Abstract
Visceral leishmaniasis (VL) is a potentially fatal parasitic disease causing high morbidity and mortality in developing countries. Vaccination is considered the most effective and powerful tool for blocking transmission and control of diseases. However, no vaccine is available so far in the market for humans. In the present study, we characterized the hypothetical protein LDBPK_252400 of Leishmania donovani (LdHyP) and explored its prophylactic behavior as a potential vaccine candidate against VL. We found reduced hepato-splenomegaly along with more than 50% parasite reduction in spleen and liver after vaccination in mice. Protection in vaccinated mice after the antigen challenge correlated with the stimulation of antigen specific IFN-γ expressing CD4+T cell (~4.6 fold) and CD8+T cells (~2.1 fold) in vaccinated mice in compared to infected mice, even after 2–3 months of immunization. Importantly, antigen-mediated humoral immunity correlated with high antigen specific IgG2/IgG1 responses in vaccinated mice. In vitro re-stimulation of splenocytes with LdHyP enhances the expression of TNF-α, IFN-γ, IL-12 and IL-10 cytokines along with lower IL-4 cytokine and IL-10/IFN-γ ratio in vaccinated mice. Importantly, we observed ~3.5 fold high NO production through activated macrophages validates antigen mediated cellular immunity induction, which is critical in controlling infection progression. These findings suggest that immunization with LdHyP mount a very robust immunity (from IL-10 towards TFN-γ mediated responses) against L. donovani infection and could be explored further as a putative vaccine candidate against VL.
Keywords: Visceral leishmaniasis, Hypothetical protein, Vaccine, CFSE, CD4/CD8+T cells
1. Introduction
Leishmaniasis represents a complex of diseases with a spectrum of severity ranging from cutaneous non-healing lesions to a potentially fatal visceralizing phenotype, depending upon the species of the Leishmania parasite involved. Visceral leishmaniasis (VL), commonly known as Kala-azar in India is the most severe form of leishmaniasis caused by Leishmania donovani on the Indian Subcontinent. World Health Organization (WHO) reports that the number of new cases of VL caused by L. donovani was 93,660 between 2014 and 2019, occurring principally in India, Nepal, Bangladesh, Sudan, South Sudan, Ethiopia and Somalia. While there has been some recent progress in the Indian subcontinent to bring the disease under control (number of cases decreased from 32,803 in 2005 to 3,128 in 2019, representing a 90% drop [1], Over 50,000 cases of VL occur worldwide per year [2], There is no vaccine yet available in the market; hence, control of disease mainly depends on the treatment with chemotherapeutic drugs like pentavalent antimonials, amphotericin B, permomycin etc. However, drug resistance, toxicity, cost and long treatment course, vector control, HIV-Co infections are some serious concerns. In spite of this appearance of sporadic cases in non-endemic areas (like Himachal Pradesh, Sikkim (India) etc) likely has another barrier towards complete elimination program of WHO [3,4]. Now-a-days, utilization of multi-drug chemotherapy is beneficial and becomes helpful for solving these problems [5–7]. However, adopting only chemotherapy strategy will not stop transmission of disease. Therefore, it is imperative to develop an immunocompetent arrangement for long term protection that would be helpful for complete eradication of leishmaniasis.
Vaccination is the safest and one of the most effective ways to fight against any infectious diseases than therapeutic drugs. Modulation of the host immune system to evade the phagocyte property of macrophages for survival and initiation of infection in a host is the hallmark of Leishmania parasite. Several significant immunosuppressive factors discovered in Leishmania that exploiting host immune system and contributed to leishmaniasis [8]. It is well documented that a T-cell mediated immune responses of host contribute to the resolution of leishmania infection although their disturbance marks progressive leishmaniasis [9]. Disturbance in Th1/Th2 cytokines balance of the host immune system contributes an important role in Leishmania infection like parasite specific Th2 immune responses, characterized by the production of IL-4, IL-10 and TGF-β associated with progression of Leishmania infection [10,11]. However, activation of Th1 type of immune response especially the production of TNF-α, IFN-γ and IL-12 followed by macrophage activation to produce nitric oxides (NO), which plays a crucial role in mediating the killing of intracellular amastigotes [12]. The induction of parasite antigen specific CD4+ Th1 cell is very critical in clearance of leishmania infection through the production of Th1 driven principle cytokine IFN-γ [11–13]. Rather than CD4+T cells, antigen specific CD8+T cells also play an important role in parasite reduction through IFN-γ production, direct cytolytic mechanism or by both [14–16]. So far, several potent antigens against leishmania have been screened as vaccine candidate eliciting CD4+T and CD8+T cell responses, such as Leish111f, Leishmune (a recombinant protein fusion of LelF, TSA and LmsTI1+Saponis), PDI, gp46, TSA, LmsTI1 and CPB [17–21] but no one yet used as an effective vaccine for human due to difficulties in manufacturing, regulatory issues and/or poor field response in the clinical trials [20]. Despite all of these hurdles, making continued studies to understand the underlying immunopathology of disease of great importance, especially in the context of advances being made in immune-therapeutics for the development of safe and effective vaccine against leishmaniasis.
Several immune-proteomic studies document many putative or hypothetical proteins (HyPs) of the parasite, which lacks sequence similarity with previous proteins and their function is also not defined. In leishmania species around 40% proteins comes under this category, which is uncharacterized [22,23]. These proteins have been analyzed through immunoproteiomics studies of a clinical isolate L. donovani soluble lysate [22–25]. Since, characterization of these HyPs and analysis of immunological parameters at the molecular level is necessary to develop suitable vaccine/diagnostic marker in future for the control of infection. Hypothetical proteins having antigenic ability become putative vaccine molecules as it does not show any cross reactivity with other organisms due to lack of high sequence similarity. We have selected LDBPK_252400 hypothetical protein of L. donovani for vaccine study against VL based on available literature [22,23]. No study have been conducted with regards to possible role of L. donovani LDBPK_252400 hypothetical protein as a vaccine candidate. Only scant attentions have been focused by Sinha and Raja et al. on the basis of immunoproteiomics and insilico approaches [22,23]. The available data allow us to assess its role as vaccine against VL.
Thus, the present study aimed to characterize first time a new L. donovani hypothetical protein, namely LDBPK_252400 (LdHyP) and evaluated the prophylactic potential of candidate protein in susceptible Balb/c mice by the series of in vivo and in vitro immune-biochemical approaches. In this direction first, we have successfully cloned, expressed, purified and structurally characterized 32.9 kDa LdHyP. Its protective efficacy correlated with partial protection in immunized challenged mice via the induction of LdHyP specific Th1 type of cellular and humoral immune responses. The present study may sedge light towards the evaluation of vaccine against leishmanial infection.
2. Results
2.1. Cloning, expression and purification of LdHyP
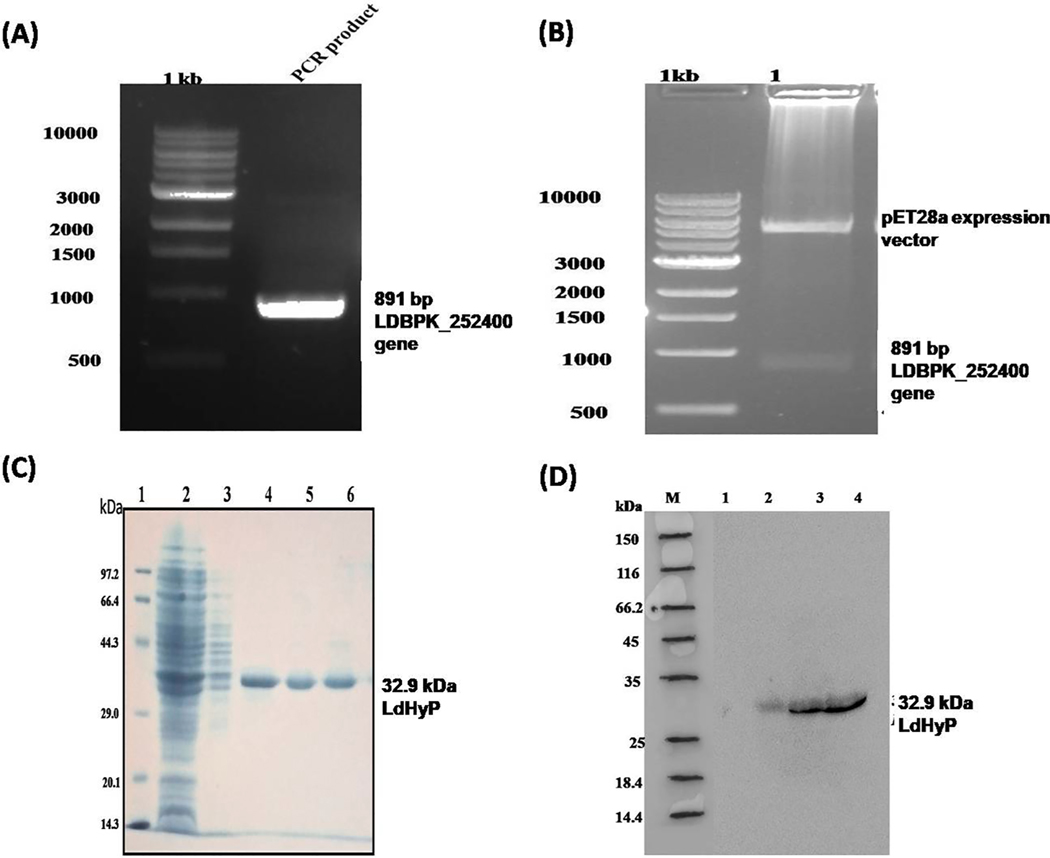
The complete gene of L. donovani encoding functional LdHyP hypothetical protein was PCR amplified using gene-specific primers and found in the single band at the position of 891 bp (Fig. 1A). The PCR amplicon were cloned into pET28a expression vector and positive clone was confirmed by restriction digestion as shown in Fig. 1B. The recombinant parasite specific membrane antigen (LdHyP) was expressed in E. coli and successfully purified 32.9 kDa LdHyP by Ni-NTA chromatography (Fig. 1C). Western blotting further confirmed the expression of 32.9 kDa 6xHis-tagged recombinant LdHyP.
