) and 24% with grade 3 or higher; meanwhile, the incidence for anti-PD-1 therapy is slightly lower than that of anti-CTLA-4 monotherapy, combination therapy, or both at about 50–60% (across all grades) and <10% (grade 3 or higher).35 With the increase of immune-related reactions from immune checkpoint inhibitors, it is of paramount importance for anaesthesiologists to recognise the risk of immune-related adverse reactions as it relates to their patient and perioperative management. For purposes of this review, we limit our discussion to the organ toxicities with the greatest potential for perioperative complications.
Endocrine toxicities
Immune-mediated endocrinopathies are common adverse reactions associated with immune checkpoint inhibitors. The most common abnormality after initiation of immune checkpoint inhibitors is pituitary dysfunction. Hypophysitis is an inflammatory condition of the pituitary gland, resulting in multiple hormone deficiencies. Hypophysitis occurs as early as 4 weeks after initiation of treatment, but the median time to onset is 11 weeks.39,40 The incidence of hypophysitis is reported as 10–15% for patients on anti-CTLA-4 therapy and <1% for patients on anti-PD-1/PD-L1 therapy.41, 42, 43 Hypophysitis has a wide spectrum of clinical presentations because patients are at risk for multiple endocrine failure from hypothyroidism, adrenal insufficiency, hypogonadism, and, in rare cases, diabetes insipidus. Endocrinologists generally recommend obtaining baseline laboratory results for thyroid stimulating hormone (TSH), free T4, and an 8 am adrenocorticotropic hormone (ACTH) and cortisol levels.44 Pituitary enlargement on MRI is the most sensitive and specific indicator of hypophysitis and can confirm abnormal laboratory findings.42 Patients with grade 2 hypophysitis (moderate symptoms) receive hormone replacement (thyroid supplementation) and high-dose steroids.43 If patients fail to recover pituitary function, then long-term steroid supplementation is warranted.
In the perioperative period, anaesthesiologists should evaluate trends in laboratory values for TSH and ACTH. If TSH, ACTH, or both progressively decline, surgery should be postponed, and an endocrine consult should be requested. For patients with a known diagnosis of immune checkpoint inhibitor-induced hypopituitarism, an endocrinologist should be involved throughout the perioperative period to determine adequate hormone and steroid replacement before and after surgery. When hypopituitarism goes undetected, it can potentially lead to major perioperative complications. Morbidity attributed to immune-mediated hypopituitarism is thought to be predominantly associated with adrenal insufficiency.43,45 The adrenal gland plays an important role in the regulation of water homeostasis, acid–base, and electrolyte balances.46 Therefore, adrenal insufficiency can manifest as electrolyte abnormalities (i.e. persistent hyponatraemia) and persistent hypotension. If patients develop intraoperative hypotension that cannot be adequately managed by conservative means (e.g. optimising depth of anaesthesia, fluid resuscitation, and the use of vasopressors), then it should raise suspicion for an adrenal crisis, and a rescue dose of 100 mg of hydrocortisone IV should be administered, followed by continued supplementation of 50 mg of hydrocortisone IV every 6 h.47,48
Although anaesthesiologists must evaluate for adrenal insufficiency in the preoperative evaluation, it is important to pay close attention to thyroid function tests. Thyroid hormone levels can fluctuate dramatically after the initiation of an immune checkpoint inhibitor. Hypothyroidism is commonly observed. The reported incidence of primary hypothyroidism with the use of anti-CTLA-4, anti-PD-1, and anti-PD-L1 as a monotherapy is 5–10%; the incidence increases to 22% when anti-CTLA-4 and anti-PD-1 are used in combination therapy.49,50 Thyrotoxicosis often precedes the onset of hypothyroidism and is typically self-limited. Hypothyroidism can develop as early as 5 months to as late as 3 yr after treatment.50 Therefore, endocrinologists recommend monitoring thyroid function tests every 2–3 weeks.44 Patients with subclinical hypothyroidism (an elevated serum TSH and normal free T4) are generally asymptomatic and unlikely to experience major anaesthetic complications during elective surgery. In moderate to severe cases of hypothyroidism, elective surgery should be postponed until an euthyroid state is achieved. For emergency surgery, patients may present with undetected hypothyroidism. The anaesthetic complications from untreated hypothyroidism include delayed emergence, hypothermia, bradycardia, low cardiac output, and an impaired hypoxic and hypercapnic respiratory drive.51 In severe cases, patients may develop myxoedema coma, a rare, life-threatening condition. Patients with myxoedema coma present with mental status changes, hypoventilation, profound hypotension, bradycardia, severe hypothermia, and electrolyte abnormalities. The mortality from myxoedema coma is reported as high as 60% if undiagnosed or left untreated.52, 53, 54 If there is any suspicion of myxoedema coma, it is recommended initially using either 200–400 μg of intravenous levothyroxine (T4) followed by 100 μg day−1 or 10–25 μg of IV triiodothyronine (T3) every 8 h with careful dosing in cardiac patients.55 The use of corticosteroid administration is recommended because myxoedema coma can have a concomitant diagnosis of adrenal insufficiency.
