Abstract
Background
COVID-19 is a novel disease that has been associated with changes in haemostasis and increased risk of thrombosis, especially in patients who are critically ill.
Case summary
a 71-year-old patient presented to the emergency department with acute respiratory failure. The patient had been discharged from the hospital 1 day before, after apparent recovery of a proven COVID-19 infection. Relevant medical history reports polycythemia vera. The diagnostic work-up included a CT-scan of the thorax, revealing bilateral sub-segmental pulmonary embolism. An echocardiogram showed a dilated right ventricle with poor systolic function and a large multi-lobar thrombus. Forty-eight hours after initiation of treatment with therapeutic anticoagulation the thrombus was no longer seen on the echocardiogram.
Discussion
This case confirms the high risk of thrombosis in COVID-19 infection as has been described in recent literature. It demonstrates the severity of the coagulopathy given the presence of both bilateral sub-segmental pulmonary embolism and right ventricular thrombus, despite treatment with prophylactic anticoagulation. Remarkable in this case is the fact that the patient had a myeloproliferative neoplasm (polycythaemia vera). This is associated with an increased risk of thrombosis, especially in the presence of erythrocytosis, leucocytosis, and/or inflammation.
Keywords: COVID-19, Pulmonary embolism, Right ventricular thrombus, polycythaemia vera, Case report
Introduction
COVID-19 is a novel disease that has been associated with changes in haemostasis and increased risk of thrombosis, especially in critically ill patients1. It is currently unclear whether this increased risk of thrombosis is even higher in patients with pre-existing myeloproliferative neoplasms (MPN) such as polycythaemia vera. In this case report, a patient with polycythaemia vera was admitted to the hospital due to acute respiratory distress. This occurred 1 day after apparent recovery from a proven COVID-19 infection. The cause was bilateral pulmonary embolism with a thrombus in the right ventricular apex.
Timeline
| 27 March 2020 | Hospital admission witd dyspnoea due to novel COVID-19 infection, confirmed by positive polymerase chain reaction (PCR). Oxygen saturation levels of 93% with 5 L. Treatment withantibiotics, chloroquine, and dalteparin 5000 IU once daily. |
| 31 March 2020 | Hospital discharge after apparent recovery from COVID-19 infection. |
| 1 April 2020 | Presentation at the emergency room with acute respiratory distress. Oxygen saturation levels of 95% with 15 L (non-rebreather mask). Electrocardiogram shows signs of right ventricular strain. Computed tomography scan thorax: bilateral sub-segmental pulmonary embolism and mass in the apex of the right ventricle. Echocardiography: dilated right ventricle with poor systolic function and mobile, multi-lobar mass in the apex suspect for thrombus. Treatment with dalteparin 12.500 IU once daily initiated. |
| 3 April 2020 | Repeat echocardiogram: thrombus right ventricular apex no longer present. Persisting respiratory distress despite maximum supplemental oxygen using OptiflowTM Nasal High Flow (FiO2 90% with 60 L). |
| 7 April 2020 | Persisting dyspnoea and hypoxia (oxygen saturation levels 87–88%) despite several days of optimal medical treatment (patient had a do not intubate policy). Palliative sedation was initiated. Patient passed away the same day. No autopsy was performed. |
Case presentation
A 71-year-old man presented to our emergency room with acute respiratory distress. The patient had been discharged from the hospital 1 day previously, after an apparent recovery from a proven (PCR positive) COVID-19 infection. During the initial hospital stay, dyspnoea had been mild with oxygen saturation levels of 95% with 5 L of additional oxygen. Medical treatment had consisted of intravenous broad-spectrum antibiotics, off-label chloroquine (loading dose of 600 mg, followed by 300 mg twice daily for a duration of 5 days) and dalteparin 5000 IU subcutaneously once daily as thrombosis prophylaxis. Over the course of 5 days, the patient recovered quickly with no dyspnoea and normal oxygen saturation levels at the time of discharge. The current respiratory distress had developed progressively over the course of 24 h.
The relevant medical history reports rheumatoid arthritis, dilating cardiomyopathy with a left ventricular ejection fraction of 35% since 2015, pulmonary emphysema and polycythaemia vera currently being treated with hydroxycarbamide 500 mg once daily. This therapy was continued during both hospital stays.
At the time of presentation, the patient was in shock with cold extremities, a tachycardia of 107 b.p.m., hypotension (86/50 mmHg), tachypnoeic (27 b.p.m.), and oxygen saturation levels of 95% with 15 L additional oxygen using a non-rebreather mask (FiO2 80%).