Fig. 1.
Cloning expression and purification of Leishamnia donovani hypothetical protein (LDBPK_252400). (A) PCR amplification of LDBPK_252400 gene. Lane 1. 500 bp DNA ladder, Lane 2. PCR product. (B) Double digestion of positive clone (pET28a+ expression vector-LdHyP genes): Lane 1. 500 bp DNA ladder, Lane 2. Double digested clone with EcoR1 and Hind III. (C) 12% SDS-PAGE analysis of purified rLDBPK _252400 protein (LdHyP). Lane 1: molecular weight markers (Takara), Lane 2: flow through fraction, Lane 3: 50 mM imidazole wash, Lane 4–6: protein eluted with 500 mM imidazole. Single band showing 32.9 kDa purified LdHyP protein. (D) Western blotting to confirmed expression of his-tagged recombinant protein. Lane M: molecular weight markers, Lane 1: Uninduced cells, Lane 2: Induced cells, Lane 3–4 eluted fraction.
2.2. Biophysical behavior of LdHyP
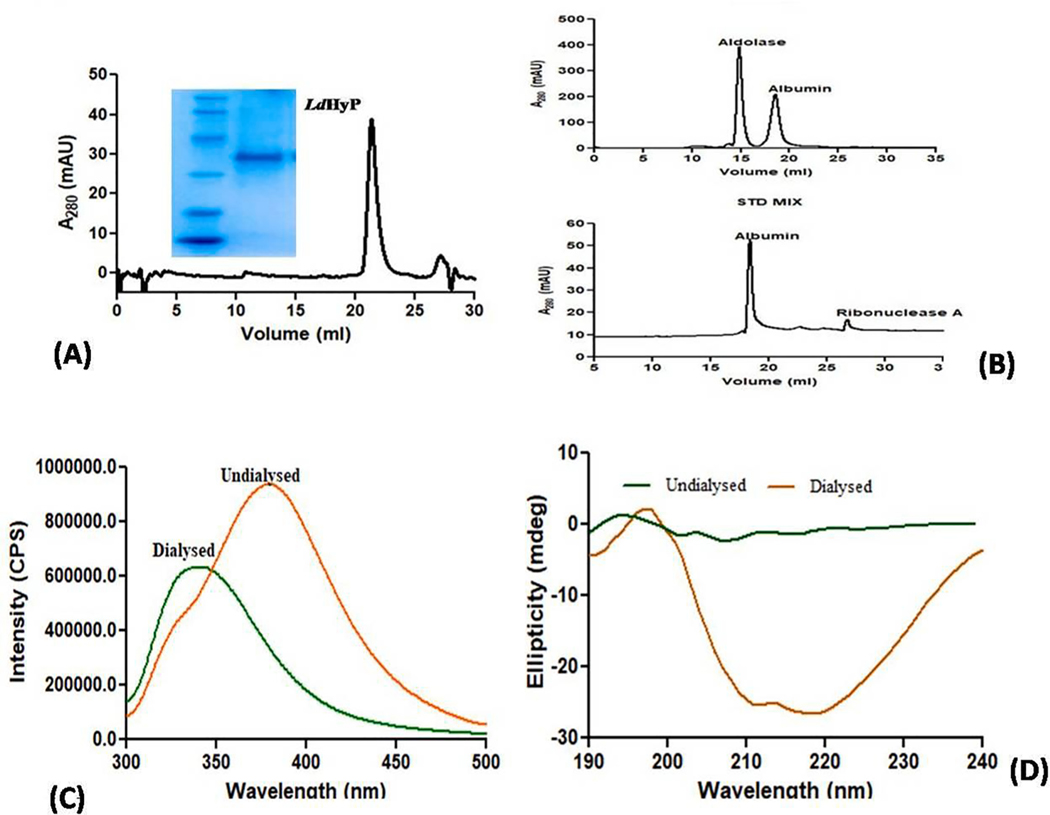
To obtain highly purified protein (>90%) for vaccination as well as identification of its native molecular mass, the dialyzed protein was further subjected to SEC. All fractions of purified recombinant LdHyP protein were pooled, dialyzed and concentrated then injected further into the SEC column (Superdex™650 10/300 GL column). The recombinant LdHyP gives a single peak at 21.3 ml elution volume (Fig. 2A) that corresponds to around 35 kDa when correlates with the molecular weight of standard markers (Fig. 2B). The data suggests that LdHyP exists as a monomeric form under native conditions. Native condition of purified protein has been validated by spectrofluorimetry. Fig. 2C indicate that the wavelength of dialyzed protein (0 M urea) was between 335 and 345 nm (emission maxima range of native protein) while non-dialyzed protein (2 M urea) was found around 350 nm (emission maxima of denatured protein ≥350 nm). Secondary structure of dialyzed protein was also observed by circular dichroism and we found that α content of dialyzed (native, 0 M urea) protein is 33% and β content is 16%. However, non-dialyzed protein gives random coil-like structure (denatured protein, 2 M urea) as shown in Fig. 2D. Altogether these data indicate that after dialysis protein was probably obtained in their native and purified form and we can use it for further studies.
Fig. 2.
(A-D): Size exclusion chromatography. (A) Native molecular mass (32.9 kDa) of LDBPK_252400 protein was determined by SEC (inset show single peak obtained at 21.3 ml). (B) Standard molecular markers: Aldolase (158 kDa), Albumin (65 kDa), Ribonuclease A (13.5 kDa). (C) Confirmation of native condition of dialyzed rLDBPK_252400; Spectrofluorimetry of dialyzed and undialyzed protein. (D) Prediction of secondary structure of dialyzed and undialyzed protein by Circular Dichroism.