The fluctuations of thyroid levels during treatment with an immune checkpoint inhibitor can also precipitate hyperthyroidism. The overall incidence of hyperthyroidism is estimated to be 2.9%, but its incidence ranges from 0.6% with PD-L1 inhibitors to 8.0% with combination therapy.56 If the patient has subclinical thyrotoxicosis/hyperthyroidism, the initiation of a beta-blocker along with methimazole or propylthiouracil several weeks before surgery is recommended. If hyperthyroidism is left untreated, patients are at risk for thyroid storm intraoperatively. Patients may present with tachycardia, fever, and, in extreme cases, cardiovascular collapse. The management of thyroid storm requires the use of intravenous beta-blockers, but treatment is mostly supportive with hydration, cooling, inotropes, and steroids as needed.57 The administration of iodine is reserved as a rescue therapy because it rapidly decreases hormone production and reduces the peripheral conversion of thyroxine to triiodothyronine. Iodine can be administered orally as 1 ml Lugol's solution every 6 h or intravenously as iapanoic acid 1 g every 8 h in the first 24 h of treatment.58
Along with thyroid abnormalities, the exocrine function of the pancreas may be impaired from immune checkpoint therapy. Diabetes from immune checkpoint inhibitors is rare with an incidence of <1% and is associated with anti-PD-1 and anti-PD-L1 therapies.59 Hyperglycaemia may be difficult to control in patients with a known history of diabetes, and adjustments to insulin regimens may be required. Although immune-related reactions are treated with steroids, corticosteroids can worsen hyperglycaemia and are generally not recommended for patients who develop diabetes during treatment. It is important to monitor for hyperglycaemia throughout the perioperative and administer insulin as necessary.
Cardiac toxicities
Cardiac complications from immune checkpoint inhibitors are rare, and the overall incidence is reported as <1%.60 The cardiotoxic effects can manifest as heart failure, cardiomyopathy, conduction abnormalities, myocardial fibrosis, myocarditis, and pericarditis. However, myocarditis is the most common adverse cardiac reaction with an incidence of 1.14%.61,62 The incidence of myocarditis is highest with the combination of ipilimumab and nivolumab (0.27%) compared with nivolumab alone (0.06%).17,63 When therapy is initiated, the median onset of myocarditis is 17 days.17 The clinical symptoms of myocarditis can vary from non-specific symptoms (i.e. fatigue) to more specific symptoms such as dyspnoea and chest pain. Most patients with grade 1 (asymptomatic) or 2 (mild) cardiac adverse cardiac events can be observed and managed medically. Patients with a grade 3 cardiac event—defined as an elevated brain natriuretic peptide (BNP), elevated troponins, or new ECG findings (QTc prolongation, new conduction disease, or ST-T wave changes)—require a cardiology consult based on recommendations by the Society for Immunotherapy of Cancer Toxicity (SITC) Management Working Group.64 Cardiologists confirm the diagnosis of myocarditis with cardiac MRI or an endomyocardial biopsy (gold standard). Severe cases of myocarditis are treated with high-dose corticosteroids with methylprednisolone 1 g day−1. Although steroids are the mainstay of treatment, immunosuppressive agents, therapy with infliximab, mycophenolate mofetil, or tacrolimus is an alternative if myocarditis fails to respond to steroids.60 Steroid and immunosuppressive therapy should continue until resolution of symptoms.60 If biopsy findings are non-specific, steroids may not be appropriate, and patients may be managed symptomatically with the use of beta-blockers, diuretic therapy, and angiotensin-converting enzyme (ACE) inhibitors.
