Abstract
Toll-like receptors (TLRs) are a family of pattern-recognition receptors that initiate signaling in innate and adaptive immune pathways. The highly conserved family of transmembrane proteins are comprised of an extracellular domain that recognizes exogenous and endogenous danger molecules and an ectodomain that activates downstream pathways in response. Recent studies suggest that continuous activation or dysregulation of TLR signaling may contribute to chronic disease states. The receptor is located not only on inflammatory cells (meningeal and peripheral macrophages) but on neuraxial glia (microglia and astrocytes), schwann cells, fibroblasts, dorsal root ganglia and dorsal horn neurons. Procedures blocking TLR functionality have shown pronounced effects on pain behavior otherwise observed in models of chronic inflammation and nerve injury. This review addresses the role of TLR4 as an emerging therapeutic target for the evolution of persistent pain and its role in non canonical signaling mediating anomalous proalgesic actions of opiates. Accordingly, molecules targeting inhibition of this receptor have promise as disease modifying and opioid sparing alternatives for persistent pain states.
Keywords: Chronic pain, TLR4, immune modulation, TAK242, Eritoran, E5564, neuropathic pain
INTRODUCTION
Globally, chronic pain affects ~1.5 billion individuals. [30]. Within the United States alone, nearly 100 million Americans experience chronic pain, more than the total number affected by heart disease, cancer, and diabetes combined [62]. Nearly a quarter of those individuals experience moderate to severe chronic pain that limits activities of daily living and reduces quality of life [62]. Notably, pain is the primary reason that Americans receive disability insurance, and societal costs are estimated at between $560 to $630 billion per year due to missed workdays and medical expenses [29].
Current treatment options range from oral and topical pharmacologic therapies to invasive procedures and surgical interventions [18]. To this day, opiates remain the most potent and reliable analgesic agents, however as we face a mounting opioid epidemic, alternative, opioid-sparing treatment options are of increasing importance [24]. Unfortunately, despite an arsenal of adjuvant therapies, the World Health Organization estimates that 80% of individuals with severe pain do not receive sufficient treatment, likely due to the complex pathophysiology underlying the transition from acute to chronic pain [79].
Current models of pain propose that acute tissue insult from thermal, chemical or mechanical injury triggers a complex cascade of immune and inflammatory interactions [94]. Cessation of acute nociceptive stimulation with full tissue recovery is necessary to terminate the pain signaling process. However, if repetitive nociceptive stimulation persists, pathophysiologic changes occurring at the peripheral, spinal and supraspinal levels may enable the transition of an acute pain state to a chronic pain condition [23]. Thus, the pain state initiated by an acute tissue injury or inflammation may develop a persistency despite wound healing or resolution of the inflammation [16,36,61].
The role of toll-like receptor-4 (TLR4) in immune function and inflammation has been well established, however the role of targeted treatment or prevention of chronic neuropathic pain with TLR4 antagonists is still under investigation [3,7,34,76]. Increasing evidence suggests that the immune system plays an integral role in the transition to and maintenance of chronic pain, but no treatment is currently available to target this pathway. Targeting TLR4 could offer a novel approach to modifying pain processing. This review will focus on the preclinical and clinical evidence for the prevention and treatment of chronic pain states with TLR4 antagonists.
TOLL-LIKE RECEPTORS
Toll-like receptors (TLRs) are a family of pattern-recognition receptors that initiate innate and adaptive immune pathways [46]. This highly conserved family of transmembrane proteins are comprised of an ectodomain characterized by leucine-rich repeats and cytosolic Toll-interleukin-1 (IL-1) receptor (TIR) domains that activate downstream pathways [10,46](Figure 1). The extracellular domain is designed to recognize pathogen associated molecular pattern (PAMP) molecules such as the endotoxin lipopolysaccharide (LPS) [67] as well as danger associated molecular pattern (DAMP) molecules released by damaged cells such as heat shock proteins, extracellular matrix degradation products, and high-mobility group box-1 (HMGB-1) protein [10].
Figure 1.