2.3. LdHyP vaccination induces protection against L. donovani infection in susceptible Balb/c mice
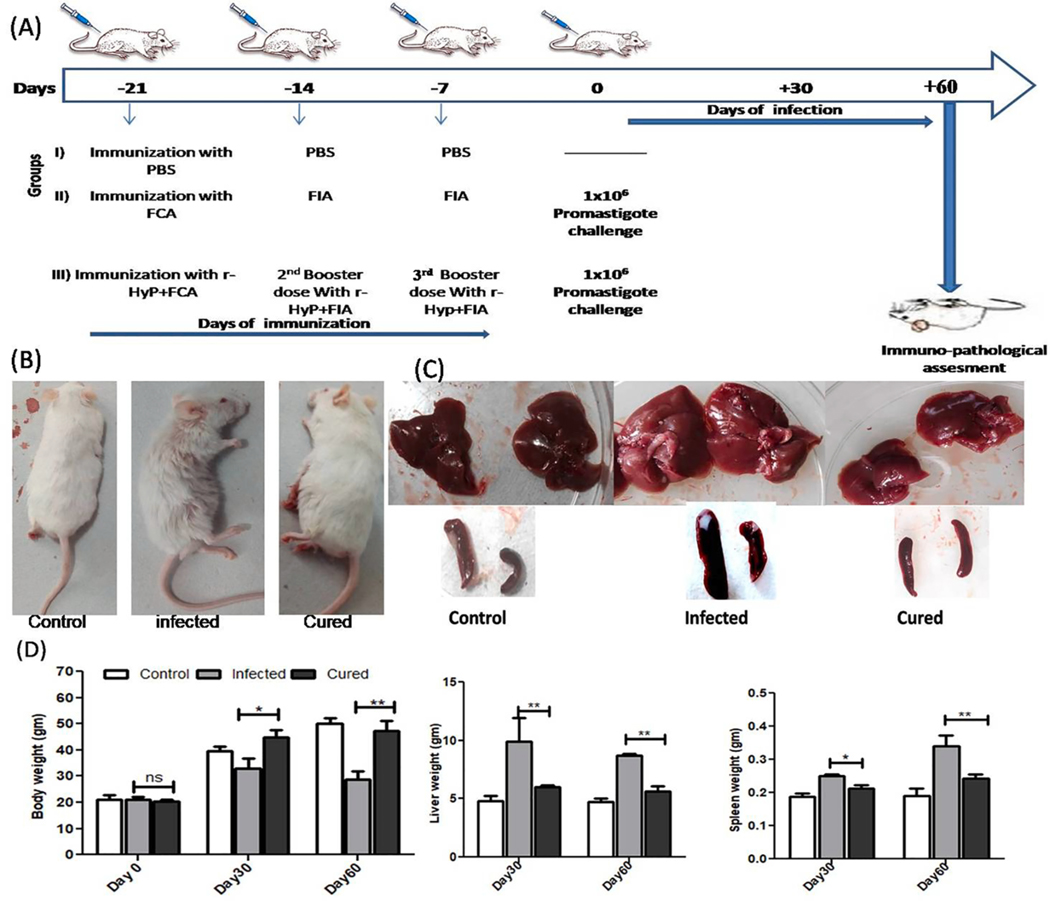
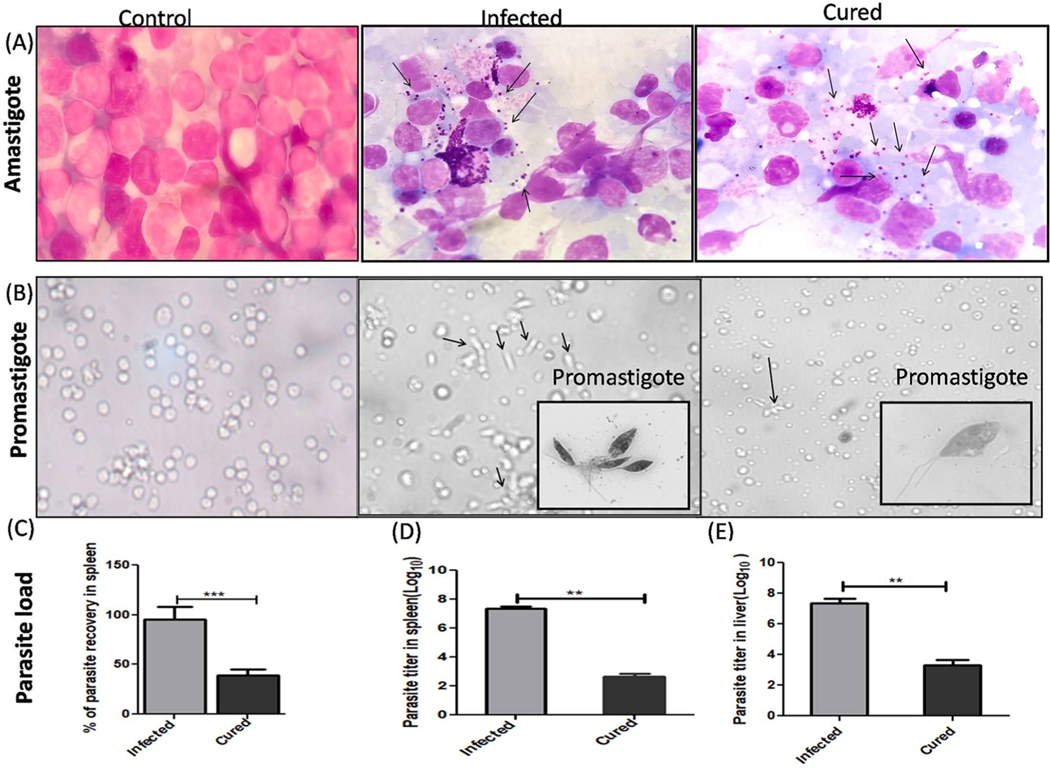
The LdHyP vaccinated mice were noticed to be survived more and healthy until the termination of study (2–3 months). Furthermore, hair loss, hepatomegaly and splenomegaly were also found to be very low as compared to the infected mice (Fig. 3B–D). After that, for the evaluation of prophylaxis potential of LdHyP protein, we first determined the parasite load in vaccinated (cured) mice and compared it with infected (lethal L. donovani) mice. A significant reduction in amastigote numbers were seen in cured/treated mice and was well correlated with body, liver and spleen weights (Figs. 3D, 4A). We performed serial dilution experiments for the confirmation of the presence of amastigotes in spleen/liver, which was converted into motile promastigotes after 5 days of incubation in serial dilution (Fig. 4B). We found that >50% reduction of parasite load in spleen and liver of LdHyP vaccinated mice (Fig. 4C–E). Altogether these observations indicate prophylactic efficacy of LdHyP in immunized mice against the progression of parasite infection.
Fig. 3.
Schematic representation of Balb/c mice immunization with LdHyP and challenge with stationary stage promastigotes of L. donovani to evaluate its prophylactic potential. (A) Study design and Immunization schedule representing the experiments. (B-C) Immunized challenged mice (Cured) showing reduced splenomegaly and hepatomegaly along with no effect on hair loss as compaired to infected mice. (D) Effect of LdHyP in immunized mice body weight, spleen weight and liver weight as compare to infected mice. Significance values represent the difference between cured and infected group of three independent experiments group (n = 4 animals per group). *p < 0.05, **p < 0.01, ns: no significant.
Fig. 4.
Effect of LdHyP on parasite load in different organs of cured Balb/c mice. (A) Presence of L. donovani amastigotes in the spleen of control infected and cured Balb/c by Giemsa staining (100X magnification). (B) L. donovani promastigotes were evaluated through serial dilution and viable promastigote observed under 40X magnification (inset 100X magnification). Bar graph represents parasite reduction in spleen and liver of cured mice as compared to infected mice by both Giemsa staining (C) and serial dilution methods (D-E). Significance value represent the difference between cured and infected group (n = 4 mice per group). *p < 0.05, **p < 0.01, ns: no significant.
2.4. Resistance to L. donovani challenged in vaccinated mice is associated with induction of protective Th1 immune response
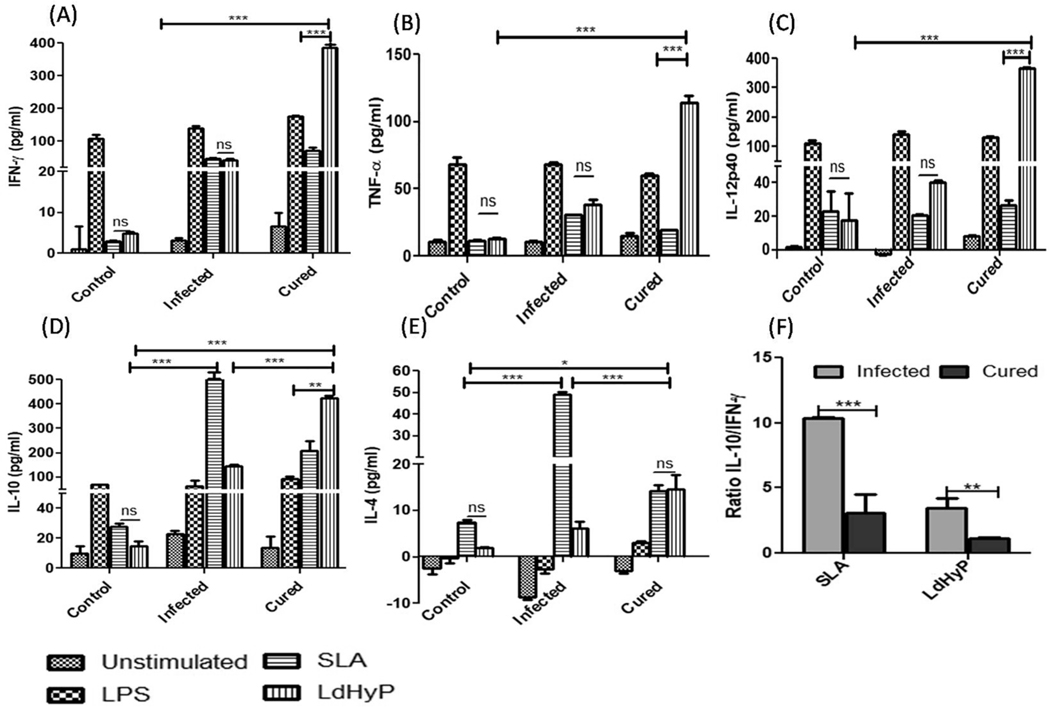
To validate the protective potential of LdHyP as a vaccine, we analyzed the Th1/Th2 specific cytokines alteration in the culture supernatant through ELISA stimulated with LdHyP/SLA/LPS (Fig. 5). Vaccinated mice induced a significantly higher level of LdHyP specific Th1 cytokines (IFN-γ, TNF-α and IL-12p40) secretion as compared to infected and control group of mice (Fig. 5A–C). The secretion of IFN-γ was found to be the most abundant cytokines in cell culture of the spleen after re-stimulation with the LdHyP based recombinant vaccine. Apart from this, LdHyP specific IL-10 level was also found to be high while IL-4 secretion was low in vaccinated mice (Fig. 5 D–E). However, SLA stimulated IL-10 and IL-4 cytokines were observed to be low in LdHyP vaccinated mice over the infected mice. The lower value of IL-10/IFN-Y ratio in cured mice indicates that LdHyP induced predominant Th1 biased cellular immune response in cured mice (Fig. 5F).
