Abstract
Background
Positive results from real-time reverse-transcription polymerase chain reaction (rRT-PCR) in recovered patients raise concern that patients who recover from coronavirus disease 2019 (COVID-19) may be at risk of reinfection. Currently, however, evidence that supports reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has not been reported.
Methods
We conducted whole-genome sequencing of the viral RNA from clinical specimens at the initial infection and at the positive retest from 6 patients who recovered from COVID-19 and retested positive for SARS-CoV-2 via rRT-PCR after recovery. A total of 13 viral RNAs from the patients’ respiratory specimens were consecutively obtained, which enabled us to characterize the difference in viral genomes between initial infection and positive retest.
Results
At the time of the positive retest, we were able to acquire a complete genome sequence from patient 1, a 21-year-old previously healthy woman. In this patient, through the phylogenetic analysis, we confirmed that the viral RNA of positive retest was clustered into a subgroup distinct from that of the initial infection, suggesting that there was a reinfection of SARS-CoV-2 with a subtype that was different from that of the primary strain. The spike protein D614G substitution that defines the clade “G” emerged in reinfection, while mutations that characterize the clade “V” (ie, nsp6 L37F and ORF3a G251V) were present at initial infection.
Conclusions
Reinfection with a genetically distinct SARS-CoV-2 strain may occur in an immunocompetent patient shortly after recovery from mild COVID-19. SARS-CoV-2 infection may not confer immunity against a different SARS-CoV-2 strain.
Keywords: COVID-19, SARS-CoV-2, reinfection, whole-genome sequencing
We conducted serial whole-genome sequencing of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Our results highlight possible SARS-CoV-2 reinfection with a genetically distinct SARS-CoV-2 strain in patients shortly after recovery from mild coronavirus disease 2019 (COVID-19).
The coronavirus disease 2019 (COVID-19) pandemic, with 15 million cases confirmed as of 24 July 2020, is presenting another challenge with regard to the follow-up of recovered patients and the question of reinfection [1]. A report in April showed that in South Korea, 116 patients retested positive after recovery from COVID-19 [2]. Reinfection has been identified in various infectious respiratory diseases, such as human coronaviruses NL63 and respiratory syncytial virus [3, 4]. With regard to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several reports of positive results from real-time reverse-transcription polymerase chain reaction (rRT-PCR) in recovered patients raise a concern that patients who recover from COVID-19 may be at risk of reinfection, which is defined as the subsequent infection of the host with the same microorganism [5–7]. Recently, 2 cases of reinfection with SARS-CoV-2 were reported, and these reports described reinfection that occurred 3–4 months after initial infection [8, 9]. However, more evidence is required to clarify the unique characteristics of SARS-CoV-2 reinfection.
Here, we describe another case of SARS-CoV-2 reinfection 26 days after initial infection in a patient who clinically recovered from COVID-19 with 2 consecutive negative rRT-PCR results 24 hours apart. We performed whole-genome sequencing of the SARS-CoV-2 RNAs consecutively obtained from the respiratory specimens at the initial infection and at the positive retest for SARS-CoV-2 in order to characterize the difference between the viral genomes at the 2 different time points and to investigate the mechanism that underlies a positive retest result for SARS-CoV-2 in recovered patients.
METHODS
Patients and Samples
Six patients who recovered from COVID-19 and retested positive for SARS-CoV-2 via rRT-PCR after discharge with 2 consecutive negative rRT-PCR results were included in this study. These patients were from 3 tertiary hospitals in South Korea (National Medical Center, Seoul Medical Center, and Keimyung University Dongsan Medical Center). The Institutional Review Board of Seoul National University Hospital approved this study. Specimen collection and diagnostic testing were performed in accordance with guidelines from the World Health Organization. Briefly, upper respiratory specimens (nasopharyngeal swabs and oropharyngeal swabs) were collected using synthetic fiber swabs at 2–3 different time points: at the initial diagnosis of COVID-19, during follow-up if specimens were available, and at the SARS-CoV-2 positive retest after recovery (Supplementary Table 1). For patients who presented with a productive cough, sputum was collected. A total of 14 clinical samples collected from the 6 patients were tested for SARS-CoV-2 via rRT-PCR, targeting RNA-dependent RNA polymerase (RdRp) and envelope (E) genes (Supplementary Table 1). Cycle threshold (Ct) values <40 for both the RdRp and E were defined as positive. All 14 samples were positive for SARS-CoV-2.
