Abstract
Objective
With the COVID-19 pandemic, there have been supply challenges necessitating that laboratories must prepare their own viral transport medium (VTM), which provides stability for clinical specimens for diagnostic viral testing.
Methods
Within a veteran affairs medical center clinical laboratory, VTM was prepared with a Hanks Balanced Salt Solution (HBSS) 500 mL bottle with phenol red, sterile heat-inactivated fetal bovine serum (FBS), gentamicin sulfate (50 mg/mL), and amphotericin B (250 μg/mL). An antimicrobial mixture was made of 50 mL each of amphotericin B and gentamicin sulfate. Ten mL of FBS and 2 mL of the antimicrobial mixture were mixed into the HBSS bottle, from which 3 mL aliquots were made. Sterility and efficacy check were assessed. These preparations were conducted at our VAMC’s clinical laboratory to assure adequate VTM supply during the COVID-19 shortage.
Results
The VTM was successfully prepared in-house, supporting uninterrupted testing for the facility and other affiliated medical facilities/centers and community living centers.
Conclusion
This quality assurance/improvement report represents the first published manuscript on feasible VTM preparation exclusively within a clinical microbiology laboratory during the COVID-19 pandemic.
Keywords: viral transport media preparation, SARS-CoV-2, emergency preparedness, molecular pathology, COVID-19, quality control, quality assurance, supply shortage, clinical pathology, laboratory workflow
From its unassuming beginnings in Wuhan, China, SARS-CoV-2, the viral agent that causes the COVID-19 disease, has become an international pandemic.1-6 With the unprecedented outbreak of the respiratory illness caused by SARS-CoV-2, there have been challenges to the maintenance of adequate supplies in terms of both personal protective equipment and testing materials.7,8 In particular, the dire nationwide shortage of commercial viral transport medium (VTM, a formulation of a buffered salt solution, complex protein and amino acids, and antimicrobial agents), a critical medium for the transport of specimens for reverse transcriptase polymerase chain reaction (RT-PCR) testing, has created a situation in which testing laboratories need to manufacture their own in-house VTM because commonly used commercial VTM is simply unavailable.7-11 Despite the shortage, VTM remains a critical and pivotal reagent for preserving stability to achieve a reliable test result.12 Maintaining the stability of the collected virus is of utmost importance to perform COVID-19 RT-PCR with optimal results because the virus must still be sufficiently present and preserved in the specimen at the time of testing.
Given the lack of supply from commercial vendors, in-house manufacture has become an uncharted route forced upon medical centers and laboratories with the intention of proceeding with testing using VTM despite the dire shortages.7-10 Consequently, a variety of transport media including saline, phosphate buffered saline, minimum essential medium, and other media with variable formulations have been shared and documented.11,13-15 In nonshortage times, VTM is generally considered the standard of care and offers advantages over other simpler formulations because of its documented ability to prevent overgrowth of bacteria/fungi and ensure prolonged stability of viral genetic material.11-15 It has an established role in ensuring the stability of viral genetic material with simpler temperature and handling needs falling within the usual standard of care compared with the other media types such as saline.11-15 Indeed, in a study by Rogers et al,15 a trend toward an increasing cycle threshold value likely resulting from the degradation of viral genetic material in a small sampling was noted with saline stored at room temperature for prolonged periods of time in contrast to storage in VTM. Therefore, although there may be some degree of equivalence in various transport media in supporting the diagnosis of viral genetic material by RT-PCR, the use of VTM is preferred and is closer to general standard practice outside of shortage times.15
Despite the extraordinary nature of the current crisis, peer-reviewed literature to guide laboratories on the emergency manufacture, initial laboratory validation, and quality control of VTM for the COVID-19 pandemic is sparse. More important, peer-reviewed literature on VTM preparation and quality control has not been published by a clinical laboratory without research department infrastructure, specifically one in a regional veteran’s hospital.1,9,12 To fill this gap in the peer-reviewed literature for this critical component, this study shares the viewpoint and experience of pathologists at a regional veteran affairs medical center (VAMC) in both manufacturing and performing quality control of its VTM. These preparations were performed at our VAMC’s clinical microbiology laboratory to assure adequate VTM supply during the COVID-19 shortage.