In the preoperative assessment, anaesthesiologists should review a preoperative ECG, baseline troponins, and BNP, and obtain a transthoracic echocardiogram for patients with a history of myocarditis. Overt signs of heart failure should prompt anaesthesiologists to postpone elective surgery until the patient is medically optimised. A cardiologist should evaluate patients before surgery to determine whether further testing is warranted. For major elective surgery, intraoperative management should include the use of invasive arterial pressure monitoring and intraoperative transoesophageal echocardiography should be considered to monitor contractility and guide fluid management. Patients on chronic steroid therapy must receive stress dose steroids to prevent an adrenal crisis.
Pulmonary toxicities
The pulmonary toxicities from immunotherapy checkpoint inhibitors vary, but pneumonitis is the most common pulmonary complication reported. The incidence of pneumonitis from PD-1 inhibitors has been reported as high as 3–7%, whereas the incidence with PD-L1 inhibitors is 1.3%.65,66 The clinical presentation characteristically involves cough, shortness of breath, wheezing, and, in severe cases, hypoxaemia. The clinical features of PD-1 inhibitor-induced pneumonitis can be very non-specific and difficult to distinguish from patients with underlying pulmonary disease. If the clinical diagnosis is unclear, a chest film and CT scan should be ordered to ensure a timely diagnosis. The most common radiologic findings of PD-1 inhibitor-related pneumonitis include ground glass opacities with peripheral multifocal consolidations, a pattern similar to cryptogenic organising pneumonia (COP) on CT scan.66
Management of pneumonitis requires the use of corticosteroids. In recalcitrant cases, the addition of infliximab, cyclophosphamide, or both may be needed for patients with a poor response to steroids.64 The chronic use of steroid therapy is relevant to preoperative planning because these patients may require steroid supplementation intraoperatively depending on the severity of illness and complexity of surgery. Patients with a diagnosis of NSCLC may receive treatment with a PD-1 inhibitor, which is associated with a high incidence of pneumonitis. NSCLC patients will require thoracic surgery during and/after their treatment, and anaesthesiologists must use strategies to minimise their risk of acute lung injury. The judicious use of fluids and the use of protective ventilation strategies to reduce lung injury in this population is essential.
Gastrointestinal toxicities
Of all toxicities, gastrointestinal complications represent the most common adverse reaction from immune check point inhibitors. Gastrointestinal reactions include diarrhoea, gastritis, enterocolitis, and hepatitis. Enterocolitis consistently remains the most common gastrointestinal complication with an incidence of 20–30%.67 Patients with grade 1–2 colitis can be managed with observation for 2–3 days but require the initiation of oral prednisone at 1 mg kg−1 day−1 if symptoms persist beyond this observation period. When patients develop grade 3 colitis, defined as severe abdominal pain associated with ≥7 bowel movements day−1, then these patients require the discontinuation of ICIs and immediate hospitalisation. In severe cases, intravenous steroids are initiated with or without the addition of immunosuppressive agents (i.e. infliximab 5 mg kg−1).64,68
Along with colitis, hepatitis is another adverse reaction from immune checkpoint inhibitor therapy with an incidence of 5–10%.69,70 The incidence of hepatitis in patients treated with anti-PD-1 therapy is estimated as 5%, but this increases to 30% in patients on a combination regimen consisting of ipilimumab and nivolumab.71,72 Medical oncologists generally screen certain susceptible populations for immune-mediated hepatitis (i.e. human immunodeficiency virus [HIV], hepatitis B and C) before the initiation of immunotherapy. Any abnormalities on routine liver function test (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and bilirubin) should prompt further workup for infection, inflammation, biliary obstruction, or progression of disease. In the absence of other hepatic disease, steroids, immunosuppressive drugs, or both are indicated for patients with grade 2 hepatitis (defined as AST/ALT levels <5×).64 In severe cases, a liver biopsy is recommended.
Anaesthesiologists may provide care for patients with gastrointestinal disorders in remote anaesthesia locations, such as the endoscopy or interventional radiology suite. The preoperative assessment should include a detailed history with attention to the patient's comorbidities, the use of immune checkpoint inhibitors, and prior immune-related reactions. With a thorough history, anaesthesiologists will be well equipped to handle unanticipated perioperative complications related to immune checkpoint inhibitors.