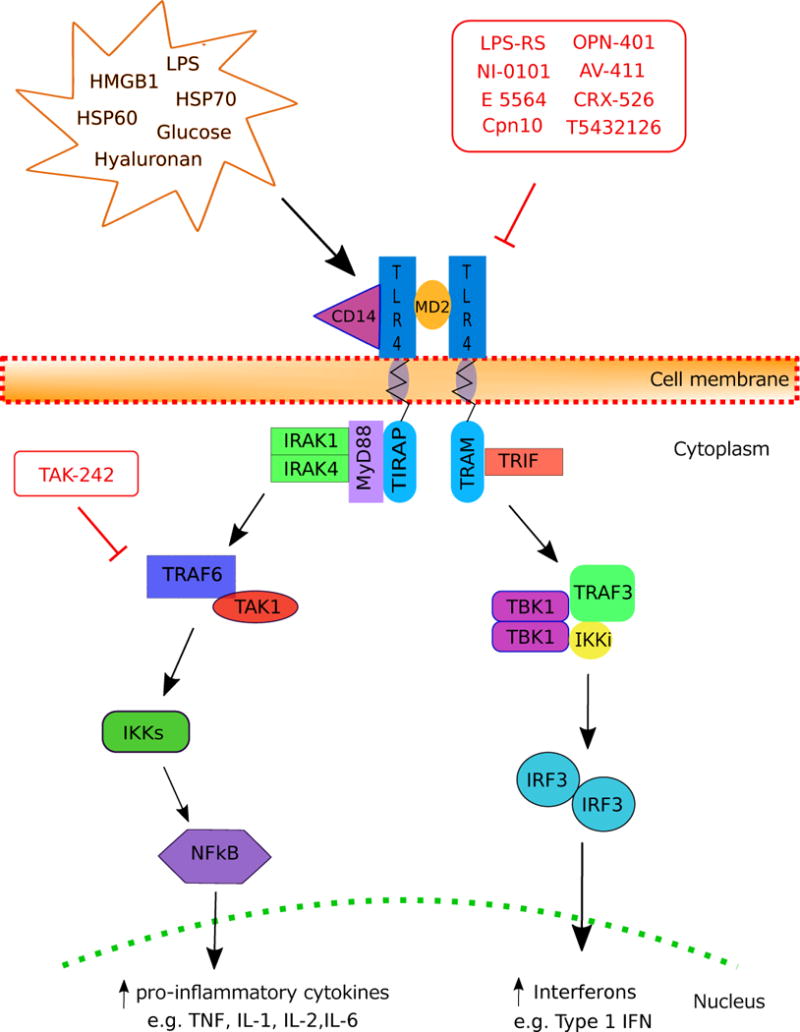
TLR4 Activation Pathways.
Distribution
In the periphery, TLR4 is widely expressed, not only on antigen-presenting cells, but also on endothelial cells, myocytes, thyroid cells, endometrial cells, mesangial cells, adipocytes, and fibroblasts [27]. Expression is modifiable and may vary in response to LPS or pro-inflammatory cytokines. In the central nervous system (CNS), TLR4 is primarily expressed on CNS residential macrophages or microglial cells and to a lesser extent on macroglial cells such as astrocytes [86]. TLR4 is also expressed on primary sensory neurons and on calcitonin-gene related peptide (CGRP) and transient receptor potential cation channel subfamily V (TRPV1)-expressing neurons [88].
Activation
TLR4 localizes, constitutively and/or upon ligand binding, to lipid rafts, which are membrane microdomains characterized by a high content of cholesterol and sphingomyelin [42,87,91]. The TLR4 receptor undergoes dimerization with the co-receptor myeloid differentiation protein 2 (MD-2) to permit binding of the ligand at the TLR4 site and initiate recruitment of intracellular TIR-adaptor molecules [14]. Cluster of differentiation 14 (CD14) also resides in lipid rafts and is involved in some, but not all, TLR4 activation [60]. Decreased diffusion rates in lipid rafts support TLR4 dimerization, an initiating step of its signaling cascade [68,82].
Signaling
Stimulation of TLR4 activates two major intracellular signaling pathways: the myeloid differentiation primary response 88 (MyD88)-dependent pathway and the TIR-domain-containing adapter-inducing interferon (IFN)-β (TRIF) pathway (Figure 1). The MyD88-dependent pathway, mediated by MyD88 and TIR domain-containing adaptor protein (TIRAP), induces NF-kB translocation and expression of pro-inflammatory cytokines (e.g. tumor necrosis factor α (TNFα), IL-1, IL-2, IL-6) and type I interferon (INF) genes (e.g. TNF, IL-12) [65]. The TRIF pathway is mediated by TRIF and TRIF-related adaptor molecule (TRAM) to activate type 1 IFN genes and delayed NF-kB via IFN regulatory factor 3 (IRF-3) [49]. The activation and inhibition of these pathways maintains balanced production of inflammatory cytokines and type 1 IFN [35].
ROLE OF TLR IN REGULATING IMMUNITY AND PAIN CONVERSION STATES
Growing evidence suggests that TLR4 plays a critical link between the innate and adaptive immune response as well as the induction, conversion, and maintenance of chronic pain states [76]. TLR expression on antigen-presenting cells (APCs) leads to subsequent priming of naïve T cells as well as B cell activation, thereby linking the recognition of pathogens with induction of adaptive immune responses [7,12,34]. While recognition of pathogens and molecules from damaged tissue is self-protective, excessive activation of TLRs leads to sustained production of pro-inflammatory mediators [26]. Continuous activation or dysregulation of TLR4 signaling may contribute to inflammatory and autoimmune diseases, including sepsis, atherosclerosis, rheumatoid arthritis, neuropathic pain, and neurodegenerative diseases [53].
Induction of Neuropathic Pain
Specifically, in neuropathic pain, spinal microglial TLR4 activation appears to be critical for pain induction after nerve injury by directly modulating the pro-inflammatory cascade and expression of IFN-γ, IL-1β, TNFα, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway after injury [39,78]. The expression of TLRs on spinal microglia and TLR mediated TNF release emphasizes the role of spinal TLRs in spinally mediated pain states [74]. TLR4 antagonists and siRNA-mediated suppression of intrathecal TLR4 signaling prevents activation of the NF-kB pathway and production of TNF and IL-1β that attenuates mechanical allodynia and thermal hyperalgesia in a chronic constriction injury (CCI)-induced pain model [20,96].
