Abstract
Introduction:
Bilateral internal thoracic arteries (BITA) based coronary bypass grafting may improve long term outcomes but is associated with increased risk of deep sternal wound infection (DSWI). We analyzed whether BITA skeletonization impacts DSWI and operative mortality (OM) using the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD).
Methods:
Primary, isolated, non-emergent/non-salvage BITA patients between July 2017 and December 2018 in the STS ACSD were divided into groups based on BITA harvesting technique: both arteries skeletonized (ssBITA) either non-skeletonized (Non-ssBITA). DSWI and OM Observed-to-Expected (O/E) ratios were compared across groups using the STS Peri-operative Risk Models. ssBITA versus Non-ssBITA DSWI and OM Adjusted Odds Ratio (AOR) were calculated by multivariable logistic regression and among propensity score matched comparison groups.
Results:
11,269 BITA patients (42.8%-ssBITA, 57.2%-Non-ssBITA) operated on by 1,448 surgeons from 770 hospitals were analyzed. The ssBITA group had a higher incidence of obesity, diabetes, hyperlipidemia, cerebrovascular disease, systolic heart failure and off-pump surgery and a longer total operative time. Overall incidence of DSWI and OM was 0.98%(O/E-5.1) and 1.72%(O/E-1.4), respectively and was 28%(p=0.129) and 23%(p=0.096) lower in ssBITA. After multivariable adjustment, ssBITA was associated with a decreased risk of DSWI [AOR(95%CI) 0.66(0.44–1.00),p=0.05]. In the 3884 matched pairs, the DSWI was also lower [AOR(95% CI) 0.60(0.36–0.09),p=0.05]. There was not difference in OM. Incidence of DSWI increased sharply in patients with increasing number of risk factors for DSWI regardless of BITA harvesting technique with a trend for higher DSWI with Non-ssBITA for all risk categories.
Conclusion:
The observed high O/E indicates that BITA is associated with increased risk of DSWI. Risk adjusted DSWI rates and relatively lower O/E ratios in case of ssBITA support a potential reduction of DSWI risk with skeletonization.
VISUAL ABSTRACT
Key Question:
Does skeletonization of internal thoracic arteries effect outcomes in bilateral internal thoracic arteries (BITA) coronary artery bypass grafting (CABG)?
Key Findings:
BITA use is associated with increased risk of deep sternal wound infections (DSWI) regardless of harvesting technique, but skeletonization mitigates that risk with no effect on peri-operative mortality.
Take-home Message:
Compared to non-skeletonized BITA grafts in CABG, skeletonization of BITA decreases the risk of DSWI.
Introduction
Multi arterial bypass grafting (MABG) using bilateral internal thoracic arteries (BITA-MABG) or radial arteries in conjunction with left internal thoracic arteries (RA-MABG) may improve long term outcomes in patients undergoing surgical myocardial revascularization [1–3]. Yet despite these benefits, the use of MABG remains under utilized in the US and, to a lesser degree, in Europe and Australia [4]. One concern about BITA-MABG possibly contributing to its under utilization is the increased risk of deep sternal wound infection (DSWI) compared to both the traditional single internal thoracic artery bypass approach and RA-MABG [2,5,6]. It is thought that excessive devascularization of the anterior chest wall inherent in BITA harvesting may contribute to wound healing complications including DSWI [7,8]. DSWI is a devastating complication associated with increased morbidity, mortality and resource utilization [9–12] with incremental costs as high as $75,000[13]. Thus, the well documented long term survival benefits of BITA-MABG[6,14,15] must be balanced against the short term higher risk of DSWI. DSWI is considered a “Never Event” by the Centers for Medicare and Medicaid Services and its costs have not been reimbursed since 2008 [10]. As such, extensive efforts have been expanded to minimize this risk[16]. Most [17–22], but not all studies [23], have shown that using skeletonization of BITA grafts may possibly mitigate this increased risk of DSWI. Since the majority of these studies are relatively small, relying on non-standardized definitions of what constitutes a DSWI as well as other variables and given the infrequent incidence of DSWI of 0.7% to 1.6% [5,9,10], there is concern that most of these studies: 1. Do not provide an accurate assessment of the benefits of skeletonization and 2. Are not powered to reliably detect differences in DSWI risk between skeletonized and non skeletonized BITA harvesting. To overcome these shortcomings, we reviewed the large nation-wide experience with BITA-MABG using the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS-ACSD), reflecting the practice patterns of the majority of American cardiac surgeons, to compare the risk of peri-operative DSWI of skeletonized versus non skeletonized BITA harvesting technique. In addition, given previous reports of surgeon experience with BITA-MABG and decreased DSWI [5,24], we also analyzed the impact of BITA harvesting on outcomes as a function of surgeon and hospital case volumes. Finally, in light of other reported benefits of skeletonization such as decreased acute and long term mortality among high risk CABG patients, increased graft blood flow resulting in decreased incidence of low flow state and lower need for an intra-aortic balloon pump, decreased post operative pain, increased graft length and size [25], we also assessed whether BITA skeletonization impacts the secondary study endpoint of perioperative : operative mortality (OM).