Preoperative evaluation
Immune-related reactions present unique challenges for anaesthesiologists and the development of an algorithm for the perioperative management of patients on immune checkpoint inhibitors may improve patient safety. In 2017, the SITC organised a multidisciplinary Toxicity Management Working Group to standardise the management of immune-related reactions.64 These recommendations offer guidance to medical oncologists to assist in the early diagnosis and management of immune related adverse reactions. Although these recommendations were not originally intended for anaesthesiologists, the content is relevant to our clinical practice and provides a framework for preoperative evaluation. Our institution follows the established guidelines from the Toxicity Management Working Group, and we have developed a list of the most common serologic tests collected at our institution before surgery (Table 4). In our experience, the multidisciplinary communication between the anaesthesiologist, medical oncologist, and surgeon improves patient safety and minimises delays in surgery.
Table 4.
Preoperative assessment of patients on immune checkpoint inhibitors. 6MWT, 6 min walk test; ACTH, adrenocorticotropic hormone; HbA1c, haemoglobin A1C; ICI, immune checkpoint inhibitor; TSH, thyroid stimulating hormone.
| Preoperative assessment |
|---|
|
History Assessment of cardiopulmonary function; detailed history of common treatment-related events (endocrine and organ-specific complications) after initiation of immune checkpoint inhibitors. |
|
Laboratory tests Basic: complete blood count, complete metabolic panel Endocrine: TSH, free T4, HbA1c, ACTH, cortisol, cortisol stimulation test (may not be feasible in all patients on ICI) Cardiac: baseline troponins at 6 weeks after treatment, B-natriuretic peptide |
Studies
|
Conclusions
Checkpoint inhibitors represent a new drug class that specifically assist the patient's own immune system in recognising and destroying certain types of cancer. Clinicians must realise that by initiating such therapies and disrupting the equipoise of the immune system, an increased occurrence of immune-related complications will be seen. These negative effects of immune checkpoint inhibitors on multiple organ systems can be profound. One of the intriguing findings from a review of the literature is that adverse events do not occur in isolation and patients can experience multiple immune-mediated reactions concurrently. Many of these immune-mediated reactions are responsive to corticosteroids, but a few adverse reactions are irreversible and unresponsive to corticosteroid therapy. Anaesthesiologists should anticipate that in the coming years many of their patients will have received or will be on active therapy with an immune checkpoint inhibitor. Consequently, it is imperative that perioperative care providers gain familiarity and recognise potential side-effects of these medications in order to optimise their patients' treatment accordingly.
Declaration of interest
The authors declare that they have no conflicts of interest.
Handling editor: Jonathan Hardman
References
- 1.Coley W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 2.Shankaran V., Ikeda H., Bruce A.T. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Dunn G., Bruce A., Ikeda H., Old L.J., Schreiber R. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 4.Elias A.W., Kasi P.M., Stauffer J.A. The feasibility and safety of surgery in patients receiving immune checkpoint inhibitors: a retrospective study. Front Oncol. 2017;7:121. doi: 10.3389/fonc.2017.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Töpfer K., Kempe S., Müller N. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy K.D., Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol. 1999;77:1–10. doi: 10.1046/j.1440-1711.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 7.Bretscher P. The two-signal model of lymphocyte activation twenty-one years later. Immunol Today. 1992;13:74–76. doi: 10.1016/0167-5699(92)90138-W. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P., Allison J. The future of immunotherapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 9.Buchbinder E., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 12.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernald K., Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620–633. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugowska I., Teterycz P., Rutkowski P. Immunotherapy of melanoma. Contemp Oncol (Pozn) 2018;22:61–67. doi: 10.5114/wo.2018.73889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavala V.A., Kalergis A.M. New clinical advances in immunotherapy for the treatment of solid tumours. Immunology. 2015;145:182–201. doi: 10.1111/imm.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riella L.V., Paterson A., Sharpe A., Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varricchi G., Galdiero M.R., Marone G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2:e000247. doi: 10.1136/esmoopen-2017-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney E.R., Walunas T.L., Karr R.W. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 19.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 20.Wang X.Y., Zuo D., Sarkar D., Fisher P.B. Blockade of cytotoxic T-lymphocyte antigen-4 as a new therapeutic approach for advanced melanoma. Expert Opin Pharmacother. 2011;12:2695–2706. doi: 10.1517/14656566.2011.629187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz A.A., Yu T.F., Leach D.R., Allison J.P. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Ginderachter J.A., Liu Y., Geldhof A. B7-1, IFN gamma and anti-CTLA-4 co-operate to prevent T-cell tolerization during immunotherapy against a murine T-lymphoma. Int J Cancer. 2000;87:539–547. [PubMed] [Google Scholar]