Conversion of Acute to Chronic Pain
TLR4 may also play an important role in the conversion of the acute to chronic pain state [54,94]. Mechanical hypersensitivity induced in mice via the K/BxN serum transfer arthritis model persists after resolution of visible inflammation. When induced in wild type (WT) and TLR4-knockout mice both swelling and inflammation were noted, however TLR4-mutant mice were found to have reversal of mechanical hypersensitivity and reduced glial cell activation after resolution of inflammation. When the WT mice were given an intrathecal (IT) TLR4 antagonist the conversion to mechanical hypersensitivity was reversed. When delivered during the acute inflammatory phase, no effect was seen on inflammation, however development of persistent pain was not seen [15].
Maintenance of Chronic Pain
TLR4 may also play an integral role in the maintenance of neuropathic pain. Sustained delivery of IT TLR4 antagonists reversed CCI-induced neuropathic pain in genetically unaltered rats and repeated administration of a TLR4 antagonist to neuropathic mice reversed both thermal hyperalgesia and mechanical allodynia in WT, but not TLR4 knockout mice [33]. Likewise, attenuation of L5 nerve transection-induced tactile and thermal hypersensitivity along with reduced spinal microglial activation and lower pro-inflammatory cytokines was shown in TLR4-knockout and point mutant mice [78]. When administered IT TLR4 antisense oligodexoxynucleotide (ODN), a dose-related attenuation of L5 nerve transection-induced tactile and thermal hypersensitivity in rats was observed along with reduced expression of mRNA for microglial markers, and reduced expression of mRNA for spinal proinflammatory cytokines [78].
MODULATION OF TLR SIGNALING
TLR4 Antagonism
Recently, Kuzmich and colleagues reviewed both natural and synthetic compounds with known TLR4 antagonist properties in models of systemic inflammation [46]. Of interest here is the preclinical and clinical data from synthetic molecules that demonstrate efficacy in modulating the downstream effects of TLR4 [40]. One popular design for TLR4 antagonists utilizes the native MD2 ligand as inspiration for the antagonist molecule to initiate competitive binding at the extracellular domain [43]. E 5564 (Eritoran) is one such molecule, which contains an N-acetylglucosamine disaccharide scaffold similar to LPS, however sits further inside the MD2 binding cavity to act as a direct TLR4 antagonist [43].
TAK-242 (Resatorvid) is the only other direct TLR4 antagonist to be studied in clinical trials. TAK-242 covalently binds to Cys747 in the intracellular TLR4 domain, rendering TLR4 completely inactive [51]. Another compound in clinical trials, CPN10, inhibits pro-inflammatory cytokine production mediated via TLR4 in macrophages [1]. Related, but distinct compounds currently in preclinical development are CRX-526, a direct TRL4 antagonist, 1A6, a monoclonal antibody that targets the TLR4/MD2 complex, and OPN-401, a viral protein-derived peptide that inhibits TLR4-dependent signaling [44,83,99]. Beta-amino alcohol derivatives including T5342126 have also demonstrated effective MD2-TLR4 binding in in silico models and early pre-clinical data have demonstrated increased latency of heat-induced withdrawal response [43].
Opioid Agonism and Antagonism
Opioid agonists have been reported to act as TLR4 agonists while opioid antagonists such as (+)-naltrexone and (+)-naloxone exert antagonist properties at the TLR4 receptor [33,89]. Glial activation intiates hyperalgesic signals that enhance opioid tolerance. Such effects occur via non-stereoselective activation by opiates at TLR4 [33,89]. In contrast to classic opioid receptors, where opiate receptor activation is limited to the (-)-opioid isomers, (+)-opioid agonists functionally antagonize (-)-opioid analgesia [97], an effect attributed to glial activation and independent of classical mu opioid receptors in knockout mice studies [98]. This non-stereoselectivity provides a means of specifically blocking opioid-induced glial activation by using drugs such as (+)-naloxone [89].
Membrane Microenvironment
Membrane cholesterol is typically regulated by endosomal ATP-binding cassette (ABC) cholesterol transporters ABCA1 and ABCG1, with high-density lipoprotein (HDL) serving as an extracellular cholesterol sink [11,38,66]. In Abca1−/−Abcg1−/− cells with abberant presence of membrane cholesterol, there is a substantial increase in inflammatory gene expression in response to TLR4 ligands, reflecting increased TLR4 dimerization [75,100]. Similiarly, Abca1−/− microglia exhibit augmented LPS-induced secretion of TNFα and decreased phagocytic activity [66]. Treatment with beta-cyclodextrins, which binds cholesterol in the plasma membrane, results in inhibition of TLR4-mediated signaling in cell culture experiments [52,69] and systemic and IT administration have been studied in neurodegenerative diseases such as Niemann-Pick type C disease [47,57,81,85]. Although the use of current beta-cyclodextrins in the general population may be limited by the high rate of serious adverse effects including fever, chemical meningitis and hearing loss, the pathway provides a potential target for future therapeutics.