Fig. 5.
Enzyme linked immunosorbent assays to analyze the LdHyP specific Th1/Th2 cytokines production. Cytokines levels of IFN-γ (A), TNF-α (B), IL-12p40 (C), IL-10 (D) and IL-4 (E) were determined in splenocytes supernatant of all experimental group of mice after 12 week of post challenge. (F) IL-10/IFN-γ ratio. Data represent mean ± SD of two independent experiments in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significant.
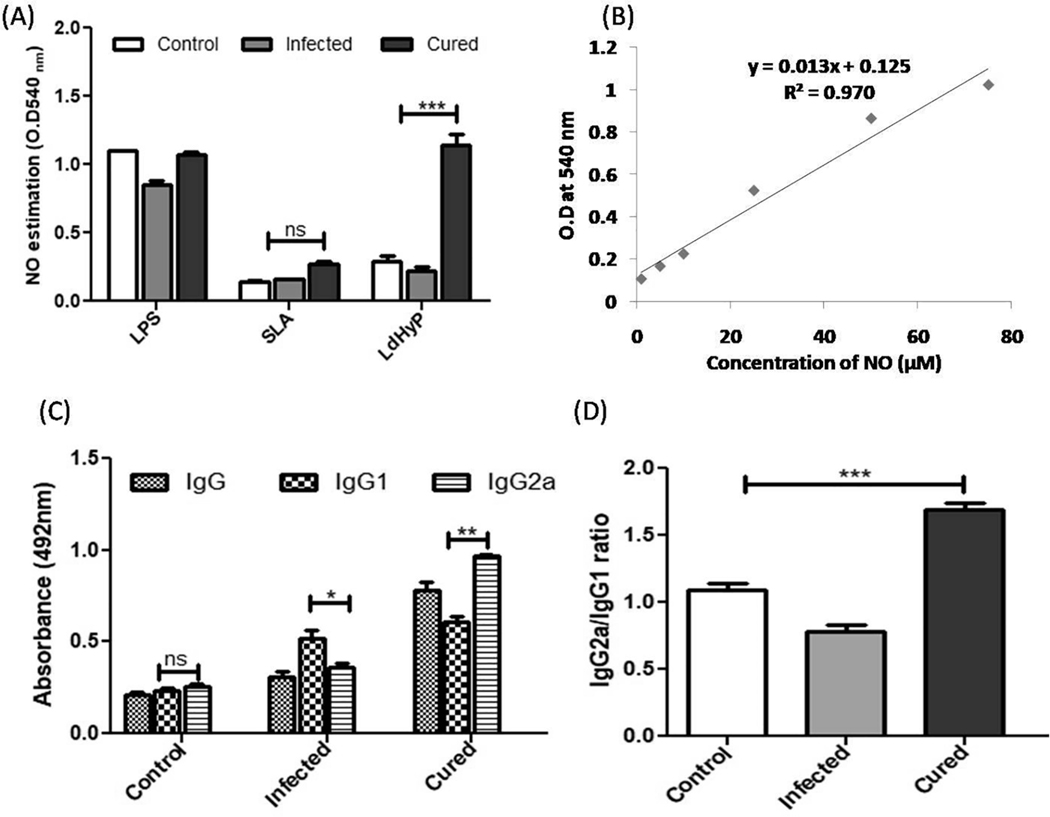
Th1 products (IFN-γ and TNF-α) mediated, macrophage activation mechanism is another effective cellular response for parasite clearance. Activated macrophages increased the production of nitric oxide (NO) to kill the intracellular parasite. In Leishmania infection, nitric oxide is an important factor to control it [13–15] and for that we estimated the NO level in the supernatant of single cell suspension of the spleen which was stimulated with LdHyP for 72 hr and found to be significantly high level (>3.5 fold) in vaccinated mice after re-stimulation with LdHyP as a comparison to infected and control (Fig. 6 A–B) group of mice. This finding substantiates the possibility of LdHyP as an effective vaccine molecule which stimulate a strong protective response in vaccinated mice to obtained significant protection from VL.
Fig. 6.
In vitro cellular and humoral immune response induced by LdHyP in vaccinated challenged mice. (A) Estimation of NO production in supernatant collected from 72 hr stimulated splenocytes of all three experimental groups of mice with LPS/SLA and LdHyP. 1:1 ratio of both supernatant and Griess reagent was used and takes reaction product absorbance at 540 nm. (B) Standard graph was generated by sodium nitrite (NaNO2). (C) LdHyP specific IgG and its isotype IgG1 and IgG2a in the sera of cured mice followed by infected mice. (D) The IgG2a versus IgG1 ratio in the sera of all experimental groups of mice. Data represent the mean ± SD of two independent experiments in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significant.
2.5. Induction of humoral immune response among LdHyP vaccinated mice
We investigated the effect of LdHyP on humoral immune response alteration in all experimental groups’ sera of mice and we found that mice vaccinated with LdHyP showed significantly higher IgG response in comparison to control and infected groups. The switching of IgG1 and IgG2a isotype is regulated by IL-4 and IFN-γ cytokines respectively [14,16]. The significant increased IgG2a antibody titer was observed in the sera of cured mice (Fig. 6C). It is well documented that higher IgG1 isotype (Th2 driven) titer was associated with high parasite load [14]. Here, the lower IgG1 antibody titer in cured mice as compared to infected mice, reflecting poor Th2 immune response generation in LdHyP vaccinated mice. The higher IgG2a/IgG1 ratio was observed in cured mice against parasite specific antigen (Fig. 6D). Both humoral as well as cellular immune response were well correlated with the parasite clearance in LdHyP immunized mice challenged with L. donovani.
2.6. Splenocyte proliferation and different T-subset response to LdHyP in vaccinated mice
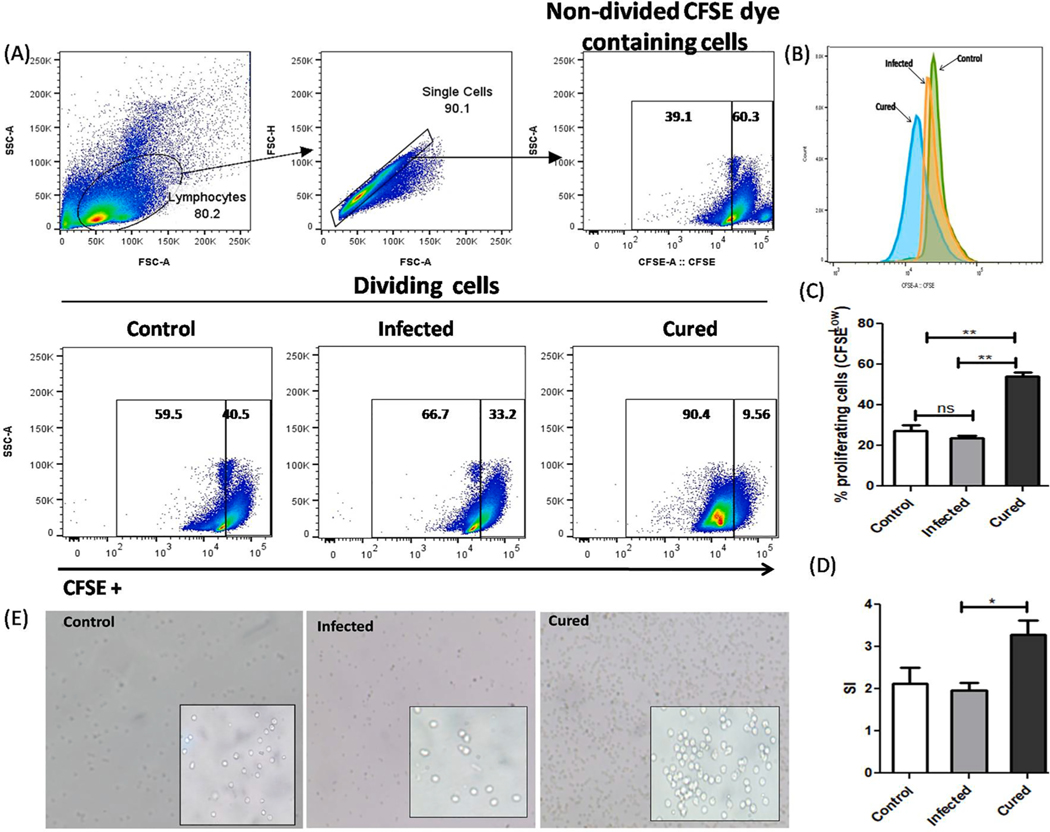
Decreased T cell response, as well as its lower proliferation is associated with VL progression which is characterized by lower production of IFN-γ through T helper 1 (CD4+/CD8+) cells [19,26,27]. Therefore, a vaccine candidate against leishmaniasis should be able to restore the T cell proliferation and its responses to produced Th1 marker cytokines. To determine the level of T-cell proliferation in all experimental groups of mice, we treated their splenocytes with CFSE dye and proliferation was analyzed through flow cytometry (Fig. 7A). A significant higher splenocyte proliferation (58 ± 6.5%) was found in cured mice over control (22 ± 5%) and infected (25 ± 2%) group of mice as clearly indicated in Fig. 7B & D. A clear peak shift in left direction was observed in cured mice as compared to both control and infected group, indicates more CFSE dilution (Fig. 7C). The stimulation index of LdHyP was also found to be significantly high in cured mice (Fig. 7E). This result was further validated by our microscopic examination, which clearly showed higher number of cells in cured mice as compared to the control and infected group (Fig. 7F).