Methods
Patients:
The STS-ACSD was queried for all patients undergoing BITA based CABG from July 2017, which corresponded to the date at which BITA harvesting technique data was first collected within the database, to December 2018. Patients undergoing primary, isolated, non-emergent/non-salvage without missing harvest or outcome data were included in the analysis. Consistent STS definitions for all variables were used in the analysis. Patient comparison groups were based on BITA harvesting technique: ssBITA (both internal thoracic arteries skeletonized) and Non-ssBITA (either one or both internal thoracic arteries pedicled). Primary outcome was deep sternal wound infection (DSWI). Secondary outcome was operative mortality (OM). All outcomes of interest were captured if they occurred within the index hospitalization or within 30 days of surgery if patient was discharged. The well validated Society of thoracic Surgeons Perioperative Risk Models were used to calculate the observed to expected ratios (O/E) of OM and DSWI among both comparison groups [26].
Statistical Analysis:
Categorical variables were reported as numbers and percentage. Continuous variables were reported as mean and standard deviation. Unadjusted 30-day DSWI and OM were compared between groups using chi-squared test. Univariate analysis was followed by multivariable logistic regression analysis. In addition, one to one propensity matching was performed based on 13 independent variables, including hospital BITA volume, listed in Table 3. Results are expressed as adjusted odds ratio with 95% confidence intervals. A 2-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Table 3.
Characteristics of ssBITA and Non-ssBITA patients before and after propensity score matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Non-SS BITA | SS BITA | SD | Non-SS BITA | SS BITA | SD | |
| Characteristic | (n=6428) | (n=4841) | (n=3884) | (n=3884) | ||
| Age,mean(sd) | 61.9±10.4 | 61.2±10.0 | 0.07 | 61.0±10.2 | 61.0±9.9 | 0.00 |
| Gender | 0.02 | −0.01 | ||||
| Male | 5487(85.4%) | 4169(86.1%) | 3353(86.3%) | 3338(85.9%) | ||
| Female | 941(14.6%) | 672(13.9%) | 531(13.7%) | 546(14.1%) | ||
| Race | 0.07 | 0.03 | ||||
| White | 5216(81.1%) | 3972(82.0%) | 3148(81.1%) | 3187(82.1%) | ||
| Black | 397(6.2%) | 270(5.6%) | 233(6.0%) | 230(5.9%) | ||
| Other | 632(9.8%) | 420(8.7%) | 380(9.8%) | 346(8.9%) | ||
| Unknown | 183(2.8%) | 179(3.7%) | 123(3.2%) | 121(3.1%) | ||
| BMI | 0.04 | 0.02 | ||||
| Underweight | 26(0.4%) | 22(0.5%) | 14(0.4%) | 16(0.4%) | ||
| Normal weigh | 1167(18.2%) | 833(17.2%) | 665(17.1%) | 681(17.5%) | ||
| Overweight | 2733(42.5%) | 2004(41.4%) | 1655(42.6%) | 1628(41.9%) | ||
| Obese | 2502(38.9%) | 1982(40.9%) | 1550(39.9%) | 1559(40.1%) | ||
| Chronic Lung Disease > Mild | 769(12.0%) | 603(12.5%) | 0.02 | 467(12.0%) | 472(12.2%) | 0.00 |
| Diabetes | 2219(34.5%) | 1841(38.0%) | 0.07 | 1427(36.7%) | 1447(37.3%) | 0.01 |
| Hemoglobin A1c > 6% | 6371(99.1%) | 4763(98.4%) | −0.07 | 3842(98.9%) | 3846(99.0%) | 0.01 |
| Hypertension | 5483(85.3%) | 4162(86.0%) | 0.02 | 3334(85.8%) | 3352(86.3%) | 0.01 |
| Cerebrovascular Disease | 1024(15.9%) | 797(16.5%) | 0.01 | 614(15.8%) | 636(16.4%) | 0.02 |
| Peripheral Arterial Disease | 702(10.9%) | 510(10.5%) | −0.01 | 394(10.1%) | 419(10.8%) | 0.02 |
| Albumin | 0.06 | 0.01 | ||||
| Normal | 4363(67.9%) | 3367(69.6%) | 2674(68.8%) | 2681(69.0%) | ||
| Low | 1099(17.1%) | 727(15.0%) | 625(16.1%) | 616(15.9%) | ||
| Very Low | 966(15.0%) | 747(15.4%) | 585(15.1%) | 587(15.1%) | ||
| Off Pump | 752(11.7%) | 787(16.3%) | 0.13 | 606(15.6%) | 563(14.5%) | −0.03 |