Whole-genome Sequencing
RNA extracted from the rRT-PCR–positive clinical samples was used for whole-genome sequencing. Briefly, cDNA was obtained through RT-PCR using the SuperScript III Reverse Transcriptase kit (Thermo Fisher Scientific, Waltham, MA). Targeting an average fragment size of 800 base pairs (bp), multiple overlapping PCR assays spanning the entire SARS-CoV-2 genome were performed (Supplementary Table 2). Individual amplicons were pooled, 500 ng of which were used for library preparation using the Nextera DNA Flex Library Prep kit (Illumina, San Diego, CA), according to the manufacturer’s instructions. The libraries were sequenced on the NextSeq 550 (Illumina). Sequencing data were further processed to generate consensus whole-genome sequences and to call the variants. After adapter sequence trimming, the remaining reads were aligned to the reference SARS-CoV-2 genome (GenBank NC_045512.2) using the Burrows-Wheeler Aligner (BWA v.0.7.17) [10]. We called the variants using Samtools v.1.10 (Genome Research Limited, Cambridgeshire, United Kingdom). All the variants were manually verified using the Integrative Genomics Viewer (Broad Institute, Cambridge, MA). A de novo genome sequence assembly was performed using CLC Genomics Workbench v.11.0 (Qiagen, Hilden, Germany) and SPAdes 3.10.1 [11]. Data obtained in this study were submitted to the Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra) under accession PRJNA663292 [12].
Phylogenetic Analysis
We attempted to characterize the genomic sequences of SARS-CoV-2 that we obtained from 2 time points (ie, initial infection and positive retest). The complete genome sequences we were able to obtain from patient 1 at these 2 time points were aligned with 23 SARS-CoV-2 genomes available from the Global Initiative on Sharing All Influenza Data database [13]. The accession IDs are included in Supplementary Table 3. Phylogenetic trees were inferred with the maximum likelihood method using the Hasegawa-Kishino-Yano nucleotide substitution model and 1000 bootstrap replicates using MEGA X software (Pennsylvania State University, PA).
Antibody Measurement
Serum samples were longitudinally collected from patient 1 at initial diagnosis, on day 11 of initial symptom onset, at second admission, 10 days after the onset of the reinfection episode, on the day of 2 consecutive rRT-PCR returning negative results, and on days 3 and 5 of the third hospitalization. SARS-CoV-2–specific immunoglobulin G (IgG) antibodies (anti-SARS-CoV-2 S1 IgG and anti-SARS-CoV-2 receptor binding domain [RBD] IgG) were tested using enzyme-linked immunosorbent assay Research Use Only kits (AdvanSure SARS-CoV-2 IgG [S1] and AdvanSure SARS-CoV-2 IgG [RBD], LG Life Science, Korea), according to the manufacturer’s instructions. We also performed a plaque-reduction neutralization test (PRNT). Neutralization titer (PRNT50) was defined as the highest serum dilution that results in a reduction of >50% of the control plaque count.
RESULTS
The demographics and clinical characteristics of the study patients are summarized in Supplementary Table 4. The median age was 29.5 years (range, 17 to 72), and 2 of the patients were male. Three of the patients had at least 1 coexisting disorder (eg, allergic rhinitis and dyslipidemia). At the positive retest, 4 patients had at least 1 symptom that was present at the initial infection.
On average, 13 million reads of 150 bp paired-end sequencing were obtained from each sample. Summary statistics of the whole-genome sequencing data are presented in Supplementary Table 5. For the specimens from the initial infection (n = 7), the genome sequences covered an average 98.1% (range, 93.22%–99.95%) of the 29 903 bp reference sequence, with an average depth of 53 529×. In contrast, the sequence reads for the majority of the specimens (6/7) from the positive retest were rarely mappable; an average of 1.99% (range, .01%–7.87%) of the reads were mappable to 1 or 2 specific fragments of the reference genome, with an average depth of 481× (Supplementary Table 5 and Figure 1). These findings indicate that these few mappable reads were not from the intact viral RNA but from the dead virus fragments in the respiratory epithelial cell that had not been cleared from the recovered patients. To exclude the possibility of these sequencing reads being rarely mappable due to a higher Ct value (ie, lower viral load) in positive retest than in initial diagnosis, we diluted the viral RNA at initial diagnosis from 2 patients (patient 2, diluted at 1:200; patient 3, diluted at 1:100; Supplementary Table 1). The sequencing reads of viral RNAs from the clinical specimens of patients at initial diagnosis covered at least 70% of the reference sequence even when diluted (data not shown).