Method of Preparation
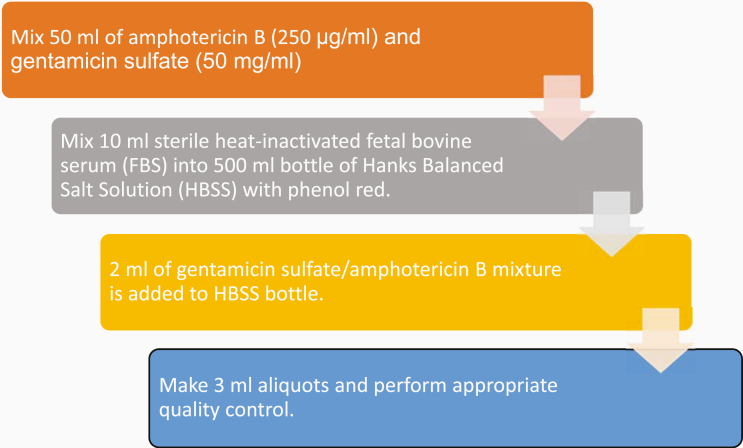
There has been variation leading to a lack of clarity in reference to VTM formulations, including the usage of phenol red and quality control processes. Phenol red, with its corresponding color change from pink to yellow in the presence of increasing acidity, has its use in the detection of potential bacterial contamination and was thus chosen to be included in the VAMC’s preparation.11 Within the clinical microbiology laboratory, VTM was prepared using a strict aseptic technique with a 500 mL bottle of Hanks Balanced Salt Solution (HBSS) with phenol red, sterile heat-inactivated fetal bovine serum (FBS), gentamicin sulfate (50 mg/mL), and amphotericin B (250 μg/ml), see Figure 1. First, 50 mL of amphotericin B and 50 mL of gentamicin sulfate were mixed into an antimicrobial mixture. Next, 10 mL of FBS was mixed with the HBSS bottle. Then 2 mL of the antimicrobial mixture was also mixed into the HBSS bottle. The HBSS bottle was then capped and mixed by inverting the bottle. Each bottle was then additionally labeled as follows: “2% FBS, 100 μg/mL Gentamicin, 0.5 μg/mL Amphotericin B, [current date], Expires [1 month from current date].”
Figure 1.
Flowchart of summary of 4 steps of VTM preparation. This figure was shown as part of an abstract presentation of these data.1 VTM, viral transport medium.
Afterward, 3 mL aliquots were made from the bottle to constitute individual tubes of VTM for clinical use. These tubes were further labeled as follows:
Viral Transport Medium
**For Transport of specimens only**
**Not to be taken internally**
Store at 2 to 8ºC. Do not freeze.
Ingredients: Hanks balanced salt solution, fetal bovine serum, gentamicin, amphotericin B
Expires [1 year after current date of manufacture]
All tubes and any remaining medium in the bottle were stored in the refrigerator (2 to 8ºC).
Method of Quality Control for Sterility
One viral transport tube aliquot per bottle was utilized for the sterility check (see Figure 2). After the first initial visual inspection to verify that the color of the liquid in the tube was appropriate (pink from the phenol red), the aliquot tube was incubated in the CO2 incubator at 37ºC for 24 hours.
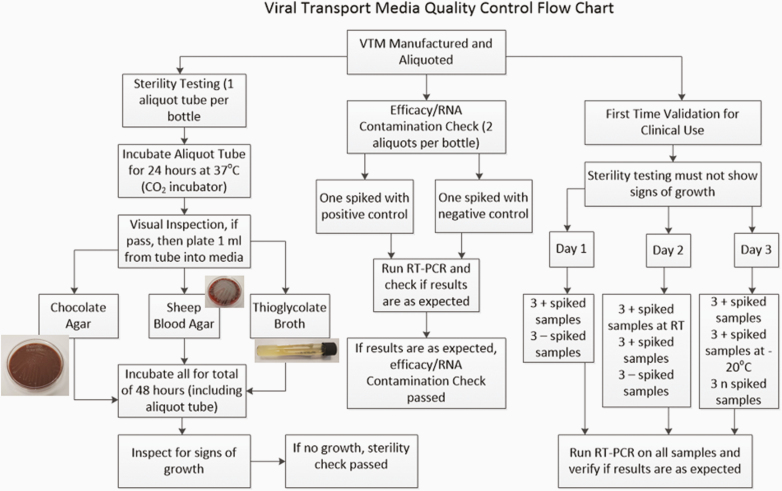
Figure 2.