POTENTIAL CLINICAL INDICATIONS BASED ON PRECLINICAL DATA
Joint Inflammation
Studies in TLR4-knockout animals have provided initial evidence for the role of TLR4 in joint inflammation and collagen-induced arthritis. Preclinical data from mice genetically predisposed to rheumatoid arthritis strongly suggest that TLR4 plays a pro-inflammatory role in multiple forms of chronic autoimmune arthritis [2]. TLR4-deficient mice demonstrate a lower incidence and much less severe course of collagen-induced arthritis with nearly no cartilage destruction and lower anti-CCP antibody and IL-17 concentrations [59].
Central and Peripheral Neuropathic Pain
Preclinical data in nerve injury and chemotherapy-induced peripheral neuropathy (CIPN) models have shown attenuation of hyperalgesia and allodynia in TLR4-mutant animals [58,73]. In addition to changes in behavioral outcomes, data from preclinical studies in paclitaxel-related CIPN showed that co-treatment with a TLR4 antagonist can prevent increases in TLR4 and its downstream signaling factors [101]. In cisplatin-related CIPN, disruption of all downstream TLR signaling by mutations in the Myd88 and TRIF adaptor genes in TLR4-mutant mice completely prevented the onset of cisplatin initiated allodynia at any time [58].
Inhibition of spinal TLR4 by delivery of an IT TLR4 antagonist was also found to decrease activation of NF-κB signaling pathways and reduce expression of IL-1β and TNFα with improvement in allodynia and hyperalgesia outcomes in CCI rats [39]. Reversal of mechanical allodynia by (+)-naltrexone has also been reported by Ellis and colleagues in a novel model for central neuropathic pain (CNP) involving T13/L1 dorsal root avulsion in rats after 2 weeks of daily systemic administration [22]. Though this is an indirect inhibitor of TLR4, it is worth noting that this is one illustration of effects of TLR4 on central neurpathic pain.
Migraine
Work in murine models of migraine (photophobia induced by mast cell degranulation), has revealed that the TLR4-mutant mouse signaling through MyD88 displayed significantly reduced photophobia (mimicking the effects of Sumitriptan- a classic anti-migraine therapeutic) [This paper is in review]. These data are apparently unique at present. In addition, cortical spreading depression (CSD) of typical migraine aura, the preceding symptoms that an individual may associate with impending or evolving migraine, are associated with microglial cell activation and hypertrophy. This downstream activation of microglia has been shown, in part, to be mediated through TLR4 modulation [77].
A recent study by Wieseler et al suggested a supradural inflammatory soup rodent model of migraines whereby facial allodynia was mediated by histamine, bradykinin, serotonin, prostaglandin E2 treatment over the dura. In the rat model following induction of facial allodynia using this inflammatory soup, the study showed that treatment with toll-like receptor 4 (TLR4) antagonist (+)-naltrexone blocked development of facial allodynia, giving an additional mechanism of action of pain mediation through TLR4 within the migrane model [92].
Lower Back Pain and Disc Extrusion
The anatomical intervertebral disc (IVD) is a minimally innervated structure [28]. Under normal circumstances, this limited innervation prevents debilitating pain states. However, during pathologic disc degeneration, the extracellular matrix is degraded, resulting in an increase in pro-inflammatory cytokines and neurotrophins which stimulates neurogenesis, resulting in a hyper-innervated disc and upregulation of TLR4 stimulating molecules [28,37]. Data from human IVDs show that upregulation of TLR4 expression is dependent on the degree of IVD degeneration. TLR4 inhibition may provide a target for modulating this proinflammatory cascade to prevent the onset of discogenic pain [37].
Opioid Induced Hyperalgesia (OIH)
Exposure to morphine (or other mu opioids) can lead to a paradoxically enhanced pain state [90]. Relevant data, although some disputed, suggest that the phenomenon is mediated by a TLR4 receptor, which is supported by evidence that the phenomenon is diminished in the absence of TLR4 [5]. Opioids can activate TLR4 on glial cells by mimicking LPS and binding to MD2 as an LPS substitute to activate TLR4 directly [43]. By acting as direct TLR4 ligands, opioid agonsists such as morphine are thought to enhance hyperalgesia in post-nerve injury pain states [21,32].
Mood and Psychological Disorders
Predisposing psychological faactors like mental health disorders such as post-traumatic stress disorder (PTSD), major depressive disorder (MDD), and anxiety have been strongly associated with chronic pain conditions [6]. Interestingly, preclinical and clinical data suggest that TLR4-related mechanisms can mediate stress-induced adaptations involved in the development of MDD [45]. Further studies have shown that the absence of TLR4 inhibition, and even TLR4 antagonism with TAK-242, reduces the development of neuroinflammation associated with brain trauma and may play a neuroprotective role in TBI in mice [101].
LIMITATIONS
TLR4 and sex differences
Clinically, it is well recognized that males and females differentially develop chronic pain conditions [9,25,72,84]. Accordingly, preclinical research is increasingly examining mechanisms that underly the development of persistent pain in female as well as male animals. Initial comparison of TLR4 signaling in male and female mice showed that, at the spinal level, the administration of a TLR4 agonist induced allodynia in male, but not female mice despite equivalent spinal TLR4 expression and similar behavior between male and female mice at the time of administration [71]. Complete Freund’s Adjuvant- and spinal nerve injury-induced allodynia also appear to be TLR4-independent in female mice [71]. A separate study found that TLR4 deficient female mice develop a more severe and persistent tactile allodynia following L5 nerve ligation while TLR4 deficient males show reduced allodynia relative to WT counterparts [73].