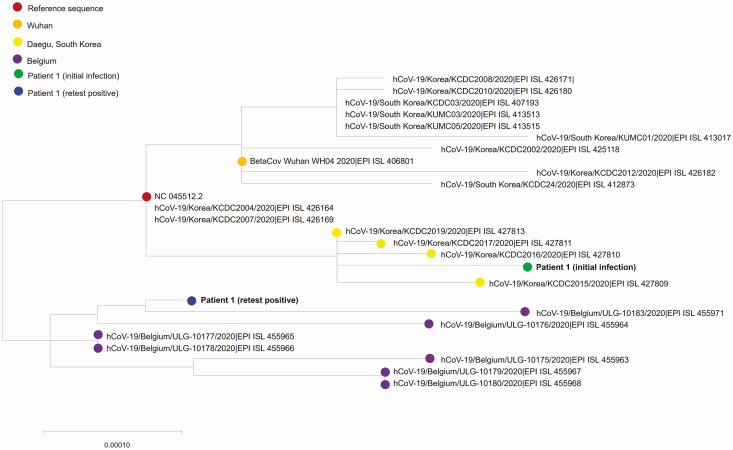
Unexpectedly, however, we were able to acquire a complete genome sequence from viral RNA collected from patient 1 at the second admission with the positive retest after recovery. The genome sequence from this viral RNA covered 99.7% of the reference sequence, with an average depth of 39 727×. To determine if there was any molecular difference between the 2 viral genomes (ie, viral genome at initial infection and viral genome at the second admission with positive retest), we constructed a phylogenetic tree using full genomic sequences at the initial and second infections. Phylogenetic analysis revealed that the viral RNA from the positive retest was clustered into a subgroup distinct from that of the initial infection, suggesting that there was a reinfection of SARS-CoV-2 with a subtype that was different from that of the primary strain (Figure 1). To exclude the possibility of contamination or patient misidentification (ie, specimen switch), we performed short tandem repeat (STR) analysis (Supplementary Methods and Supplementary Figure 2). The results of all 16 STR markers showed identical allele size between the 2 specimens (at initial diagnosis and at reinfection), which excluded contamination by nucleic acid from a different host and also confirmed that these clinical specimens at the 2 time points were collected from the same patient (ie, patient 1).
Figure 1.
Phylogenetic analysis of severe acute respiratory syndrome coronavirus 2 strains. The maximum likelihood method was used with branch lengths measured in the number of base substitutions per site. Each color indicates each sampling location or sampling time point.
To gain insight into the major differences in the sequences that determine a distinct lineage from each other, we further analyzed the variant profiles of viral RNA from patient 1 at 3 time points (at initial diagnosis, during follow-up, and at reinfection after recovery). A total of 8 variants were identified with a variant allele frequency (VAF) greater than 99.0% in the viral genome from the primary infection (Table 1). Of these, nsp6 L37F and ORF3a G251V were the key substitutions that characterized clade “V” [13]. The variant profile of this primary strain remained unchanged during the hospitalization period. In the second infection, however, 14 new variants emerged. Notably, the substitution D614G in S protein (VAF, 84.8%) was detected, which implies that this viral genome corresponds with another clade (“G”) [13]. Four additional substitutions (VAF, 79.4%–92.1%) that are almost always accompanied by S D614G (5’UTR 241C>T [VAF, 79.4%], nsp3 F106F [VAF, 88.7%], P323L [VAF, 92.1%], and ORF3a Q57H [VAF, 80.0%]) were also detected in the viral genome from the second infection, which suggests the majority of viral genome in the second infection was clade “G.” We also found that 9 variants (ie, nsp1 R124C, nsp2 T85I, nsp3 L744F, nsp3 L1035F, nsp4 V407fs, nsp4 S481L, nsp9 L42P, nsp13 T115I, N T165=) emerged in the second infection with low VAF (20.5%–66.0 %). The substitutions (ie, nsp1 Q87D, nsp3 M951I, nsp3 N1181=, nsp3 T1334A, nsp6 L37F, nsp12 Y455=, ORF3a T223I, and ORF3a G251V) that constituted the majority of viral genomes from the primary infection were still detected but with lowered VAF (8%–59.4%) in the viral RNA from the second infection.