| Hospital BITA Volume | 0.66 | 0.03 | ||||
| 1–20 | 2732(42.5%) | 955(19.7%) | 942(24.3%) | 954(24.6%) | ||
| 21–40 | 1413(22.0%) | 872(18.0%) | 909(23.4%) | 872(22.5%) | ||
| 41–60 | 782(12.2%) | 519(10.7%) | 532(13.7%) | 518(13.3%) | ||
| 61–80 | 521(8.1%) | 747(15.4%) | 521(13.4%) | 553(14.2%) | ||
| 80+ | 980(15.2%) | 1748(36.1%) | 980(25.2%) | 987(25.4%) | ||
Results
Unadjusted outcomes
A total of 1,448 individual surgeons working in 770 institutions performed BITA-CABG in the 11,269 patients who were included in the analysis. 4,841(42.8%) had both internal thoracic arteries harvested via a skeletonized technique (ssBITA) while 6,428 (57.2%) had both (41.5%) or one (15.7%) their BITA grafts harvested in a pedicled fashion (Non-ssBITA). Surgeons performing ssBITA (527) were fewer than surgeons performing Non-ssBITA cases (1264) as were hospital performing ssBITA (354) and Non-ssBITA (713) The mean (SD) and median (IQR) cases per institution and surgeon was higher for ssBITA [Institution:[13.7(25.3) and 4.0(2.0–12.3)], Surgeon: [9.2(15.2) and 3.0(1.0–11.0)] than Non-ssBITA [Institution: [9.1(14.5) and 4.0(2.0–10.0)], Surgeon: [5.1(6.5) and 2.0(1.0–5.0). Compared to Non-ssBITA patients, ssBITA patients were more obese with a higher incidence of diabetes, dyslipidemia, systolic heart failure, triple vessel coronary artery disease, cerebrovascular disease, hemoglobin A1c > 6%, off pump surgery and a lower incidence of chronic lung disease and blood transfusions. Both total operative and cross clamp times were longer in ssBITA patients. (Table 1)
Table 1.
Baseline characteristics of ssBITA and Non-ssBITA comparison groups.
| Patient Factor | ssBITA | Non-ssBITA | p |
|---|---|---|---|
| (n=4,841) | (n=6,428) | ||
| Age (mean(SD),years) | 61.2±10.0 | 61.9±10.4 | 0.070 |
| Male | 86.1% | 85.4% | 0.234 |
| Obese | 40.6% | 38.7% | 0.037 |
| Diabetes Mellitus | 38.1% | 34.6% | 0.001 |
| Hypertension | 85.9% | 85.2% | 0.635 |
| Dyslipidemia | 92.6% | 91.0% | 0.010 |
| Systolic Heart Failure | 45.8%% | 38.9% | 0.014 |
| 3ple Vessel Disease | 83.2% | 81.1% | 0.021 |
| Cerebrovascular Disease | 16.4% | 15.9% | 0.010 |
| Chronic Lung Disease (>mild) | 5.3% | 6.1% | 0.002 |
| Hemoglobin A1c > 6% | 45.8% | 43.0% | 0.005 |
| Off Pump | 16.3% | 11.6% | < 0.001 |
| Intra-op PRBC Transfusion | 18.9% | 21.7% | < 0.001 |
| Post-op PRBC Transfusion | 23.2% | 27.3% | < 0.001 |
| OR Time (mean(SD), minutes) | 387±96 | 364±94 | < 0.001 |
| X-Clamp Time (mean(SD), minutes) | 82.0±33.0 | 77.8±33.5 | <0.001 |
| CPB Time (mean(SD), minutes) | 105.8±41.6 | 105.0±42.6 | NS |
| BITA cases/hospital (mean(SD)) | 13.7±25.3 | 9.1±14.5 | <0.001 |
| BITA cases/surgeon (mean(SD)) | 9.2±15.2 | 5.1±8.4 | <0.001 |
The overall operative mortality (OM) and deep sternal wound infections (DSWI) among the entire BITA group were 1.72% with a corresponding O/E ratio of 1.4 and 0.98% with a corresponding O/E ratio of 5.1, respectively. Both OM and DSWI rates were higher in the Non-ssBITA group [1.90%(O/E 1.5) and 1.11%(O/E 5.9), respectively] than in the ssBITA group [1.47%(O/E 1.2) and 0.80 (O/E 4.1), respectively]. The 28% and 23% relative reduction in unadjusted DSWI and OM rates with ssBITA, however, were not statistically significant with a value of p=0.129 and p=0.096, respectively (Table 2). The mean (SD) and median (IQR) number of cases per surgeon in the entire BITA, the ssBITA and the Non-ssBITA were 7.8(12.9) and 3.0(1.0–8.0), 13.7(25.3) and 3.0(1.0–11.0), 5.1(8.4) and 2.0(1.0–5.0). There were no systematic differences in DSWI and OM between high and low BITA utilization institutions or surgeons.
Table 2.