Unfortunately, the sex differences related to the induction and maintenance of pain states is complex and likely model, site and gender dependent. For instance, other studies have found that male and female TLR4 deficient animals are both protected from developing delayed-onset tactile allodynia when exposed to intraplantar formalin in the presence of a TLR4 antagonist (TAK-242) and equally protected from cisplatin-induced tactile allodynia [95]. Further research focused on in vitro effects of TLR4 antagonism has provided conflicting data on the variance in sex-related TLR4 expression in resident leukocyte populations, pro-inflammatory cytokine release, and even cell type thought to mediate pain pathways [48,50].
In addition to differences in cell types and innate immune signaling, recent evidence has identified sex differences in TLR4 signaling related to estrogen and testosterone. Specifically, the involvement of TLR4 in pain-like behaviors in male mice is dependent on testosterone [71], and estrogens have been shown to enhance the production of pro-inflammatory mediators and cytokines upon TLR4 activation [13]. Further, microglia co-incubated with estradiol show sexually dimorphic effects: estradiol in male microglia produces anti-inflammatory effects, but pro-inflammatory effects in female microglia [48]. This work indicates the need for carefully controlled studies examining the role of TLR4 and sex differences in persistent pain states.
Limited Clinical Data Using Anti-TLR4 Therapies
Despite a growing list of potential clinical indications for anti-TLR4 therapies given the extensive role TLR4 plays in immune and inflammatory-mediated pathologic conditions, limited clinical data for TLR4 antagonism is currently available. E 5564 and TAK-242 have been studied in large randomized trials for the treatment of sepsis with outcomes that demonstrate acceptable safety and tolerability profiles [56,63,80]. E 5564 also demonstrated limited efficacy, but favorable tolerability in a Phase II trial in patients undergoing cardiopulmonary bypass [8]. Data have also been collected, but not published in Phase II clinical trial for NI-101 to evaluate for pharmacokinetic, pharmacodynamics, tolerability, and efficacy data in patients with rheumatoid arthritis [no data available]. Further studies are needed to investigate the clinical efficacy of targeting the TLR4 receptor for the management of pain states.
Redundant and/or Alternative Pathways
TLR4 may be a more effective target than previously studied immunomodulators because of its upstream position in many immune-modulating pathways. For instance, despite promising preclinical data, modulators of TNF showed limited effect in clinical studies for the treatment of neuropathic pain, including sciatica and discogenic lumbar radiculopathy [4,17,70,93]. Although not fully clear, one reason for the limited translation in humans is that TNF is just one of many downstream acute response cytokines activated by nerve injury so even when directly targeted, separate immune pathways persist. Targeting TLR4 would enable indirect modulation of multiple downstream immune pathways with more widespread effects on the inflammatory response to neuropathic pain.
Other targets along the TLR pathway also show promise. Ibudilast (AV-411) is a non-selective phosphodiesterase inhibitor known to suppress glial cell activation that attenuates pain symptoms in diverse neuropathic pain models in rats and reduces opioid withdrawal symptoms in opioid-dependent volunteers [19,41]. Other potential targets with modulatory effects on TLR activity include prostaglandin D (PGD), vasoactive intestinal polypeptide (VIP), the peroxisome proliferator-activated receptor γ (PPARγ) and adenosine 2A pathways, and bone morphogenetic protein 7 (BMP-7), which are reviewed in detail by Gomez and colleagues as targets for disease-modifying osteoarthritis drugs [31].
CONCLUSION
The role of inflammation can be protective, however the process, when inadequately regulated can also lead to pathologic states such as chronic neuropathic pain. Although the underlying mechanism of neuropathic pain is multifactorial, neuroinflammation is a central factor in the initiation and maintenance of the persistent pain state. Given the central role of TLR4 in the inflammatory pathway, it is a potential target for therapeutic agents in an array of inflammatory conditions. Preclinical findings suggest a positive link in neuropathic pain and clinical studies have demonstrated a positive safety profile in both healthy volunteers and pathologic conditions.
Prior clinical trials provide useful data on medication dosing as well as time course and routes of administration. IV infusion of the TLR4 antagonist Eritoran given in doses up to 105 mg total over 6 days has been reasonably well-studied. Except for self-limiting phlebitis, the safety profile is favorable, however the efficacy in previously studied pathologic conditions including severe sepsis and inflammation after cardiopulmonary bypass has not proven significant [8,55,64,80]. Currently, there is no clinical data for TLR4 antagonists in neuropathic pain, however preclinical data suggest that a more targeted intrathecal approach to delivery may be appropriate. Further data will be necessary for this indication and route of administration.