Table 1.
Allele Frequency Changes Between the Severe Acute Respiratory Syndrome Coronavirus 2 Genomes in Patient 1
| Gene | Protein | Nucleotide Change | Amino Acid Change | Variant Allele Frequency (%) | ||
|---|---|---|---|---|---|---|
| Initial Infection | Reinfection | |||||
| Initial Diagnosis | Follow-up | |||||
| 5’UTR | 241C>Ta | .0 | .0 | 79.4 | ||
| ORF1ab | nsp1 | 526G>T | Q87D | 99.9 | 99.1 | 19.0 |
| ORF1ab | nsp1 | 635C>T | R124C | .0 | .0 | 28.6 |
| ORF1ab | nsp2 | 1059C>T | T85I | .0 | .0 | 66.0 |
| ORF1ab | nsp3 | 3037C>Ta | F106= | .0 | .0 | 88.7 |
| ORF1ab | nsp3 | 4951G>C | L744F | .0 | .0 | 20.5 |
| ORF1ab | nsp3 | 5572G>T | M951I | 99.9 | NAb | 45.7 |
| ORF1ab | nsp3 | 5822C>T | L1035F | .0 | NAb | 23.3 |
| ORF1ab | nsp3 | 6262T>C | N1181= | 99.9 | 99.8 | 26.7 |
| ORF1ab | nsp3 | 6719A>G | T1334A | 99.8 | 99.9 | 24.9 |
| ORF1ab | nsp4 | 9773delT | V407fs | .0 | .0 | 25.2 |
| ORF1ab | nsp4 | 9996C>T | S481L | .0 | .0 | 23.3 |
| ORF1ab | nsp6 | 11083G>Tc | L37F | 99.5 | 99.2 | 8.0 |
| ORF1ab | nsp9 | 12810T>C | L42P | .0 | .0 | 32.0 |
| ORF1ab | nsp12 | 14408C>Ta | P323L | .0 | .0 | 92.1 |
| ORF1ab | nsp12 | 14805C>T | Y455= | 99.7 | 99.4 | 59.4 |
| ORF1ab | nsp13 | 16580C>T | T115I | .0 | .0 | 34.5 |
| S | S | 23403A>Ga | D614G | .0 | .0 | 84.8 |
| ORF3a | ORF3a | 25563G>Ta | Q57H | .0 | .0 | 80.0 |
| ORF3a | ORF3a | 26060C>T | T223I | 99.8 | 99.5 | 10.0 |
| ORF3a | ORF3a | 26144G>Tc | G251V | 99.9 | 98.6 | 10.0 |
| N | N | 28768A>G | T165= | .0 | .0 | 47.5 |
Abbreviation: NA, not available.
aThe variant that characterizes the clade G.
bNot available because the region between 5214 and 5931 of the reference genome was not well covered by aligned sequencing reads.
cThe variant that characterizes the clade V.
Patient 1, a 21-year-old woman with a history of allergic rhinitis and who was otherwise healthy, reported having a sore throat and cough with a small amount of sputum on 5 March 2020, and the symptoms persisted for a week. Upper respiratory specimens (ie, nasopharyngeal swabs and oropharyngeal swabs) and sputum specimens were collected from the patient and tested positive for SARS-CoV-2 on 11 March 2020 (Figure 2). The patient had mild illness. On admission the same day, the patient showed stable vital signs without any abnormal findings in chest radiograph and computed tomography. Her laboratory findings including serum levels of C-reactive protein (CRP) and procalcitonin (CRP, 1.9 mg/L, normal range, <5.0 mg/L; procalcitonin, <0.02 ng/mL, normal range, <0.046 ng/mL) were within normal range. The patient received symptomatic care with oral antitussives and esomeprazole, as needed, but did not receive corticosteroids, other immunomodulators, or antivirals. On day 15 of hospitalization (25 March 2020), the patient’s symptoms had nearly disappeared. The patient tested negative via rRT-PCR on 26 March 2020 and 27 March 2020 and was discharged home on 30 March 2020. On the day of discharge, the patient reported residual upper airway symptoms, that is, nonproductive cough and sore throat.
Figure 2.