Flowchart of quality control program for the VTM including sterility testing in the lefthand branch, efficacy check in the middle branch, and the first-time validation in the righthand branch. For the validation, all spiked specimens without a specified temperature were done at refrigerator temperature (4 to 10ºC temperatures). RT, room temperature; RT-PCR, reverse transcriptase polymerase chain reaction; VTM, viral transport medium. This figure was shown in an abstract presentation of these data.1
After incubation for 24 hours, the tubes were visually inspected for signs of growth such as color change (with phenol red specifically, a yellowing of the initial pink color indicative of acidification, frequently because of bacterial growth), turbidity, or presence of floccules when tapped or vortexed. If no signs of growth were observed, then within a biosafety cabinet, 1 mL of the viral transport medium was inoculated into a sheep blood agar plate, a chocolate agar plate, and a thioglycolate broth vial. Both the remaining aliquot tube and the plated medium were returned to the CO2 incubator for incubation at 37ºC for 48 hours. At both the 24- and 48-hour marks, plates and tubes were examined for signs of bacterial growth. Any observed growth at any stage of this sterility testing were fully worked up to identify the contaminating organism(s). All the bottle tests for sterility conducted by the VAMC clinical laboratory passed with no growth observed.
Initial Laboratory Validation
Additional RT-PCR tests were performed on the first bottle received for testing to confirm that the in-house manufacture of VTM was efficacious for clinical use (see Figure 2). These validations were performed just once before in-house VTM was authorized for clinical use. These additional runs were performed over 3 days and included both positive and negative aliquots spiked with corresponding control material from the Seracare (Milford, MA) AccuPlex SARS-CoV-2 Reference Material Kit. The runs were performed on the Abbott (Abbott Park, IL) RealTime SARS-CoV-2 Assay on the Abbott m2000 testing platform and on the Cepheid (Sunnyvale, CA) Xpert Xpress SARS-CoV-2 Assay. Because VTM would be utilized clinically and might be placed temporarily in conditions other than refrigerator temperatures, positive spiked specimens were stored temporarily at room temperature, refrigerator temperatures (2 to 8ºC), and frozen temperatures (–20ºC) to verify that RNA material was still preserved in the VTM for testing at the various temperatures.
The first day of testing involved 3 positively spiked and 3 negatively spiked specimens stored in the refrigerator routinely. The second day involved 3 positively spiked specimens stored at room temperature before testing, 3 positively spiked specimens kept in the refrigerator, and 3 negatively spiked specimens kept in the refrigerator. The third day involved 3 positively spiked specimens kept in the refrigerator, 3 positively spiked specimens frozen at –20ºC before testing, and 3 negatively spiked specimens kept in the refrigerator. In this first-time validation, all test results were as expected and thus achieved validation for using in-house VTM for clinical application.
Method of Quality Control for Efficacy and RNA Contamination
Positive efficacy checks were performed to ensure that the in-house VTM from each bottle did not lead to degradation of viral RNA that would preclude accurate testing. Negative RNA contamination tests were performed to verify that the manufacturing process did not introduce viral RNA that could lead to false positive results. Two aliquots from each bottle of VTM were thus taken (see Figure 2). One aliquot was spiked with positive control material and the other aliquot with negative control material. The laboratory staff then performed RT-PCR testing on each aliquot to confirm that the expected results were obtained (positive in the aliquot spiked with positive control material, negative in the aliquot spiked with negative control material).