Although TLR4 has been identified as the major target for modulating the immune response in persistent pain states, it is just one of 14 identified receptors within the TLR family. Given the complexity of the pathway, it is likely that other TLR targets exist. Other possible TLR targets involved in directly or indirectly modulating signaling through MyD88-dependent and/or MyD88-independent pathways include TLR2, TLR3, and TLR5 [73]. In addition, although TLR4 has been implicated as a critical player in the development of neuropathic pain in male rodents, this may not be universally true in females. Although progress has been made in understanding sex-dependent variations in the expression and function of TLR4, additional research is needed to fully understand the significance [95].
The pro-inflammatory response is robust and it is possible that other redundant inflammatory pathways will predominate if TLR4 is antagonized. For instance, peripheral TLR4 stimulation acts as a transient counter-regulatory mechanism for inflammatory pain in vivo, and increases the release of opioid peptides from monocytes in vitro. Therefore, it is possible that TLR4 antagonists might unexpectedly, transiently enhance pain by impairing peripheral opioid analgesia. Future studies are needed to elucidate the specific effect of targeting TLR4 in humans with neuropathic pain.
Acknowledgments
Financial Support Disclosure: The authors declare no financial support with regards to this body of work.
Footnotes
Authorship Statement: The authors declare no conflict of interest with regards to this body of work.
References
- 1.Chaperonin 10 as a putative modulator of multiple Toll-like receptors for the treatment of inflammatory diseases. Expert Opinion on Therapeutic Patents. 2007;17:1299–1308. [Google Scholar]
- 2.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG, van den Berg WB. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2007;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 4.Andrade P, Hoogland G, Del Rosario JS, Steinbusch HW, Visser-Vandewalle V, Daemen MA. Tumor necrosis factor-alpha inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J Neurosci Res. 2014;92:1490–1498. doi: 10.1002/jnr.23432. [DOI] [PubMed] [Google Scholar]
- 5.Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu JT. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull. 2014;30:936–948. doi: 10.1007/s12264-014-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and Pain Comorbidity. Archives of Internal Medicine. 2003;163:2433. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 7.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Current Opinion in Immunology. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 8.Bennett-Guerrero E, Grocott HP, Levy JH, Stierer KA, Hogue CW, Cheung AT, Newman MF, Carter AA, Rossignol DP, Collard CD. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2007;104:378–383. doi: 10.1213/01.ane.0000253501.07183.2a. [DOI] [PubMed] [Google Scholar]
- 9.Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 10.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56:1312–1319. doi: 10.1002/glia.20699. [DOI] [PubMed] [Google Scholar]
- 11.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski W, Hahmann H, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genetics. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 12.Booth J, Wilson H, Jimbo S, Mutwiri G. Modulation of B cell responses by Toll-like receptors. Cell Tissue Res. 2011;343:131–140. doi: 10.1007/s00441-010-1031-3. [DOI] [PubMed] [Google Scholar]
- 13.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, Gourdy P. 17-Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Fu W, Zheng L, Wang Y, Liang G. Recent progress in the discovery of myeloid differentiation 2 (MD2) modulators for inflammatory diseases. Drug Discov Today. 2018 doi: 10.1016/j.drudis.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115:428–442. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SP, Galvagno SM, Plunkett A, Harris D, Kurihara C, Turabi A, Rehrig S, Buckenmaier CC, 3rd, Chelly JE. A multicenter randomized controlled study evaluating preventive etanercept on postoperative pain after inguinal hernia repair. Anesth Analg. 2013;116:455–462. doi: 10.1213/ANE.0b013e318273f71c. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 19.Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, Manubay JM, Martinez DM, Jones JD, Saccone PA, Comer SD. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol. 2016;21:895–903. doi: 10.1111/adb.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eidson LN, Murphy AZ. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33:15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, Ayala M, Hutchinson MR, Falci S, Rice KC, Maier SF, Watkins LR. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain, Behavior, and Immunity. 2016;58:348–356. doi: 10.1016/j.bbi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis A, Wieseler J, Favret J, Johnson KW, Rice KC, Maier SF, Falci S, Watkins LR. Systemic administration of propentofylline, ibudilast, and (+)-naltrexone each reverses mechanical allodynia in a novel rat model of central neuropathic pain. J Pain. 2014;15:407–421. doi: 10.1016/j.jpain.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feizerfan A, Sheh G. Transition from acute to chronic pain. Continuing Education in Anaesthesia Critical Care & Pain. 2015;15:98–102. [Google Scholar]
- 24.Fields HL. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex gender pain: a review of recent clinical experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao W, Xiong Y, Li Q, Yang H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garay-Malpartida HMR, Mantovani M, Santos I, Sogayar M, Goldberg A. Toll-like receptor 4 (TLR4) expression in human and murine pancreatic beta-cells affects cell viability and insulin homeostasis. BMC Immunology. 2011;12 doi: 10.1186/1471-2172-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Cosamalon J, del Valle ME, Calavia MG, Garcia-Suarez O, Lopez-Muniz A, Otero J, Vega JA. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Global Pain GIA. Market Management to Reach US$60 Billion by 2015, According to a New Report by Global Industry Analysts, Inc. PR Web. 2011 [Google Scholar]