Fig. 7.
LdHyP specific splenocytes cell proliferation in cured mice followed by control and infected mice. (A) Single cell suspension of spleen (5×106) from all three experimental groups of mice were labeled with CFSE dye and stimulated for 3 days as mentioned in material and methods section for cell proliferation by FACS. (B) Histogram of CFSE labeled proliferating cell (CFSE low) in all three groups. (C) Percentage of splenocytes cells proliferation (CFSE low) and stimulation index of LdHyP (D) in all three groups. (E) Microscopic evaluation of cell proliferation in all three groups of mice under 10X magnification (inset 40X magnification). Data represent the mean ± SD of two independent experiments. *p < 0.05, **p < 0.01, ns: no significant.
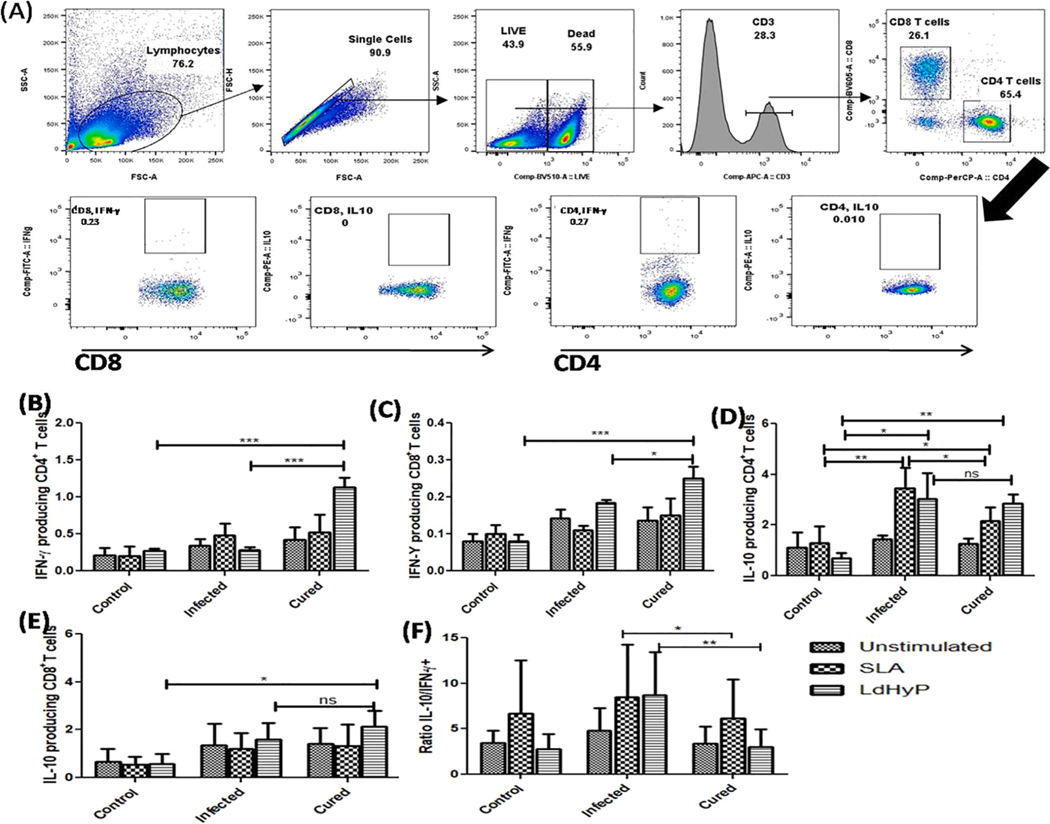
A higher level of IFN-γ producing CD4+ and CD8+ T cells in Leishmania infected host contribute to preventing the VL progression [28]. IFN-γ produced by these cells activates macrophages to kill the intracellular amastigotes [29]. To evaluate the contribution of IFN-γ expressing CD4+ and CD8+ T cells in LdHyP vaccinated challenged mice against Leishmania infection, we isolated spleen from all experimental groups of mice and analyzed T cell response by intracellular cytokines staining (Fig. 8A). A significant higher (~4.6 fold) LdHyP specific IFN-γ producing CD4+ (1.4 ± 0.2%) and ~2.1 fold higher CD8+ T cells (0.4 ± 0.05%) was found in cured mice as compared to IFN-γ+ CD4+ (0.3 ± 0.11%) and CD8+ T cells (0.19 ± 0.2%) of infected mice (Fig. 8B–C). However, we did not observe significant induction with SLA stimulation. Altogether, these data suggest that LdHyP immunization stimulates cell proliferation as well as induced polarization of IFN-γ expressing T cells. Apart from this, we also observed elevated level of LdHyP specific IL-10+ CD4+ and CD8+ T cells in cured (2.5 ± 1%, 2.1 ± 1.5%) and infected (3.0 ± 2%, 1.6 ± 1.1%) group of mice both over control (0.7 ± 0.1%, 0.5 ± 0.1%) group of mice (Fig. 8D–E). SLA specific IL-10 expressing CD4+T cells were found to be high in the infected group of mice. While the ratio of IL-10/IFN-γ was found to be lower in cured mice as compared to infected mice (Fig. 8F), indicates that LdHyP vaccinated mice induced prominent Th1 biased cellular immune response that correlates to parasite clearance in cured mice. Further investigation towards the multifunctional role of both CD4+ and CD8+ T cells needs to better understand their involvement in parasite clearance in LdHyP immunized mice.
Fig. 8.
Evaluate the contribution of CD4+ and CD8+ T cells towards parasite clearance in cured mice. (A) Gating strategy used in this study is as mentioned in material and methods section. (B-C) Bar graph showing the percentage of IFN-γ expressing CD4+T cells and CD8+T cells in various groups. (D-E) Bar graph depicting percentage of IL-10+CD4+T cells and IL-10+CD8+T cells in all three groups. (F) Ratio of IL-10/IFN-γ in cured group over infected group. Data represent the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significant.
3. Discussion
VL is a deadliest infectious disease in the world that is caused by L. donovani and its treatment depends on a few drugs with some limitations. Vaccination is an effective, affordable and safest way to combat Leishmania infection in comparison to chemotherapeutic approaches. Still, there is no vaccine against VL available in the market for humans, while some recombinant vaccines are in pipeline and 2nd and 3rd phase of clinical trials [17–21]. It makes basic study necessary towards the identification of good vaccine candidates which should have the capability to induce strong Th1 biased cellular immune response.
Here, in the present study we first time characterized and evaluate the prophylactic and antigenic potential of L. donovani hypothetical protein LDBPK_252400 (LdHyP) in the susceptible Balb/c mice model against VL. LdHyP shows more than 95% sequence identity with the hypothetical proteins of L. infantum (XM_001466233.1) and L. major (XM_001683939.1). The native molecular mass (32.9 kDa) of purified dialyzed LdHyP indicated its monomeric nature (Fig. 2A–B). The emission maxima of dialyzed protein was found to be between 335 and 345 nm and more than 350 nm of un-dialyzed protein upon excitation of 290 nm, suggesting that we obtained native form of LdHyP after dialysis (Fig. 2C). It is well documented that the native protein gives emission maxima between 334 and 345 nm while emission maxima ≥350 nm (red shift) of any protein indicated its denatured form [30,31].