Differences in Preparation
Methods to produce VTM have been made publicly available previously.9,11 The primary similarity among these methods is that VTM is ultimately a mixture of a buffered salt solution, complex protein and amino acids, and antimicrobial agents to ensure the stability of the viral specimen for later nucleic acid testing. However, given the different circumstances of each practice setting and laboratory/clinical needs, there are also important differences. For instance, some preparations of VTM exclude phenol red for simplicity, but at the VAMC laboratory it was determined that including this quality marker was important to ensure the lack of bacterial contamination of the specimens produced (see Image 1).9,11 In addition, a CO2 incubator at an increased temperature of 37ºC was utilized to provide a stable conducive environment to efficiently detect bacterial or fungal contamination compared to a lower room or refrigerator temperature incubation.10,15
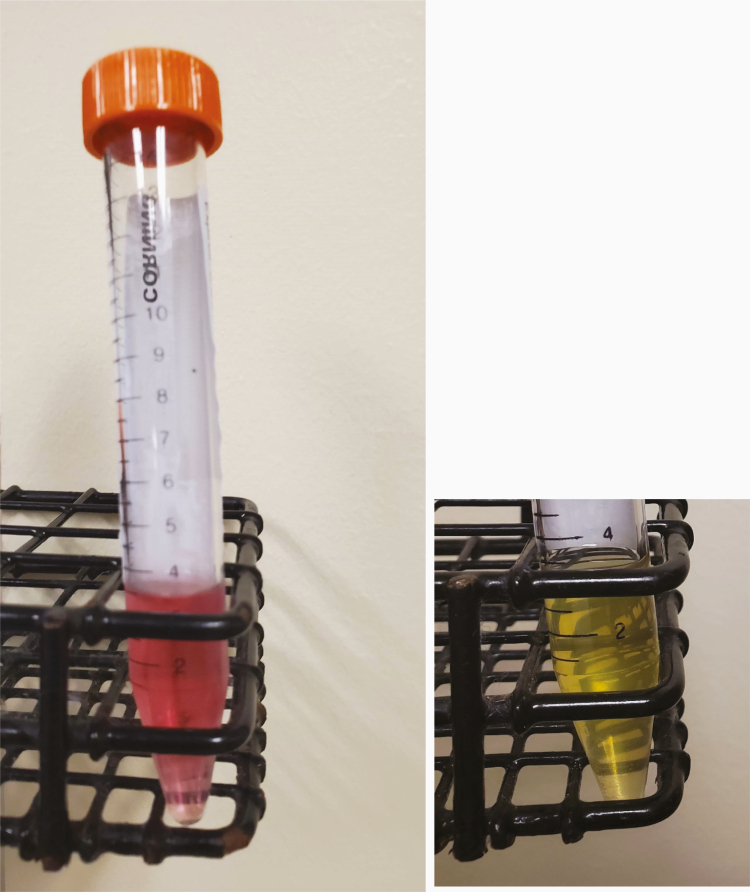
Image 1.
Left: One of the prepared aliquots of transport media. Note the pink color because of the phenol red. In the presence of acidity (perhaps because of bacterial growth), this color would show yellowing, and thus this coloration remains a quality control tool. This image was shown in an abstract presentation of these data.1 Right: One aliquot of prepared VTM was generously inoculated with a large quantity of Escherichia coli and then incubated in the CO2 incubator at 37ºC for 24 hours. Because of bacterial growth, the VTM has a yellow appearance.
Methods to validate and perform quality control have varied from institution to institution and in the literature (both peer-reviewed and non-peer-reviewed).9 One publicly available standard operating procedure by the Centers for Disease Control and Prevention (CDC), for instance, does not include an efficacy check to verify that viral genetic material is preserved by the media created and that the medium is not inadvertently contaminated by interfering genetic material.9 Although the CDC has a publicly available protocol with its viewpoint, there is no peer-reviewed consensus in reference to sterility quality control.9,11 Other institutions have found quality testing for efficacy/genetic material to be important and have included it in their algorithms, with variation in specifics.11 Three culture mediums (sheep blood agar, chocolate agar, and thioglycolate broth) were used at the VAMC for thoroughness in sterility testing and to ensure sensitivity in detecting microorganism contamination. The length of time that the products of each step of the preparation process can be kept before expiration has also lacked clarity among multiple institutions; given the CDC protocol and the standard length of time available for commercial VTM before expiration, the VAMC laboratory established the expiration date of each aliquot as being 1 year after manufacture. The expiration date of any intermediate products (ie, the bottle mixture to be aliquoted) was set at 1 month so that intermediate steps will be completed expediently.
Finally, it is important to note that the preparation of VTM in this report took place within the clinical microbiology laboratory of a regional VAMC. This preparation setting led to the selection of the batch size of 1 bottle, or approximately 500 mL; under a prolonged shortage, other larger laboratories can potentially consider scaling up solutions to include larger batches and multiple reagents, and supporting quality assurance common to good manufacturing practice. Other than Petersen et al,1 no published reports are available in the peer-reviewed literature for VTM preparation in the clinical laboratory of a regional VAMC. We have found only a single study on the preparation of VTM in a university hospital setting using a well-equipped research division and laboratories.11 Published reports of a clinical laboratory preparing VTM in these unprecedented times present a unique perspective and act as a reference for other laboratories faced with the similar need for preparation.