- 31.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis–finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 32.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:E3441–E3450. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 37.Klawitter M, Hakozaki M, Kobayashi H, Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T, Meier U, Sekiguchi M, Konno S, Boos N, Ferguson SJ, Wuertz K. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J. 2014;23:1878–1891. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- 38.Klucken J, Buchler C, Orso E, Kaminski W, Porsch-Ozcurumez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, Allikmets R, Schmitz G. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. PNAS. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang X, Huang Y, Gu HF, Zu XY, Zou WY, Song ZB, Guo QL. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur J Pharmacol. 2012;676:51–56. doi: 10.1016/j.ejphar.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 2017;5 doi: 10.3390/vaccines5040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 42.Levitan I, Shentu TP. Impact of oxLDL on Cholesterol-Rich Membrane Rafts. J Lipids. 2011;2011:730209. doi: 10.1155/2011/730209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Csakai A, Jin J, Zhang F, Yin H. Therapeutic Developments Targeting Toll-like Receptor-4-Mediated Neuroinflammation. ChemMedChem. 2016;11:154–165. doi: 10.1002/cmdc.201500188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin M, Yiu WH, Li RX, Wu HJ, Wong DW, Chan LY, Leung JC, Lai KN, Tang SC. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83:887–900. doi: 10.1038/ki.2013.11. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Buisman-Pijlman F, Hutchinson MR. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front Neurosci. 2014;8:309. doi: 10.3389/fnins.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez AM, Terpack SJ, Posey KS, Liu B, Ramirez CM, Turley SD. Systemic administration of 2-hydroxypropyl-beta-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function. Clin Exp Pharmacol Physiol. 2014;41:780–787. doi: 10.1111/1440-1681.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12–27. doi: 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 52.Meng G, Liu Y, Lou C, Yang H. Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-kappaB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol. 2010;161:1628–1644. doi: 10.1111/j.1476-5381.2010.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohammad Hosseini A, Majidi J, Baradaran B, Yousefi M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv Pharm Bull. 2015;5:605–614. doi: 10.15171/apb.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, Vincent JL. Effect of Eritoran, an Antagonist of MD2-TLR4, on Mortality in Patients With Severe Sepsis: The ACCESS Randomized Trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 56.Opal SMLP, Francois B, LaRosa S, Angus D, Mira J, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang C, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil A, Nuffelen M, Lynn M, Rossignol D, Gogate J, Roberts M, Wheeler J, Vincent J. Effect of Eritoran an Antagonist of MD2-TLR4 on Mortality in Patients With Severe Sepsis: The ACCESS Randomized Trial. JAMA. 2013;309:54–62. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 57.Ottinger E, Kao ML, Carillo-Carasco N, Yanjanin NM, Shankar RK, Janssen M, Brewster M, Scott I, Xu X, Cradock J, Terse P, Dehdashti S, Marugan J, Zheng W, Portilla L, Hubbs A, Pavan WJ, Heiss J, Vite CH, Walkley SU, Ory DS, Silber SA, Porter FD, Austin CP, McKew JC. Collaborative Development of 2-Hydroxypropyl-β-Cyclodextrin for the Treatment of Niemann-Pick Type C1 Disease. Curr Top Med Chem. 2014;14:330–339. doi: 10.2174/1568026613666131127160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park HJ, Stokes JA, Corr M, Yaksh TL. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother Pharmacol. 2014;73:25–34. doi: 10.1007/s00280-013-2304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierer M, Wagner U, Rossol M, Ibrahim S. Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen-induced arthritis in DBA1J mice. PLoS One. 2011;6:e23539. doi: 10.1371/journal.pone.0023539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajaiah R, Perkins DJ, Ireland DD, Vogel SN. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc Natl Acad Sci U S A. 2015;112:8391–8396. doi: 10.1073/pnas.1424980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J. 2014;90:222–227. doi: 10.1136/postgradmedj-2013-132215. [DOI] [PubMed] [Google Scholar]
- 62.Reuben DB, Alvanzo AA, Ashikaga T, Bogat GA, Callahan CM, Ruffing V, Steffens DC. National Institutes of Health Pathways to Prevention Workshop: the role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162:295–300. doi: 10.7326/M14-2775. [DOI] [PubMed] [Google Scholar]
- 63.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, Ii M, Matsuda H, Mouri K, Cohen J. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 64.Rossignol DP, Wong N, Noveck R, Lynn M. Continuous pharmacodynamic activity of eritoran tetrasodium, a TLR4 antagonist, during intermittent intravenous infusion into normal volunteers. Innate Immunity. 2008;14:383–394. doi: 10.1177/1753425908099173. [DOI] [PubMed] [Google Scholar]