- 23.Kwon E.D., Hurwitz A.A., Foster B.A. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beer T.M., Kwon E.D., Drake C.G. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 25.Govindan R., Szczesna A., Ahn M. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non–small-cell lung cancer. J Clin Oncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 26.Kulangara K., Zhang N., Corigliano E. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 27.Ghandi L., Rodriguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 28.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 29.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 30.Laurence M.S., Stojanov P., Polak P. Mutational heterogeneity in cancer and the search for new cancer genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varecki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immuno Ther Cancer. 2018;6:157. doi: 10.1186/s40425-018-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan T.A., Yarchoan M., Jaffee E. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman A.M., Kato S., Bazhenova L. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melendez B., Van Campenhout C., Rorive S. Methods of measurement for tumor mutational burden in tumour tissue. Transl Lung Cancer Res. 2018;7:661–667. doi: 10.21037/tlcr.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kottschade L.A. Incidence and management of immune-related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep. 2018;20:24. doi: 10.1007/s11912-018-0671-4. [DOI] [PubMed] [Google Scholar]
- 36.Friedman C.F., Proverbs-Singh T.A., Postow M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V., Chaudhary N., Garg M., Floudas C.S., Soni P., Chandra A.B. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winer A., Bodor J.N., Borghaei H. Identifying and managing the adverse effects of immune checkpoint blockade. J Thorac Dis. 2018;10:S480–S489. doi: 10.21037/jtd.2018.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahzari M., Liu D., Arnaout A., Lochnan H. Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol Diabetes. 2015;8:21–28. doi: 10.4137/CMED.S22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillard T., Yedinak C.G., Alumkal J., Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 41.Albarel F., Gaudy C., Castinetti F. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti- CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172:195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 42.Faje A.T., Sullivan R., Lawrence D. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078–4085. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 43.Byun D.J., Wolchok J.D., Rosenberg L.M., Girotra M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;134:195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girotra M., Hansen A., Farook A. The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr. 2018;2:pky021. doi: 10.1093/jncics/pky021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Postow M.A. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;76–83 doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 46.Rassam S.S., Counsell D.J. Perioperative electrolyte and fluid balance. CEACCP. 2005;5:157–160. [Google Scholar]
- 47.Bornstein S.R., Allolio B., Arlt W. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M., Reidy A., Saatee S., Collard C. Perioperative steroid management: approaches based on current evidence. Anesthesiology. 2017;127:166–172. doi: 10.1097/ALN.0000000000001659. [DOI] [PubMed] [Google Scholar]
- 49.Sznol M., Postow M.A., Davies M.J. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70–76. doi: 10.1016/j.ctrv.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Ryder M., Callahan M., Postow M.A., Wolchok J., Fagin J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palace M.R. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10 doi: 10.1177/1178632916689677. 1178632916689677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt W., Mann K. Incidence, clinical picture, and treatment of hypothyroid coma: results of a survey. Med Klin. 1997;92:521–524. doi: 10.1007/BF03044925. [DOI] [PubMed] [Google Scholar]
- 53.Dutta P., Bhansali A., Masoodi S., Bhadada S., Sharma N., Rajput R. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit Care. 2008;12:R1. doi: 10.1186/cc6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am. 2006;35:687–698. doi: 10.1016/j.ecl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Bennett-Guerrero E., Kramer D.C., Schwinn D.A. Effect of chronic and acute thyroid hormone reduction on perioperative outcome. Anesth Analg. 1997;85:30–36. doi: 10.1097/00000539-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Barroso-Sousa R., Barry W.T., Garrido-Castro A.C. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farling P.A. Thyroid disease. Br J Anaesth. 2000;85:15–28. doi: 10.1093/bja/85.1.15. [DOI] [PubMed] [Google Scholar]
- 58.Carroll R., Matfin G. Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab. 2010;1:139–145. doi: 10.1177/2042018810382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haanen J., Carbonnel F., Robert C. ESMO guidelines committee, management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:119–142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 60.Ganatra S., Neilan T.G. Immune checkpoint inhibitor associated myocarditis. Oncologist. 2018;23:879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immuno Ther Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishino M., Chambers E.S., Chong C.R. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res. 2016;4:289–293. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khunger M., Rakshit S., Pasupuleti V. Incidence of pneumonitis with use of PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 67.Stucci S., Palmirotta R., Passarelli A. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett. 2017;14:5671–5680. doi: 10.3892/ol.2017.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. NEJM. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 69.Robert C., Schachter J., Long G.V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 70.Larkin J., Chiarion Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spain L., Diem S., Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Villadolid J., Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]