Some hypothetical proteins of Leishmania species have been already evaluated as a DNA vaccine or recombinant protein vaccine against Leishmania infection [25,32]. Here, we have shown that LdHyP is safe and induced partial protection in Balb/c mice challenged with L. donovani stationary stage promastigotes. We also report that clearance of the parasite burden in vaccinated challenged mice is correlated with LdHyP induced predominant Th1 biased cellular immune response. Apart from this, we did not observe any splenomegaly and hepatomegaly in LdHyP vaccinated mice as compared to infected mice. The prophylactic efficacy of LdHyP as immunogen was validated by significant decrease in parasite load in liver and spleen of vaccinated challenged mice.
Several studies have been reported that parasite antigen that elicits T helper 1 cellular immune response shows strong protection against VL in susceptible Balb/c mice model [28,29] and IFN-γ is the marker cytokines of Th1cellular immune response. On the contrary, the production of Th2 driven cytokines (IL-4 and IL-10) promotes progression of Leishmania infection in Balb/c mice model after parasite challenge [13,16,29]. Therefore, the production of IFN-γ from splenocytes of all three experimental group of mice were analyzed. Apart from this, other Th1 and Th2 driven cytokines (Th1: IL-12p40, TNF-α., Th2: IL-4, IL-10) were also analyzed. The production of IFN-γ was found to be the most abundant in the supernatant followed by IL12p40 and TNF-α in vaccinated challenged group (cured) compared to the challenged group (infected). IL-12p40 is mainly responsible for the differentiation of Th1 cells and promotes the production of IFN-γ cytokines [33] while IFN-γ and TNF-α cytokines induced parasite killing by the activation of macrophages [34]. The production of Th2 driven cytokines IL4 was found to be significantly low in cured mice while IL-10 was observed high in both cured and infected mice over the control mice. However, the ratio of IL-10 versus IFN-γ cytokines was found to be significantly low in cured mice. Collectively, our results show that LdHyP immunization conferred protective ability by triggering the Th1 biased cellular immune response in cured mice as compared to infected mice challenged at the same time point. Furthermore, the higher level of NO production in cured mice also confirmed the efficacy of LdHyP to elicit the cellular immune response towards Th1.
Additionally, in the current study, we observed a robust IgG2 antibody titer against LdHyP in cured mice while IgG1 antibody titer was found to low. Humoral immune responses against LdHyP in cured mice sera are consistent with our cellular immune responses. IFN-γ cytokines regulate the switching of IgG2 isotypes while IgG1 by IL-4. The elevated ratio of IgG2/IgG1 antibodies in cured mice might be contributed to parasite clearance. These data suggest that LdHyP immunization generate all protective cellular immune responses to achieve significant protection against Leishmania infection.
It is well documented that any vaccine that can alter the cellular immune response towards CD4+Th1 type are essential to control the progression of VL disease [21,28,29] due to its role in the secretion of IFN-γ. Along with this CD8+T cells also contributes to the reduction of parasite burden either by the production of IFN-γ which activates macrophages or through direct killing of parasite containing macrophages or by both mechanisms [21,29]. Our flow cytometry data validate these findings and confirm the protective efficacy of LdHyP in cured mice. We observed the higher level of LdHyP specific IFN-γ+ CD4+T (4.6 fold) and IFN-γ+ CD8+T (2.1 fold) cells in cured mice as compared to infected mice. In our previous insilico study, we found that LdHyP contains both MHC-I and MHC-II (6 CTL, 2 HTL and 10 B cell epitopes) regions which may contribute for production of both types of T cells in immunized mice [35]. Apart from this, we also observed IL-10+ CD4+T and IL-10+ CD8+T production in cured mice but slightly low than infected mice. The lower ratio of IL-10/IFN-γ producing T cells in cured mice again confirmed Th1 biased immune response production by LdHyP in immunized mice. Further investigations are needed in this direction to better understand and characterized the mode of action of different subtype of both T cell responses in LdHyP immunized mice.
In conclusion, we conjecture that LdHyP is safe, protective and immunogenic in murine model. It is well documented that in the case of VL patients the cure and protection against Leishmania infection are associated with dominant Th1 type and CD8+T cells response. A specific type of cytokines balance is essential for the suppression of infection progression. Thus our findings suggest that LdHyP behaves as a good prophylactic efficacy, it induced predominant Th1 biased cellular immune response in susceptible Balb/c mice. In vitro re-stimulation with LdHyP enhanced the production of higher percentage of IFN-γ+ CD4+T and IFN-γ+ CD8+T cells both which indicates its long-term protective efficacy since in future it might be utilized as a vaccine candidate against Leishmania infection. However, more study needs in this direction to better understand the mode of action of LdHyP towards subtypes of T cells.
4. Materials and methods
4.1. Animals and parasite culture
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Institute of Medical Sciences (IMS), Banaras Hindu University, Varanasi (CAEC number: Dean/2014/CAEC/615). Balb/c mice (7–8 weeks old) were purchased from Central Drug Research Institute (CDRI), Lucknow. All study experiments on mice followed the guidelines of Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India.
For parasite culture, splenic aspirates of VL patients were collected in 250 μl of RPMI 1640 medium and transferred into the blood agar tube. Tube was kept at 25 °C BOD incubator to observe promastigotes after 3 days up to 7 days as described earlier [36]. Soluble Leishmania antigen (SLA) was prepared using these stationary promastigote parasites as described by Singh et al. [37] and stored at – 80 °C until use.
4.2. Cloning, expression and purification of LdHyP
The ORF of L. donovani LdHyP gene (NCBI ID of LDBPK_252400: XM_003861522.1) was PCR amplified from L. donovani genomic DNA using gene specific forward 5′-CCGAATTCATGCAGACGGCCACCGCCCCT-3′and reverse 5′-CCGAAGCTTCGGCGGCTCCTTGGCGACGTA-3′ primers with restriction enzyme EcoRI (GAATTC) at the 5′end and Hind III (AAGCTT) site at the 3′ end. PCR product (891 bp amplified LDBPK_252400 gene) was cloned into the MCS site of pET28a expression vector. The cloned vector was transformed into BL21 (DE3) E. coli host cells for overexpression by 1 mM IPTG induction. Induced culture, supplemented with 50 μg/ml kanamycin were grown for 16–18 hr at 18 °C, shaking at 180 rpm. After lysis of induced cells in lysis buffer (20 mM Trish-HCl buffer (pH 8.8), 100 mM NaCl, 2 M Urea) by sonication and centrifugation at 12000 rpm for 30 min, the pellets of inclusion bodies were re-suspended in 50 mM Tris-HCl buffer (pH 8.8) containing 100 mM NaCl, 3 mM βME and 8 M urea and then incubate overnight. After centrifugation supernatants were allowed to bind to Ni-NTA agarose matrix pre-equilibrated with lysis buffer along with 10 mM imidazole. Washing was performed by two subsequent washing buffer containing lysis buffer with 30 mM imidazole (W1) and 50 mM imidazole (W2) to remove contaminated protein. Finally protein was eluted by 500 mM imidazole in the lysis buffer and overnight dialyzed with 20 mM Tris-HCl buffer (pH 8.8), 100 mM NaCl. The purity and subunit mass of dialyzed protein was confirmed by 12% SDS-PAGE and western blotting using anti-His primary antibody [38].
4.3. Size exclusion chromatography (SEC)
Furthermore, for native molecular weight (oligomerization) determination of dialyzed LdHyP protein, gel filtration was carried out using a Superdex™ 650 HR, 10/300 column on an AKTA-FPLC (GE Healthcare Biosciences). The SEC column was calibrated first with standard molecular weight markers (Amershan). The column was pre-equilibrated with dialyzed buffer and then injected 500 μl protein samples at low rate of 0.7 ml/min (25 °C) and elution was observed at 280 nm absorbance [38].
4.4. Fluorescence spectroscopy and circular dichroism (CD)
Fluorescence spectra of dialyzed LdHyP protein were recorded on a spectrofluorometer at 25 °C for identification of its tertiary structure. 3 μM protein sample were excited at 290 nm and then monitored emission spectra in the range of 300–400 nm wavelength. Thereafter, the secondary structure was estimated by far-UV circular dichroism (J-1500 spectropolarimeter) spectropolarimeter. All parameters were the same as for fluoromax. CD spectra of 3 μM protein sample were recorded in a 1 mm path length qurze cuvette and spectra were averaged over 2–4 scans at a scan speed of 10 nm/min [30].
5. Evaluation of the prophylactic potential of LdHyP
5.1. Immunization, challenge infection and assessment of parasite load
Balb/c mice (n = 10, per group) used for vaccination were divided into three groups; group 1 mice were normal control (PBS/ Adjuvants), group 2 mice were immunized with LdHyP (50 μg/100 μl per mice) and challenged (cured/vaccinated), group 3 mice were challenged with L. donovani (infected control). Two booster doses were given at 2-week intervals. Four weeks after the final immunization group 2 and 3 were infected intra-peritonial (i.p.) by 1×106 stationary-phase promastigotes of L. donovani.