Unadjusted operative outcome and corresponding Observed to Expected DSWI and OM rates based on the Society of Thoracic Surgeons Peri-operative CABG Risk Models in Any BITA, ssBITA and Non-ssBITA groups.
| ANY BITA | ss-BITA | Non-ss-BITA | p* | ||||
|---|---|---|---|---|---|---|---|
| (%) | O/E | (%) | O/E | (%) | O/E | ||
| DSWI | 0.98 | 5.1 | 0.80 | 4.1 | 1.11 | 5.9 | 0.129 |
| Operative Morality | 1.72 | 1.4 | 1.47 | 1.2 | 1.90 | 1.5 | 0.096 |
The unadjusted incidence of DSWI or any wound infection (superficial and deep) and the corresponding odds ratios calculated in an univariate analysis increased significantly in patients with multiple risk factors for DSWI regardless of whether these risk factors were evaluated restrictively or liberally (Figure 1). For a given number of risk factors, the risk of wound infections was generally higher in the the Non-ssBITA group compared to the ssBITA group.
Figure 1.
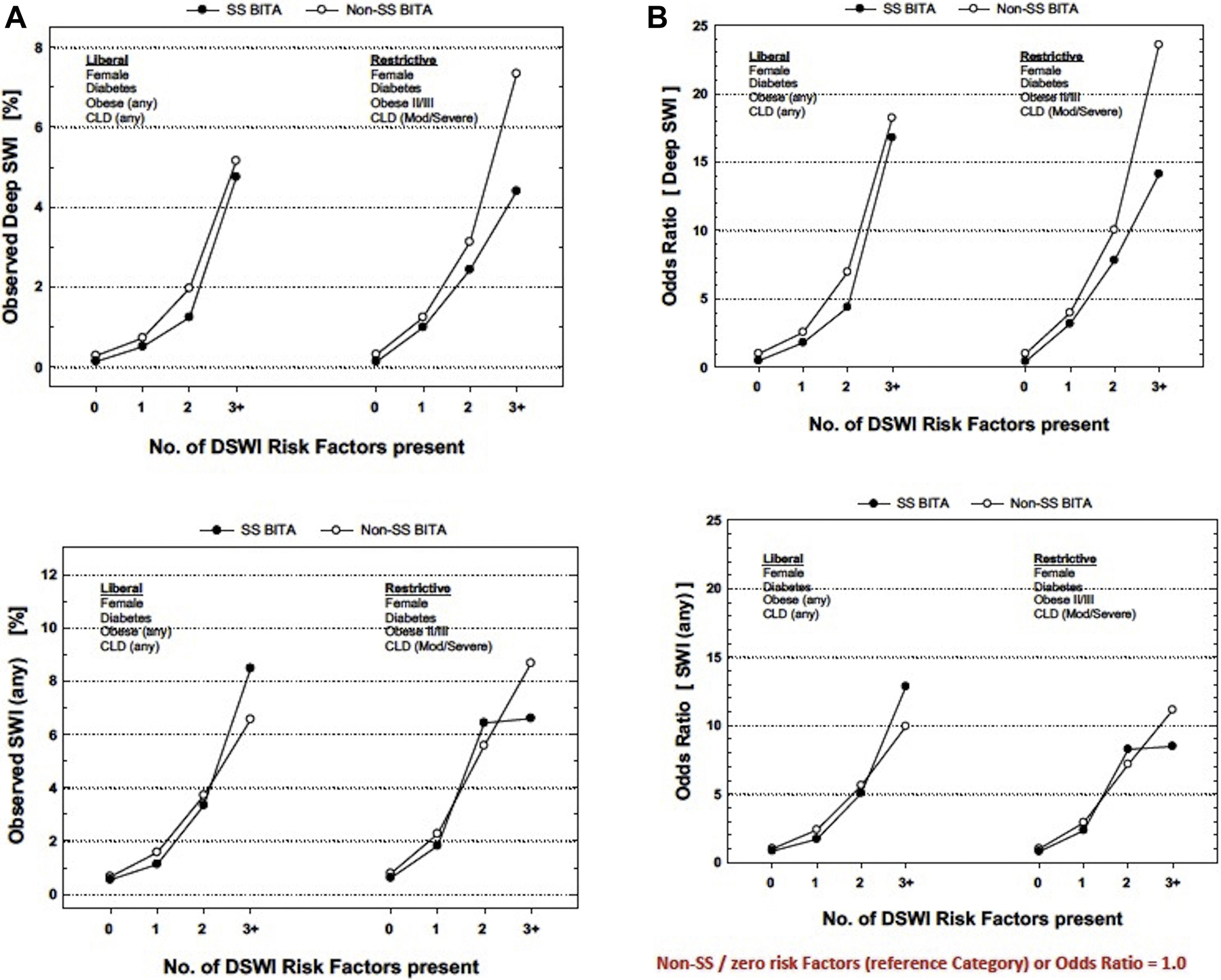
A. Absolute incidence of DSWI or Any Wound Infection (superficial or deep) with incremental number of risk factors applied liberally (left) or restrictively (right).
B. Odds ratios derived from a univariate analysis for DSWI or Any Wound Infection (superficial or deep) with incremental number of risk factors applied liberally (left) or restrictively (right).