Conclusion
In this first publication (other than the abstract presentation of these data1) on VTM manufacture, validation, and quality control within a regional VAMC’s clinical microbiology laboratory during this unprecedented crisis of COVID-19, VTM production and related quality testing/validation have proven to be feasible and highly useful.1 It is possible for VTM adequate to meet the needs of the regional VAMC to be prepared with appropriate sterility and efficacy to meet the unprecedented demand for SARS-CoV-2 testing with its concomitant severe supply shortages. This report presents the first published experience in the preparation and quality control of VTM within the clinical laboratory of a medical center specifically for SARS-CoV-2 testing during the COVID-19 pandemic; this information is a potentially useful reference point for other similar regional medical centers faced with this ongoing pandemic and worldwide health emergency. The in-house preparation of VTM in the face of severe supply shortages remains critical, and maintaining the stability of the virus to be detected is of utmost importance to ensure optimal laboratory results, appropriate laboratory diagnosis to confirm COVID-19 infection, and successful performance of testing in-house. LM
Acknowledgments
Data from this study were presented as a virtual poster at the American Society for Clinical Pathology National Conference; September 9 to September 12, 2020 (see reference 1).
Glossary
Abbreviations
- VTM
viral transport medium
- HBSS
Hanks Balanced Salt Solution
- FBS
fetal bovine serum
- RT-PCR
reverse transcriptase polymerase chain reaction
- VAMC
veteran affairs medical center
- CDC
Centers for Disease Control and Prevention
References
- 1. Petersen J, Dalal S, Jhala D. In-House Viral Transport Medium (VTM) manufacture in the time of shortage, supply and crisis of COVID-19 at Veteran Affairs Medical Center (VAMC). Am J Clin Pathol. 2020;154(Supplement_1):S161–S162. [Google Scholar]
- 2. Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020 World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed April 16, 2020.
- 3.Outbreak of 2019 Novel Coronavirus (2019-nCoV) in Wuhan, China.Updated January 20, 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/csels/dls/locs/2020/outbreak-of-2019-novel-coronavirus-2019-ncov-in-wuhan-china.html. Accessed April 16, 2020.
- 4.Country and technical guidance—coronavirus disease (COVID-19) World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance. Accessed April 16, 2020.]
- 5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, Mcgoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 7. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18):e41. [DOI] [PubMed] [Google Scholar]
- 8. Thomas K Coronavirus test obstacles: a shortage of face masks and swabs [Internet] New York Times; https://www.nytimes.com/2020/03/18/health/coronavirus-test-shortages-face-masks-swabs.html. Published March 18, 2020; updated July 23, 2020. Accessed April 13, 2020. [Google Scholar]
- 9. Preparation of viral transport medium Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medium.pdf. Accessed April 10, 2020.
- 10. Callahan CJ, Lee R, Zulauf KE, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 Testing Bottleneck. Preprint. Posted online May 7, 2020. medRxiv 20065094. doi: 10.1101/2020.04.14.20065094 [DOI] [PMC free article] [PubMed]
- 11. Smith KP, Cheng A, Chopelas A, et al. Large-scale, in-house production of viral transport media to support SARS-CoV-2 PCR testing in a multihospital health care network during the COVID-19 pandemic. J Clin Microbiol. 2020;58(8):e00913-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson FB Transport of viral specimens. Clin Microbiol Rev. 1990;3(2):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodino KG, Espy MJ, Buckwalter SP, et al. Evaluation of saline, phosphate buffered saline, and minimum essential medium as potential alternatives to viral transport media for SARS-CoV-2 testing. J Clin Microbiol. 2020;58(6):e00590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Druce J, Garcia K, Tran T, Papadakis G, Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J Clin Microbiol. 2012;50(3):1064–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogers AA, Baumann RE, Borillo GA, et al. Evaluation of transport media and specimen transport conditions for the detection of SARS-CoV-2 by use of real-time reverse transcription-PCR. J Clin Microbiol 2020;58(8):e00708-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cote RJ Media composition, microbial, laboratory scale. In: Flickinger, MC, ed. Encyclopedia of Industrial Biotechnology. John Wiley and Sons; 2010. [Google Scholar]