To evaluate the protective potential of protein after 8–12 weeks of post infection, mice of all groups were sacrificed, and liver as well as spleen tissue were collected for parasite quantification through tissue impression smears on slides as per reported protocol [11–13]. Amastigotes parasites burden were counted by the microscopic examination of Giemsa-stained tissue smears. The parasite load was expressed as the number of amastigotes nuclei per 100 cell nuclei. Percentage of parasite recovery: amastigotes/100 nuclei.
5.2. Limiting dilution assay (LDA)
We performed LDA for the validation of viable L. donovani promastogote in the mice liver and spleen as reported previously [13,16]. Spleen and liver from both group 3 and 4 were weighted, homogenized and cultured (Schnieder’s insect media with 10% FBS) in tenfold serial dilutions at 24 °C for 5–7 days in 24-well tissue culture plates (Nunc). Presence of motile promastigotes in all wells were examined after 5 days by microscope under 40X magnification and promastigotes images were captured under 100X magnification. Viable promastigote titer was calculated as the highest dilution at which parasite was visible [13].
5.3. Evaluation of extracellular Th1/Th2 cytokines and NO level
Th1/Th2 cytokines levels were evaluated through ELISA. Briefly, Spleen from all experimental groups of animals was isolated and placed in PBS. For single cell suspension formation, spleen cells mashed and pass from 70 μM nylon cell strainer (USA) into PBS. Thereafter, RBC lysis buffer (Sigma) used for lysis of erythrocytes and then centrifuges at 1800 rpm, 5 min. Cells were washed with FACS buffer (1% FBS + PBS), stained with trypan blue and count by Neubauer chamber. Cells at a density 1 × 106 cells/ml were cultured in tube alone or stimulated with 10 μg/ml SLA/2μg/ml LPS and 20 μg/ml antigen (LdHyP) for 72 hr at 37 °C and 5% CO2. After incubation of 72 hr, Th1/Th2 cytokines have been determined in the supernatant using BD Biosciences mouse cytokines ELISA kit. The same experimental set was also utilized for the estimation of NO through Griess reagents (Invitrogen). For NO estimation, 72 hr incubated culture supernatants were mixed with Griess reagent in 1:1 ratio and incubated for 15–20 min in dark. Now add 2 N H2SO4 to stop the reaction. The O. D. was taken at 540 nm in ELISA plate reader. A standard graph was generated by sodium nitrite (NaNO2).
5.4. Determination of IgG isotypes through ELISA
Sera from a different group of mice were used for the estimation of IgG and its isotype antibody by ELISA [13,16]. ELISA plate was coated with 5 μg/well LdHyP in carbonate buffer (pH 9.2) overnight at 4 °C. Next day, after three times washing with PBST (PBS + 0.05% tween 20), wells were blocked with 3% BSA and incubated for 2 hr at room temperature. Wells were then washed and added mice serum (1:500 dilutions) of different groups for 3–4 hr at room temperature. After that IgG isotype rabbit anti-mouse IgG1, IgG2a antibody conjugated with peroxidase (HRP) were added and incubated for 2–3 hr followed by addition of HRP substrate OPD (o-phenylenediamine dihydrochloride) for 15–20 min. Reaction was stopped by adding 2 N H2SO4 and absorbance was taken at 490 nm on ELISA plate reader.
5.5. Cell proliferation tracking by carboxyfluorescein succinimidyl ester (CFSE) dye
Antigen-specific splenocyte cell proliferation assay was performed using CellTrace™ CFSE cell proliferation kit (BD Biosciences cat no #565082) as described previously with some modifications [28,29]. In brief, single cell suspensions of spleen from all three groups were stained with CFSE dye (5 μM) as mentioned in the BD Biosciences protocol. Thereafter, an equal number of cells (5 × 106 cells/ml) were cultured in complete RPMI 1640 media (10% FBS) and stimulated with antigen (LdHyP: 20 μg/ml), PHA (5 μg/ml, Sigma Cat# 61764) and SLA (10 μg/ml). Unstimulated cells were taken as a negative test control and PHA stimulated cells as a positive control. The cells were incubated in CO2 incubator (Sanyo, Japan) for 72 hr at 37 °C and 5% CO2. Cells were washed 2 times with PBS and re-suspended in the FACS stain buffer (5% FCS in 1X PBS) to monitor proliferation by flow cytometry (BD LSRFortessa) at the indicated times (72 hr) and data were analyzed using FlowJov10.6.2 software (Tree Star, Ashland, OR). Unstimulated cells were used to set the gate position. An equivalent volume of cells from the same series of experiments was used to monitor the number of cells by microscopic examination.
5.6. Lymphocyte phenotyping and intracellular cytokine analysis in the spleen
The phenotypic characterization and intracellular cytokine analysis of T cells present in mouse spleen cells was done by FACS staining. CD3, CD4, CD8 expression was measured by surface staining whereas IFN-γ and IL-10 was measured by intracellular staining. Fixable Zombie Aqua™fixable viability kit (Biolegend, cat no #423102) with some modifications was used to exclude dead cells from the analysis [13]. Briefly, for each sample, 2 × 106 spleen cells/ml were distributed in three 5 ml polypropylene round bottom culture tube (Corning, Ref No. 352063, Mexico) in each and treated with 10 μl PBS (Control or unstimulated tube), SLA (10 μg/ml) and LdHyP antigen (20 μg/ml) respectively. Cell culture was then incubated for 24 hr at 37 °C, 5% CO2. GolgiStop™ (BD Biosciences cat No #51–2092KZ) was added to the culture during last 4 hr of culture to stop the release of intracellular cytokines. Thereafter, cells were washed with PBS at 1800 rpm for 6 min. Cells were transferred to 5 ml polystyrene round bottom FACS staining tube (Corning, Ref No 352054, Mexico) and incubated in dark at room temperature for 30 min in 100 μl PBS with Zombie Aqua™dye for dead cells staining. 2 ml FACS staining buffer (5% FCS in 1 × PBS) was added in each tube and washed 2 times. Surface staining was done in FACS stain buffer with CD3 APC (BD Biosciences cat no# 565643), CD4 PerCp (Biosciences cat no# 550954) and CD8 BV-605 (BD Biosciences cat no# 563152) for 30 min in dark. After surface staining, intracellular staining was done. For this BD Cytofix/Cytoperm™Fixation/Permeabilization kit (BD Cat No 554714) was used. The cells were washed two times with 2 ml 1 × perm wash buffer and 250 μl Cyto Fix/Perm buffer was added for permeabilization and fixation of cells. Cells were incubated for 20 min at 4 °C and washed two times with 2 ml 1 × perm wash buffer. Intracellular staining was done in per wash buffer with IFN-γ-FITC (BD Biosciences cat no# 554411) and IL-10 PE (BD Biosciences cat no #55446) Cells were incubated for 30 min at 4 °C. Finally, stained cells were washed two times with 2 ml perm wash buffer and 300 μl FACS stain buffer was added. Freshly stained cells were acquired by Flow cytometer (BD LSR Fortessa flow cytometer, BD Biosciencec, USA) using FACS Diva software. FACS data was analyzed by FlowJo version 10 software (Tree Star BD)
5.7. Statistical analysis
All in vitro experiments were performed in triplicate. A minimum of 10 mice per group were used for in vivo experiments. The statistical significance was determined by Graph Pad Prism software version 8.0 (San Diego, USA), using student’s t test and two way ANOVA followed by Tukey’s multiple comparison test. P value of <0.05 was considered statistically significant. All data in the bar graphs represented as the mean ± SD.
Acknowledgements
The authors (SY, JP, VKD) are thankful to the IIT-BHU for infrastructural support. The author (SY) gratefully acknowledges the financial support from SERB, India for providing an N-PDF fellowship (PDF/2016/003186) and CD facility at IIT Guwahati. JP thankfully acknowledges fellowship support from CSIR for providing CSIR-SRA (Pool scientist scheme: 13(9076-A) 2019 pool). SS is supported by the Department of Science & Technology (SR/NM/NS-57/2016), New Delhi, India (Under nano-mission), and in part by the Extramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, United States (TMRC grant number U19AI074321). The funders had no role in the design, decision to publish, or preparation of the report.