Non-ssBITA/zero risk factors (reference category) – Odds Ratio = 1.0
Obese; Body mass index > 30kg/m2
Obese II; Body mass index > 35kg/m2
Obese III; Body mass index > 40kg/m2
CLD; chronic lung disease
Risk adjusted outcomes
Independent predictors of DSWI for the entire BITA study group were: ssBITA, female gender, chronic lung disease > mild, diabetes mellitus and off pump surgery. Neither obesity nor peripheral vascular disease were associated with DSWI. The corresponding AOR are listed in Table 5 for selected variables.
ssBITA patients had a statistically significantly lower risk of DSWI with an AOR(95% CI) of 0.66(0.44–1.00), p=0.05.Compared to Non-ssBITA patients, ssBITA patients had an equivalent risk of OM with an AOR(95%CI) of 0.92(0.67–1.27), p=0.12.
The corresponding ssBITA vs Non-ssBITA AOR(95% CI) for DSWI and OM among 3,884 propensity matched ssBITA and Non-ssBITA pairs were consistent with the multivariable analysis: DSWI - 0.60(0.36–0.99), p=0.05, OM – 0.85(0.60–1.21), p=0.37.
Discussion
This retrospective review of patients undergoing BITA-based CABG within the Society of Thoracic Surgeons Adult Cardiac Surgery Database, reflecting the practice patterns of the majority of American cardiac surgeons, indicates that BITA grafting, although can be accomplished with a less than 1% rate of DSWI, and less than 2% rate of OM, is nevertheless associated with an increased risk of DSWI and OM regardless of the specific harvesting technique compared to non-BITA CABG, as evidenced by a substantially elevated O/E DSWI ratios (between 4.1 to 5.9) and O/E OM ratios (1.2–1.5) based on the well validated STS Peri-operative Risk Models. The noted DSWI risk maybe partially mitigated by harvesting both internal thoracic arteries in a skeletonized fashion. Using multivariable logistic regression and propensity matched analysis, our results indicate that compared to patients undergoing harvesting of one or both internal thoracic arteries in a pedicled fashion, skeletonization of both internal thoracic arteries is associated with a decreased risk of DSWI. As such, skeletonization of internal thoracic artery grafts in patients undergoing BITA-based multi-arterial grafting strategy should be part of a comprehensive and multi disciplinary efforts to decrease DSWI [16]. Yet, less than 50% of BITA cases undergo skeletonization in contemporary American practice.
The overall observed BITA associated DSWI rate reported herein (0.98%) compares favorably with other reports. Puskas reported a 1.2% DSWI with BITA use in CABG[6]. In a study from the 1990’s, Borger et al noted a 14.3% DSWI rate in diabetic BITA patients [19]. Importantly, our data indicates that the risk of DSWI rises substantially with increasing number of patient risk factors known to be associated with DSWI such as diabetes, obesity and chronic lung disease (Figure 1). Despite, the low observed DSWI rate among the entire BITA cohort, our risk adjusted findings corroborate the increased incidence of DSWI associated with BITA grafting, compared to single internal thoracic artery based CABG reported in most retrospective single institutional and randomized prospective studies as well as in meta-analyses. This risk was highest in the Non-ssBITA group (O/E-5.9) lowest in the ssBITA group (O/E-4.9) and intermediate in the entire BITA study group (O/E-5.1). Such a BITA associated increased DSWI risk was also noted by Marzouk et al who reported that BITA use was an independent predictor of DSWI based on a retrospective review of a single institutional experience [27]. Gatti et al [28], in a retrospective analysis of 2936 patients undergoing skeletonized BITA grafting found a DSWI rate of 4.4%, a six fold increase over 0.73% DSWI rate noted in patients undergoing traditional single left internal thoracic artery (LITA) based CABG within the STS ACSD [5]. Our previous review of a decade long experience in over 1.4 million CABG patients in the STS database identified a risk adjusted two fold increased rate of DSWI associated with BITA grafting compared to LITA grafting [5]. Although the DSWI rates were not reported in the ART trial, the sternal reconstruction rate among BITA patients was 1.9% versus 0.6% for single internal thoracic artery patients [relative risk (95%CI) 3.24(1.54–6.83)] [27]. Similarly, in a post hoc analysis of the ART study, Benedetto found an 80% increase in DSWI rates with pedicled BITA grafting compared to pedicled LITA grafting [17]. In a meta-analysis of 32 individual studies, Dai et al, found a significantly decreased risk of DSWI with LITA vs BITA grafting with a risk ratio [RR(95%CI)] of 0.62(0.55–0.77). The three to four fold increased risk of mortality associated with DSWI [9,10] and its incremental cost, which is frequently non reimbursed, may constitute an appreciable barrier to a more enthusiastic adoption of BITA by cardiac surgeons. Yet there are notable exceptions to the majority of literature reporting increased risk of DSWI in BITA grafting. Notably, neither Puskas et al [6], nor Iribarne et al [29], found an increased risk of DSWI with BITA vs LITA grafting among diabetics, although both studies noted higher rates of DSWI for both LITA and BITA patients in their diabetic study populations. The specific practice patterns underlying these exceptional contrarian results warrant further analysis.