Temporal profile of the viral load in patient 1 with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection. WGS of SARS-CoV-2 from respiratory specimens was performed at 4 time points (at first admission with initial diagnosis, during follow-up, at second admission with reinfection, at third admission with retest positive again). A lower Ct value corresponds to a higher viral load. The values under the dashed line were interpreted as negative for SARS-CoV-2. Viral culture was conducted by inoculating upper (triangle) or lower (star) respiratory tract specimens onto Vero cells. Blue indicates that SARS-CoV-2 was culturable and gray indicates that SARS-CoV-2 was not culturable. *No intact viral genome was observed at third admission. Abbreviations: Ct, cycle threshold; rRT-PCR, real-time reverse-transcription polymerase chain reaction; WGS, whole-genome sequencing.
Six days after discharge (5 April 2020), the patient reported aggravation of cough combined with sputum. A day later, upper respiratory specimens and sputum specimens were collected from the patient, of which the upper respiratory specimens retested positive. The Ct value was 32.36 (E gene), which was higher than the Ct value at the initial evaluation at the time of initial infection (Figure 2). The patient had mild signs and symptoms as was the case at the time of the initial infection. Her laboratory results were normal as was the case at the time of the initial infection. During the second hospitalization, the patient received symptomatic care for mild symptoms (eg, cough and sputum), and these symptoms almost disappeared on day 4 of the second hospitalization (9 April 2020). The patient tested negative via rRT-PCR on 17 April 2020 and 19 April 2020 and was discharged on 25 April 2020.
Five days after discharge (30 April 2020), during her self-quarantine, the patient reported having a sore throat and cough with sputum. Upon hospital revisit, the patient had positive rRT-PCR results. We additionally performed whole-genome sequencing of this clinical sample at positive retest, but the sequence reads were rarely mappable. Only .26% of total sequence reads were mappable to 3 small fragments of the reference genome with an average depth of 25 × (Supplementary Table 5 and Figure 1). We found that the variant nsp1 Q87D detected in the initial infection was still observed in a few mappable sequence reads, while the variants that emerged in reinfection (ie, second hospitalization) were not detected (Supplementary Table 6). This finding implies that the positive retest at the patient’s third admission might be due to prolonged clearance of viral gene fragments of the initial infection. The patient’s symptoms subsided the day after admission, and she remained asymptomatic thereafter. The next 2 rRT-PCR results (on 4 May 2020 and 6 May 2020) were negative, and the patient was discharged home on 11 May 2020.
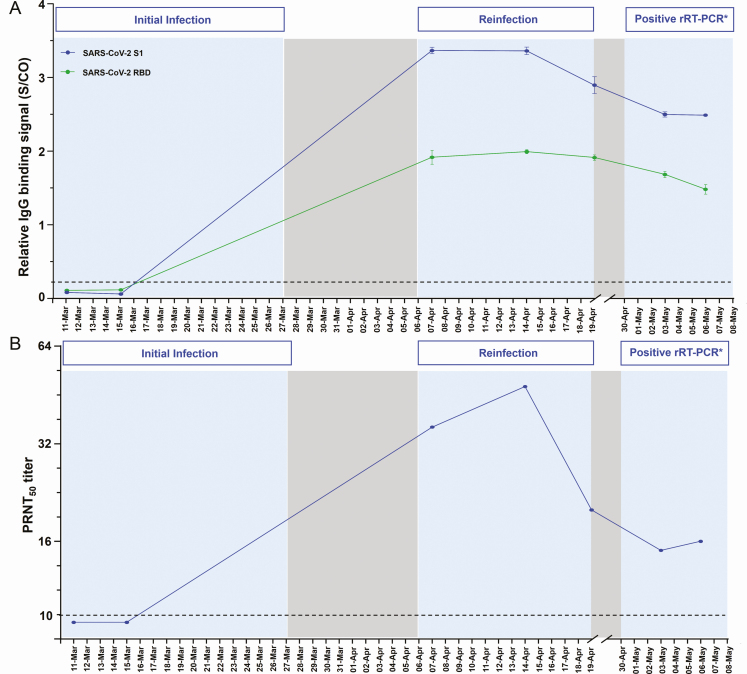
The longitudinal profiles of IgG antibodies and neutralizing antibodies (NAbs) against SARS-CoV-2 in patient 1 showed that the patient was seronegative at the early stage of the initial infection but remained seropositive during the second infection (Figure 3). The antibody levels increased approximately 10 days after onset of the patient’s reinfection episode, then decreased but remained positive within the following 3 weeks.