- 65.Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, Bishnoi S, Baig MS. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol. 2016;40:79–89. doi: 10.1016/j.intimp.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 66.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette J, Deleuze J, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nature Genetics. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 67.Sauer RS, Hackel D, Morschel L, Sahlbach H, Wang Y, Mousa S, Roewer N, Brack A, Rittner H. Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation. Molecular Pain. 2014;10 doi: 10.1186/1744-8069-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitz G, Orso E. CD14 signalling in lipid rafts: new ligands and co-receptors. Curr Opin Lipidol. 2002;2002:513–521. doi: 10.1097/00041433-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Shridas P, Bailey WM, Talbott KR, Oslund RC, Gelb MH, Webb NR. Group X secretory phospholipase A2 enhances TLR4 signaling in macrophages. J Immunol. 2011;187:482–489. doi: 10.4049/jimmunol.1003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. Journal of Peripheral Nervous System. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 71.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorge RE, Totsch SK. Sex Differences in Pain. J Neurosci Res. 2017;95:1271–1281. doi: 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 73.Stokes JA, Cheung AT, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. Journal of Neuroinflammation. 2013;10:148. doi: 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stokes JA, Corr M, Yaksh TL. Spinal toll-like receptor signaling and nociceptive processing: regulatory balance between TIRAP and TRIF cascades mediated by TNF and IFNbeta. Pain. 2013;154:733–742. doi: 10.1016/j.pain.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 77.Takizawa T, Shibata M, Kayama Y, Shimizu T, Toriumi H, Ebine T, Unekawa M, Koh A, Yoshimura A, Suzuki N. High-mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. J Cereb Blood Flow Metab. 2017;37:890–901. doi: 10.1177/0271678X16647398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor A. Addressing the global tragedy of needless pain: Rethinking the United Nations single convention on narcotic drugs. Journal of Law, Medicine and Ethics. 2007;35:556–570. doi: 10.1111/j.1748-720X.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 80.Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM, Eritoran Sepsis Study G Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010;38:72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 81.Tortelli B, Fujiwara H, Bagel JH, Zhang J, Sidhu R, Jiang X, Yanjanin NM, Shankar RK, Carillo-Carasco N, Heiss J, Ottinger E, Porter FD, Schaffer JE, Vite CH, Ory DS. Cholesterol homeostatic responses provide biomarkers for monitoring treatment for the neurodegenerative disease Niemann-Pick C1 (NPC1) Hum Mol Genet. 2014;23:6022–6033. doi: 10.1093/hmg/ddu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation? Mediators Inflamm. 2011;2011:186093. doi: 10.1155/2011/186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A, Xu R, Sotolongo J, Espana C, Zaias J, Elson G, Mayer L, Kosco-Vilbois M, Abreu MT. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1167–1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Unruh A. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 85.Vance JE, Karten B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res. 2014;55:1609–1621. doi: 10.1194/jlr.R047837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wadachi R, Hargreaves KM. Trigeminal Nociceptors Express TLR-4 and CD14: a Mechanism for Pain due to Infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber L, Yeomans DC, Tzabazis A. Opioid-induced hyperalgesia in clinical anesthesia practice: what has remained from theoretical concepts and experimental studies? Curr Opin Anaesthesiol. 2017;30:458–465. doi: 10.1097/ACO.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 91.White CR, Smythies LE, Crossman DK, Palgunachari GM, Anantharamaiah GM, Datta G. Regulation of Pattern Recognition Receptors by the Apolipoprotein A-I Mimetic Peptide 4F. Arterioscler Thromb Vasc Biol. 2012;32:2631–2639. doi: 10.1161/ATVBAHA.112.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wieseler J, Ellis A, McFadden A, Stone K, Brown K, Cady S, Bastos LF, Sprunger D, Rezvani N, Johnson K, Rice KC, Maier SF, Watkins LR. Supradural inflammatory soup in awake and freely moving rats induces facial allodynia that is blocked by putative immune modulators. Brain Res. 2017;1664:87–94. doi: 10.1016/j.brainres.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams NH, Lewis R, Din NU, Matar HE, Fitzsimmons D, Phillips CJ, Sutton A, Burton K, Hendry M, Nafees S, Wilkinson C. A systematic review and meta-analysis of biological treatments targeting tumour necrosis factor alpha for sciatica. Eur Spine J. 2013;22:1921–1935. doi: 10.1007/s00586-013-2739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woller SA, Eddinger KA, Corr M, Yaksh TL. An overview of pathways encoding nociception. Clin Exp Rheumatol. 2017;35:S40–S46. [PMC free article] [PubMed] [Google Scholar]
- 95.Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: The role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun. 2016;56:271–280. doi: 10.1016/j.bbi.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu F, Bian J, Huang S, Xu X, Gong D, Sun Y, Lu Z, Yu W. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci. 2010;7:251–259. doi: 10.7150/ijms.7.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu H-e, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharmacol. 2007;571:145–151. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu HE, Sun HS, Terashivili M, Schwasinger E, Sora I, Hall FS, Uhl GR, Tseng LF. Dextro- and levo-morphine attenuate opioid delta and kappa receptor agonist produced analgesia in mu-opioid receptor knockout mice. Eur J Pharmacol. 2006;531:103–107. doi: 10.1016/j.ejphar.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, Deng C, Fan C, Di S, Sun Y, Yi W. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016;7:e2234. doi: 10.1038/cddis.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang D, Li H, Li T, Zhou M, Hao S, Yan H, Yu Z, Li W, Li K, Hang C. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: implication in the treatment of human brain injury. Neurochem Int. 2014;75:11–18. doi: 10.1016/j.neuint.2014.05.003. [DOI] [PubMed] [Google Scholar]