Our results showed a 28% reduction in the observed DSWI with ssBITA compared to non-ssBITA, but this did not reach statistical significance. Following risk adjustment, ssBITA was associated with decreased DSWI risk (Table 4). Such favorable impact of BITA skeletonization has been also noted by other investigators. Hu noted a 32% reduction of DSWI with BITA skeletonization [25]. Benedetto and colleagues noted that an 80% increase risk of pedicled BITA grafting compared to LITA grafting, while the DSWI risk of skeletonized BITA was equivalent to LITA [17]. Saso, in a meta analysis of 13 studies, also found skeletonized BITA to be associated with reduced risk of DSWI and this was particularly evident in diabetics [21]. DePaulis, retrospectively reviewed 450 BITA cases and found no difference in the DSWI risk between skeletonized BITA and LITA [22]. Since its first description by Keely in 1987 [30], skeletonization of BITA grafts has been found to be safe and to offer multiple benefits including a reduction in the rate of DSWI [25]. The mechanism behind the protective effects of skeletonized BITA grafting against DSWI remains undefined. Traditionally it has been hypothesized that skeletonization of BITA grafts minimizes sternal devascularization. Although, intuitively and intellectually appealing, the available literature on this topic is sparse, with only a limited number of small studies published to date and whose conclusions are contradictory, with some investigators reporting less sternal devasularization with skeletonization [7,8] while others finding no differences in the decrement of sternal perfusion regardless of the specific harvesting technique [23]. Interestingly the decreased risk of DSWI with ssBITA compared to Non-ssBITA that is identified occurred despite a significantly longer total operative time in case of ssBITA, a well known risk factor for surgical site infections, (Table 1).
Table 4.
Comparison of DSWI and OM outcomes via adjusted odds ratios and 95% confidence intervals [AOR(95%)]: Unadjusted and Multivariable logistic regression analysis in the entire study group and in 3884 propensity score matched pairs.
| DSWI | OM | |||
|---|---|---|---|---|
| AOR (95%CI) | p | AOR (95%CI) | p | |
| Unadjusted | 0.72(0.48–1.06) | 0.10 | 0.78(0.58–1.05) | 0.10 |
| Multivariable Logistic Regression | 0.66(0.44–1.00) | 0.05 | 0.92(0.67–1.27) | 0.12 |
| Propensity Score Matching | 0.60(0.36–0.99) | 0.05 | 0.85(0.60–1.21) | 0.37 |
Skeletonization of BITA grafts and off pump surgery were protective factors against DSWI, while female gender, chronic lung disease > mild and diabetes mellitus were independent predictors of increased DSWI risk among the entire BITA study population (Table 5). Borger et al [9] identified BITA grafting, male sex and diabetes as independent predictors of DSWI in all CABG patients. Gatti and colleagues [28] found that female sex, obesity diabetes, poor glycemic control chronic lung disease and urgent surgical status were independently associated with DSWI risk among BITA patients. Marzuk et al noted that female sex, diabetes, chronic obstructive lung disease and peripheral vascular disease were predictors of DSWI. Importantly, neither peripheral arterial disease nor obesity was a predictor of DSWI in our analysis.
In contrast to the meta analyses of 23 studies of skeletonized vs pedicled BITA by Hu et al [25] showing improved perioperative survival, our results showed equivalent survival with either harvesting strategy. Surprisingly, given the noted elevated O/E ratio for OM based on the STS perioperative risk models, our study may have identified a signal for a higher risk of operative mortality with BITA grafting. This risk was highest for Non-ssBITA patients (O/E-1.5), intermediate for the entire BITA study cohort (O/E-1.4) and lowest for ssBITA patients (O/E-1.2). Importantly, the O/E ratios were derived principally from a LITA based CABG population within the STS database with BITA patients constituting very small minority of this group. In a previous report from the STS database of patients undergoing BITA vs LITA based CABG, we also noted a marginally statistically significant increased risk of OM with BITA grafting compared to LITA with an AOR(95%CI) of 1.14(1.00–1.30), p=0.05. Although the proximate cause behind tis increased risk of OM remains undefined, the role of surgeon experience with BITA grafting needs further evaluation in light of the reported inverse association between DSWI as well as mortality and surgeon case volumes [5,24]. In the current study, we noted relatively modest surgeon case volumes with median number of BITA cases per surgeon over the 18 month study period of only between 2–3. In our analysis, were unable to identify any systematic differences in DSWI and OM between high and low BITA utilization institutions or surgeons.