Figure 3.
Temporal profile of antibodies in patient 1 with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection. Each antibody was measured at 7 time points. A, IgG antibodies against SARS-CoV-2 S1 (blue) and receptor binding domain (green) protein. The cutoff values for IgG antibody assay were S/CO = 1. B, PRNT titers of neutralizing antibody. A PRNT50 titer was defined as the highest serum dilution that results in a reduction of >50% of the control plaque count. A PRNT50 titer of ≥10 was considered protective. Abbreviations: IgG, immunoglobulin G; PRNT, plaque-reduction neutralization test; rRT-PCR, real-time reverse-transcription polymerase chain reaction; S/CO, signal to cutoff. *No intact viral genome was observed at third admission.
DISCUSSION
In this study, we provide the genomic characteristics of SARS-CoV-2 at the retest-positive time point. The majority of the cases that retested positive for SARS-CoV-2 with rRT-PCR did not have an intact viral genomes; rather, a specific fragment of the dead virus genome was amplified. However, in 1 case, evidence of SARS-CoV-2 reinfection was identified.
In patient 1, we confirmed that viral RNA from the positive retest clustered in clade “G” as defined by the S D614G substitution, while the viral RNA from the initial infection was found to be clade “V,” as defined by the ORF3a G251V substitution. Clade “V” and clade “G” represent different geographic distributions and temporal evolutions of the SARS-CoV-2 genome [14]. Clade V, which comprises a subset of the SARS-CoV-2 genomes in Asia and Europe [15], was dominated by genomes from the Daegu outbreak (in South Korea) from late February 2020 to early March 2020 [16]. On the other hand, clade “G” is the other type of viral strain that started to be collected in South Korea in early April 2020. These evolutionary findings of SARS-CoV-2 match the timeline of infection in patient 1 who had an initial infection with clade “V” in early March and a second infection with clade “G” in late March or early April (Figure 2).
In the viral RNA from the clinical specimen of patient 1 at reinfection, no read sequences containing substitutions 5’UTR 241C>T and nsp1 R124C, which represent reinfection, contained the substitution nsp1 Q87D initially detected in the initial infection and still observed with lowered VAF in reinfection (Supplementary Figure 3). We could not exclude the possibility of the coexistence of 2 different viral genomes in reinfection. We speculate that the viral strain from the initial infection could have been present in minute amounts, and the reason that patient 1 showed consecutive negative results on rRT-PCR may be due to intermittent viral shedding [17–19]. Multiple variants emerged with low allele frequency in the viral RNA from the second infection. The effect of these variants on the functionality of the viral genome is still unclear. We speculate that these variants represent viral quasispecies that result from the evolutionary dynamics of RNA viruses, which have been previously described for SARS-CoV, Middle East respiratory syndrome coronavirus, and SARS-CoV-2 infection [1, 20–25].
Detectable IgG antibodies and NAb titers were confirmed at the second admission. The interval between reinfection and initial infection was quite short at 26 days, thus it is still unclear whether the patient’s seropositive status at the second admission was related to the patient’s adaptive immunity against initial infection or related to the antibody response during reinfection. The antibody levels were shown to increase 10 days after the onset of the patient’s reinfection episode. Recently, it has been recurrently observed that in patients with clinically mild COVID-19, the IgG and NAbs are detected within 2 weeks of onset and begin to decline within 30 days of onset of symptoms [26–30]. In this regard, antibody dynamics in patient 1 might be evidence that supports SARS-CoV-2 reinfection.
The majority of patients with clinically mild COVID-19 can develop neutralizing antibodies against SARS-CoV-2 spike [27, 28, 31–35]. However, whether these antibodies can neutralize every clade of SARS-CoV-2 and guarantee immunity to subsequent infection with these mutated viruses remains to be determined. At reinfection in our patient, S D614G variant, located in the external spike protein of SARS-CoV-2, was observed. A recent study reported that 7% of sera from convalescent patients showed decreased antibody neutralization S D614G pseudovirus [8, 36]. Further studies are necessary to identify lowered neutralizing activity against S G614-bearing SARS-CoV-2 in reinfection cases.
An important limitation of our study is that the source of reinfection in patient 1 and whether there was transmission from patient 1 at the time of reinfection were not clarified because the patient could not be reached by telephone after discharge. However, during this pandemic, the infection can be exposed anywhere in the community, and the source of the infection is often unknown.