Footnotes
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- [1].W. H. Organization, Eliminating visceral leishmaniasis: India takes decisive steps to overcome last-mile challenges, 5 March 2020. [Google Scholar]
- [2].Tedla Dawit Gebremichael, Bariagabr Fsahatsion Hailemariam, HadguAbreha Hagos, Incidence and trends of leishmaniasis and its risk factors in Humera, Western Tigray, J. Parasitol. Res 2018; 8463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dhiman RC, Emerging vector-borne zoonoses: eco-epidemiology and public health implications in India, Front. Publ. Health 30 (2014) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khanra S, Datta S, Mondal D, Saha P, Bandopadhyay SK, et al. , RFLPs of ITS, ITS1 and hsp70 amplicons and sequencing of ITS1 of recent clinical isolates of Kala-azar from India and Bangladesh confirms the association of L. tropica with the disease, Acta Trop. 124 (2012) 229–234. [DOI] [PubMed] [Google Scholar]
- [5].Krayter L, Bumb RA, Azmi K, Wuttke J, Malik MD, Multilocus microsatellite typing reveals a genetic relationship but, also, genetic differences between Indian strains of Leishmania tropica causing cutaneous leishmaniasis and those causing visceral leishmaniasis, Parasites Vectors 7 (2014) 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sundar S, Singh A, Rai M, Chakravarty J, Single-dose indigenous liposomal amphotericin B in the treatment of Indian visceral leishmaniasis: A phase 2 study, Am. J. Trop. Med. Hyg 92 (2015) 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thakur CP, Ahmed S, Observations on amphotericin B treatment of kala-azar given in a rural set up in Bihar, India, Indian J. Med. Res 113 (2001) 14–18. [PubMed] [Google Scholar]
- [8].Gupta G, Steve Oghumu R Satoskar Abhay, Mechanisms of immune evasion in leishmaniasis, Adv. Appl. Microbiol 82 (2013) 155–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gurunathan S, Stobie PC, Sacks D, Glaichenhaus N, Iwasaki A, Fowell DJ, Locksley RM, Chang JT, Wu CY, Seder RA, Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells, J. Immunol 165 (2000) 915–924. [DOI] [PubMed] [Google Scholar]
- [10].Das Nahid Ali Amrita, Vaccine development against Leishmania donovani Front. Immunol. 3 10.3389/fimmu.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nico Dirlei, Claser Carla, Borja-Cabrera Gulnara P., Travassos Luiz R., Palatnik Marcos, da Silva Irene, Martins Soares Mauricio, Rodrigues Clarisa B., Palatnik-de-Sousa, Adaptive immunity against Leishmania nucleoside hydrolase maps its C-terminal domain as the target of the CD4+ T cell-driven protective response, PLoS NTD 4 (2010), e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bhowmick Sudipta, Ravindran Rajesh, Ali Nahid, IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine, BMC Microbiol. 14 (2014) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sabur Abdus, Bhowmick Sudipta, Chhajer Rudra, Sarfaraz Ahmad Ejazi Nicky Didwania, Asad Mohammad, Bhattacharyya Anirban, Sinha Utsa, Ali Nahid. Liposomal elongation factor-1α triggers effector CD4 and CD8 T cells for induction of long-lasting protective immunity against visceral leishmaniasis. Front. Immunol 2018, 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anand Sneha, Madhubala Rentala, Genetically engineered ascorbic acid-deficient live mutants of Leishmania donovani induce long lasting protective immunity against visceral leishmaniasis, Sci. Rep 5 (2015) 10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mary C, Auriault V, Faugere B, Dessein AJ, Control of Leishmania infantum infection is associated with CD8(+) and gamma interferon- and interleukin-5-producing CD4(+) antigen-specific T cells, Infect. Immun 67 (1999) 5559–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Manuel S, Laura C, Esther G, Ramírez Laura, Iniesta Virginia, Bonay Pedro, Gómez-Nieto Carlos, González Víctor M., Martín M. Elena, Alonso Carlos, Coelho Eduardo A.F., Barral Aldina, Barral-Netto Manoel, Iborra Salvador, Coadministration of the three antigenic Leishmania infantum poly (A) binding proteins as a DNA vaccine induces protection against Leishmania major Infection in BALB/c mice, PLoS Negl. Trop. Dis 9 (2015), e0003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain Keerti, Jain NK, Vaccines for visceral Leishmaniasis: A review, J Immunol. Methods 422 (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [18].Chakravarty Jaya, Kumar Subodh, Trivedi Sundar, Piazza Franco Shyam, A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine for use in the prevention of visceral leishmaniasis, Vaccine 29 (2011) 3531–3537. [DOI] [PubMed] [Google Scholar]
- [19].Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME, New vaccines for neglected parasitic diseases and dengue, Transl. Res 162 (2013) 144–155. [DOI] [PubMed] [Google Scholar]
- [20].Ghorbani M, Farhoudi R, Leishmaniasis in humans: drug or vaccine therapy? Drug Des. Devel. Ther 12 (2018) 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stager Simona, Rafati Sima, CD8+T cells in Leishmania infections: friends or foes? Front. Immunol 3 (2012) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sinha AK, Singh P, Prakash A, Pal D, Dube A, Kumar A, Putative drug and vaccine target identification in Leishmania donovani membrane proteins using naive Bayes probabilistic classifier, IEEE/ACM Trans. Comput. Biol. Bioinform 14 (2017) 204–211. [DOI] [PubMed] [Google Scholar]
- [23].Nirujogi Raja Sekhar, Pawar Harsh, Milind Patole, Pandey Akhilesh, Moving from unsequenced to sequenced genome: Reanalysis of the proteome of Leishmania donovani, J. Proteomics 97 (2014) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gupta SK, Sisodia BS, Sinha S, Hajela K, Naik S, Shasany AK, Dube A, Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigote, Proteomics 7 (2007) 816–823. [DOI] [PubMed] [Google Scholar]
- [25].Ribeiro PAF, Dias DS, Lage DP, Costa LE, Martins VT, Tavares GSV, Mendonça DVC, Lima MP, Oliveira JS, Steiner BT, Roatt BM, Coelho EAF, Evaluation of a Leishmania hypothetical protein administered as DNA vaccine or recombinant protein against Leishmania infantum infection and its immunogenicity in humans, Cell Immunol. 331 (2018) 67–77. [DOI] [PubMed] [Google Scholar]
- [26].Seder Robert A., Darrah Patricia A., Roederer Mario, T-cell quality in memory and protection: implications for vaccine design, Nat. Immunol 8 (2008) 247. [DOI] [PubMed] [Google Scholar]
- [27].Dayakar Alti, Chandrasekaran Sambamurthy, Kuchipudi Suresh V., Kalangi Suresh K., Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy, Front. Immunol 10 (2019) 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ruben Jurjen M., García-Romo Gina Stella, Breman Eytan, van der Kooij Sandra, Redeker Anke, Arens Ramon, Cees van Kooten, Human plasmacytoid dendritic cells acquire phagocytic capacity by TLR9 ligation in the presence of soluble factors produced by renal epithelial cells, Kidney Int. 93 (2018) 355–364. [DOI] [PubMed] [Google Scholar]
- [29].Xi Chen, Ai Xiaojie, Chunlian Wu., Wang Heyong, Zeng Gang, Yang Peixin, Liu Gentao, A novel human IL-2 mutein with minimal systemic toxicity exerts greater antitumor efficacy than wild-type IL-2, Cell Death Dis. 9 (2018) 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yadav S, Gupta S, Saxena JK, Monitoring thermal and chemical unfolding of Brugia malayi calreticulin using fluorescence and Circular Dichroism spectroscopy, Int. J. Biol. Macromol 102 (2017) 986–995. [DOI] [PubMed] [Google Scholar]
- [31].Gupta S, Yadav S, Suryanarayanan V, Singh SK, Saxena JK, Investigating the folding pathway and substrate induced conformational changes in B. malayi Guanylate kinase, Int. J. Biol. Macromol 94 (2017) 621–633. [DOI] [PubMed] [Google Scholar]
- [32].Martins VT, Lage DP, Duarte MC, Costa LE, Garde E, Rodrigues MR, Coelho EA, A new Leishmania-specific hypothetical protein, LiHyT, used as a vaccine antigen against visceral leishmaniasis, Acta Trop. 154 (2015) 73–81. [DOI] [PubMed] [Google Scholar]
- [33].Park AY, Hondowicz BD, Scott P, IL-12 is required to maintain a Th1 response during Leishmania major infection, J. Immunol 165 (2000) 896–902. [DOI] [PubMed] [Google Scholar]
- [34].Liew FY, Li Y, Millott S, Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide, J. Immunol 145 (1990) 4306–4310. [PubMed] [Google Scholar]
- [35].Yadav S, Prakash J, Shukla H, Charan DK, Timir T, Dubey VK, Design a multiepitope subunit vaccine for immune-protection against Leishmania parasite, Pathog. Glob. Health (2020) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maurya R, Singh RK, Kumar B, Salotra P, Rai M, Sundar S, Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure, J. Clin. Microbiol 43 (2005) 3038–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singh OP, Stober CB, Singh AK, Blackwell JM, Sundar S, Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis, PLoS Negl. Trop. Dis 6 (2012), e1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yadav S, Gupta S, Selvaraj C, Doharey PK, Verma A, Singh SK, Saxena JK, In silico and in vitro studies on the protein-protein interactions between Brugiamalayi immunomodulatory protein calreticulin and human C1q, PLoS One 9 (2014), e106413. [DOI] [PMC free article] [PubMed] [Google Scholar]