There are a number of important limitations to our study including its retrospective nature with its inherent risk of allocation biases and hidden confounders that can not be controlled by any statistical methodologies. In addition, DSWI are known to occur later than 30 days post-operatively and thus these would not be captured within the 30 day post operative reporting window that is part of the STS ACSD. Furthermore, we are unable to ascertain if, and to what degree, any of the known specific guidelines to mitigate the risk of DSWI were implemented in the study group: methicillin resistant staph areous nasal decontamination, topical antibiotic use on sternal edges, degree of glycemic control, additional sternal fixation methodologies (sternal plating), whether electrocautery or harmonic scalpels were used in the BITA harvesting process, the specific post-operative wound care process such as negative pressure dressings or antibiotic impregnated dressing were used. If these were deployed at different rates in the comparison groups, that be a source of bias. We are also unable to assess whether the increased complexity of skeletonization of the BITA grafts may have led to inadvertent graft injury and thus may have precluded such patients from being classified as receiving a BITA-based CABG and thus not being included in our study group. More importantly, however, such patients would not realize the long term survival benefits of BITA grafting while being exposed to the risk of DSWI. Similarly, we are unable to comment on the patency rates of the ssBITA vs the Non-ssBITA patients and how any differences, if present, may have impacted not only short term results of our study but also long term survival.
Supplementary Material
Footnotes
Presented at the 33rd EACTS Annual Meeting, Lisbon, Portugal 3–5 October 2019
References:
- [1].Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD, et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069–77. doi: 10.1056/NEJMoa1716026. [DOI] [PubMed] [Google Scholar]
- [2].Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N Engl J Med 2019;380:437–46. doi: 10.1056/NEJMoa1808783. [DOI] [PubMed] [Google Scholar]
- [3].Goldstone AB, Chiu P, Baiocchi M, Wang H, Lingala B, Boyd JH, et al. Second Arterial Versus Venous Conduits for Multivessel Coronary Artery Bypass Surgery in California. Circulation 2018;137:1698–707. doi: 10.1161/CIRCULATIONAHA.117.030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwann TA, Tatoulis J, Puskas J, Bonnell M, Taggart D, Kurlansky P, et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004–2014: A Tale of 2 Continents. Semin Thorac Cardiovasc Surg 2017;29:273–80. doi: 10.1053/j.semtcvs.2017.05.018. [DOI] [PubMed] [Google Scholar]
- [5].Schwann TA, Habib RH, Wallace A, Shahian DM, O’Brien S, Jacobs JP, et al. Operative Outcomes of Multiple-Arterial Versus Single-Arterial Coronary Bypass Grafting. Ann Thorac Surg 2018;105:1109–19. doi: 10.1016/j.athoracsur.2017.10.058. [DOI] [PubMed] [Google Scholar]
- [6].Puskas JD, Sadiq A, Vassiliades TA, Kilgo PD, Lattouf OM. Bilateral Internal Thoracic Artery Grafting Is Associated With Significantly Improved Long-Term Survival, Even Among Diabetic Patients. Ann Thorac Surg 2012;94:710–6. doi: 10.1016/j.athoracsur.2012.03.082. [DOI] [PubMed] [Google Scholar]
- [7].Boodhwani et al. - 2006. - Skeletonized Internal Thoracic Artery Harvest Redu.pdf n.d
- [8].Kamiya H, Akhyari P, Martens A, Karck M, Haverich A, Lichtenberg A. Sternal microcirculation after skeletonized versus pedicled harvesting of the internal thoracic artery: A randomized study. J Thorac Cardiovasc Surg 2008;135:32–7. doi: 10.1016/j.jtcvs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [9].Borger MA, Rao V, Weisel RD, Ivanov J, Cohen G, Scully HE, et al. Deep Sternal Wound Infection: Risk Factors and Outcomes. Ann Thorac Surg 1998;65:1050–6. doi: 10.1016/S0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- [10].Sears ED, Wu L, Waljee JF, Momoh AO, Zhong L, Chung KC. The Impact of Deep Sternal Wound Infection on Mortality and Resource Utilization: A Population-based Study. World J Surg 2016;40:2673–80. doi: 10.1007/s00268-016-3598-7. [DOI] [PubMed] [Google Scholar]
- [11].Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: Validation of existing risk scores. J Thorac Cardiovasc Surg 2007;133:397–403. doi: 10.1016/j.jtcvs.2006.10.012. [DOI] [PubMed] [Google Scholar]
- [12].Braxton JH, Marrin CAS, McGrath PD, Morton JR, Norotsky M, Charlesworth DC, et al. 10-Year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg 2004;16:70–6. doi: 10.1053/j.semtcvs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [13].Marzouk M, Mohammadi S, Baillot R, Kalavrouziotis D. Rigid Primary Sternal Fixation Reduces Sternal Complications Among Patients at Risk. Ann Thorac Surg 2019;108:737–43. doi: 10.1016/j.athoracsur.2019.03.046. [DOI] [PubMed] [Google Scholar]