This study highlights possible SARS-CoV-2 reinfection with a genetically distinct SARS-CoV-2 strain in patients shortly after recovery from mild COVID-19. SARS-CoV-2 infection may not confer immunity against a different SARS-CoV-2 strain.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge the authors from the originating laboratories who were responsible for obtaining the specimens and those at the submitting laboratories where genetic sequence data were generated and shared via the Global Initiative on Sharing All Influenza Data Initiative (Supplementary Table 3).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report–186 (24 July 2020) Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200724-covid-19-sitrep-186.pdf?sfvrsn=4da7b586_2. Accessed 11 August 2020.
- 2. South Korea reports more recovered coronavirus patients testing positive again Available at:https://www.reuters.com/article/us-health-coronavirus-southkorea/south-korea-reports-more-recovered-coronavirus-patients-testing-positive-again-idUSKCN21V0JQ. Accessed 11 August 2020
- 3. Kiyuka PK, Agoti CN, Munywoki PK, et al. Human coronavirus NL63 molecular epidemiology and evolutionary patterns in rural coastal Kenya. J Infect Dis 2018; 217:1728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 5. Luo A Positive SARS-Cov-2 test in a woman with COVID-19 at 22 days after hospital discharge: a case report. J Tradit Chin Med Sci 2020. In press. [Google Scholar]
- 6. Yoo SY, Lee Y, Lee GH, Kim DH. Reactivation of SARS-CoV-2 after recovery. Pediatr Int 2020; 62:879–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. J Med Virol 2020; 92:2366–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. To KK, Hung IF, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Elslande J, Vermeersch P, Vandervoort K, et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nurk S, Bankevich A, Antipov D, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol 2013; 20:714–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sequence Read Archive (SRA) Available at: https://www.ncbi.nlm.nih.gov/sra.
- 13. Global Initiative on Sharing All Influenza Data home page Available at: https://www.gisaid.org/. Accessed 11 August 2020
- 14. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A 2020; 117:9241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mercatelli D, Giorgi FM. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol 2020; 11:1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JH, An J, Min PK, Bitton A, Gawande AA. How South Korea responded to the Covid-19 outbreak in Daegu. NEJM Catalyst. 2020. Available at: https://catalyst.nejm.org/doi/pdf/10.1056/CAT.20.0159.
- 17. Lee TH, Lin RJ, Lin RTP, et al. Testing for SARS-CoV-2: can we stop at two? Clin Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Memoli MJ, Han A, Walters KA, et al. Influenza A reinfection in sequential human challenge: implications for protective immunity and “universal” vaccine development. Clin Infect Dis 2020; 70:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu X, Xing Y, Jia J, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ 2020; 728:138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog 2010; 6:e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregori J, Perales C, Rodriguez-Frias F, Esteban JI, Quer J, Domingo E. Viral quasispecies complexity measures. Virology 2016; 493:227–37. [DOI] [PubMed] [Google Scholar]
- 22. Capobianchi MR, Rueca M, Messina F, et al. Molecular characterization of SARS-CoV-2 from the first case of COVID-19 in Italy. Clin Microbiol Infect 2020; 26:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park D, Huh HJ, Kim YJ, et al. Analysis of intrapatient heterogeneity uncovers the microevolution of Middle East respiratory syndrome coronavirus. Cold Spring Harb Mol Case Stud 2016; 2:a001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Z, Xiao Y, Kang L, et al. Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with coronavirus disease 2019. Clin Infect Dis 2020; 71:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu D, Zhang Z, Wang FS. SARS-associated coronavirus quasispecies in individual patients. N Engl J Med 2004; 350:1366–7. [DOI] [PubMed] [Google Scholar]
- 26. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 27. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Heide V Neutralizing antibody response in mild COVID-19. Nat Rev Immunol 2020; 20:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020; doi: 10.1101/2020.03.30.20047365. [DOI]
- 30. Crawford KH, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. medRxiv 2020; doi: 10.1101/2020.08.06.20169367. [DOI] [PMC free article] [PubMed]
- 31. Ota M Will we see protection or reinfection in COVID-19? Nat Rev Immunol 2020; 20:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9:386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis 2020; 26:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020; doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 36. Zhang L, Jackson CB, Mou H, et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv 2020; doi: 10.1101/2020.06.12.148726. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.