- [14].Lytle BW, Blackstone EH, Sabik JF, Houghtaling P, Loop FD, Cosgrove DM. The Effect of Bilateral Internal Thoracic Artery Grafting on Survival During 20 Postoperative Years. Ann Thorac Surg 2004;78:2005–14. doi: 10.1016/j.athoracsur.2004.05.070. [DOI] [PubMed] [Google Scholar]
- [15].Kurlansky PA, Traad EA, Dorman MJ, Galbut DL, Zucker M, Ebra G. Thirty-Year Follow-Up Defines Survival Benefit for Second Internal Mammary Artery in Propensity-Matched Groups. Ann Thorac Surg 2010;90:101–8. doi: 10.1016/j.athoracsur.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [16].Lazar HL, Salm TV, Engelman R, Orgill D, Gordon S. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg 2016;152:962–72. doi: 10.1016/j.jtcvs.2016.01.060. [DOI] [PubMed] [Google Scholar]
- [17].Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Pawlaczyk R, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152:270–6. doi: 10.1016/j.jtcvs.2016.03.056. [DOI] [PubMed] [Google Scholar]
- [18].Fouquet O, Tariel F, Desulauze P, Mével G. Does a skeletonized internal thoracic artery give fewer postoperative complications than a pedicled artery for patients undergoing coronary artery bypass grafting?: Table 1: Interact Cardiovasc Thorac Surg 2015;20:663–8. doi: 10.1093/icvts/ivv026. [DOI] [PubMed] [Google Scholar]
- [19].Peterson MD, Borger MA, Rao V, Peniston CM, Feindel CM. Skeletonization of bilateral internal thoracic artery grafts lowers the risk of sternal infection in patients with diabetes. J Thorac Cardiovasc Surg 2003;126:1314–9. doi: 10.1016/S0022-5223(03)00808-0. [DOI] [PubMed] [Google Scholar]
- [20].Deo SV, Shah IK, Dunlay SM, Erwin PJ, Locker C, Altarabsheh SE, et al. Bilateral Internal Thoracic Artery Harvest and Deep Sternal Wound Infection in Diabetic Patients. Ann Thorac Surg 2013;95:862–9. doi: 10.1016/j.athoracsur.2012.11.068. [DOI] [PubMed] [Google Scholar]
- [21].Saso S, James D, Vecht JA, Kidher E, Kokotsakis J, Malinovski V, et al. Effect of Skeletonization of the Internal Thoracic Artery for Coronary Revascularization on the Incidence of Sternal Wound Infection. Ann Thorac Surg 2010;89:661–70. doi: 10.1016/j.athoracsur.2009.08.018. [DOI] [PubMed] [Google Scholar]
- [22].De Paulis R, de Notaris S, Scaffa R, Nardella S, Zeitani J, Del Giudice C, et al. The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: The role of skeletonization. J Thorac Cardiovasc Surg 2005;129:536–43. doi: 10.1016/j.jtcvs.2004.07.059. [DOI] [PubMed] [Google Scholar]
- [23].Nishi H, Mitsuno M, Tanaka H, Ryomoto M, Fukui S, Miyamoto Y. Decreasing sternum microcirculation after harvesting the internal thoracic artery. Eur J Cardiothorac Surg 2011;40:240–4. doi: 10.1016/j.ejcts.2010.10.027. [DOI] [PubMed] [Google Scholar]
- [24].Gaudino M, Bakaeen F, Benedetto U, Rahouma M, Di Franco A, Tam DY, et al. Use Rate and Outcome in Bilateral Internal Thoracic Artery Grafting: Insights From a Systematic Review and Meta‐Analysis. J Am Heart Assoc 2018;7. doi: 10.1161/JAHA.118.009361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu X, Zhao Q. Skeletonized Internal Thoracic Artery Harvest Improves Prognosis in High-Risk Population After Coronary Artery Bypass Surgery for Good Quality Grafts. Ann Thorac Surg 2011;92:48–58. doi: 10.1016/j.athoracsur.2011.03.067. [DOI] [PubMed] [Google Scholar]
- [26].Shahian DM, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1—Background, Design Considerations, and Model Development. Ann Thorac Surg 2018;105:1411–8. doi: 10.1016/j.athoracsur.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [27].Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu L-M, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART) n.d.:12. [DOI] [PubMed] [Google Scholar]
- [28].Gatti G, Dell’Angela L, Barbati G, Benussi B, Forti G, Gabrielli M, et al. A predictive scoring system for deep sternal wound infection after bilateral internal thoracic artery grafting. Eur J Cardiothorac Surg 2016;49:910–7. doi: 10.1093/ejcts/ezv208. [DOI] [PubMed] [Google Scholar]
- [29].Iribarne A, Westbrook BM, Malenka DJ, Schmoker JD, McCullough JN, Leavitt BJ, et al. Should Diabetes Be a Contraindication to Bilateral Internal Mammary Artery Grafting? Ann Thorac Surg 2018;105:709–14. doi: 10.1016/j.athoracsur.2017.08.054. [DOI] [PubMed] [Google Scholar]
- [30].Keeley SB. The Skeletonized Internal Mammary Artery. Ann Thorac Surg 1987;44:324–5. doi: 10.1016/S0003-4975(10)62088-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