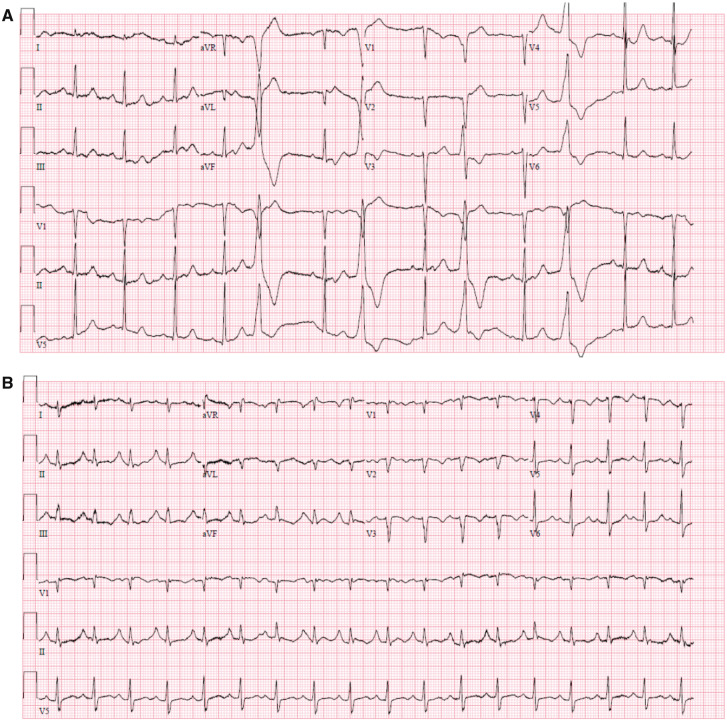
The electrocardiogram showed sinus tachycardia of 107 b.p.m., right axis, P pulmonale, incomplete right bundle branch block, prolonged QTc interval (520 ms), and negative T-waves in the precordial leads (Figure 1). These electrocardiographic findings were new compared to previous electrocardiograms. The changes were suggestive of possible pulmonary embolism, and the prolongation of the QTc interval was probably related to the previous use of chloroquine.
Figure 1.
Electrocardiogram at first hospital admission (A) and second hospital admission (B).
The laboratory findings included haemoglobin 16.3 g/dL, haematocrit 0.58 L/L, platelet count 1600 × 109/L, leucocyte count 45.6 × 109/L, lymphocyte count 1.7 × 109/L, prothrombin time 22.2 s, D-dimer 3.3 mg/L, international normalized ratio 1.6, lactate dehydrogenase 833 U/L, and C-reactive protein 35 mg/L. These findings pointed to a pro-thrombotic state.
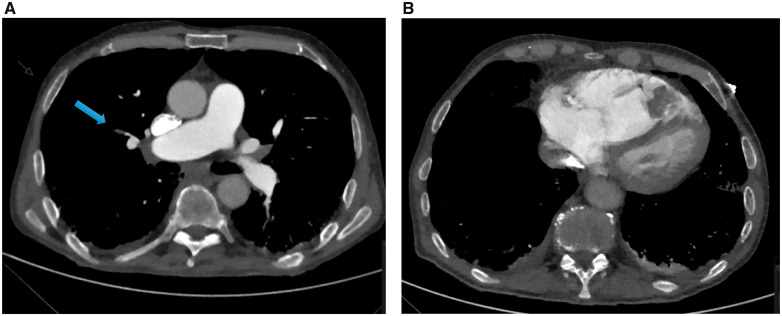
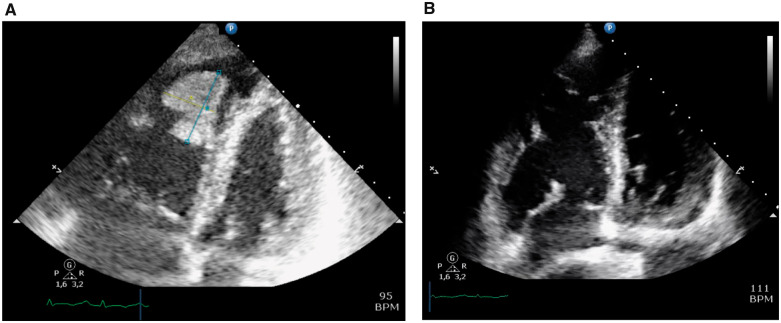
Based on these initial findings, pulmonary embolism was suspected. The diagnosis of bilateral sub-segmental pulmonary embolism was confirmed on the computed tomography scan (Figure 2A). Additionally, a contrast opacification of 3 cm in diameter in the right ventricular apex was noted (Figure 2B). Based on this finding, the cardiologist was consulted and an echocardiogram was performed. The right ventricle was severely dilated with severely decreased systolic function. In the apex of the right ventricle, a large, mobile, multi-lobar mass (26 mm × 36 mm) was seen (Figure 3A and Video 1). This was most likely thrombus given the bilateral sub-segmental pulmonary embolism in a COVID-19 patient. As treatment therapeutic dosage of dalteparin (12.500 IU subcutaneously once daily) was initiated. After 48 h, the echocardiogram was repeated and the thrombus was no longer present (Figure 3B and Video 2).
Figure 2.
Computed tomography scan: (A) sub-segmental pulmonary embolism, (B) mass in right ventricular apex.
Figure 3.
Echocardiogram at presentation (A 26 mm × B 36 mm) (A) and during follow-up (B).
Over the course of several days, the dyspnoea persisted despite high levels of supplemental oxygen therefore OptiflowTM Nasal High Flow therapy (FiO2 90%, 60 litres per minute) was initiated. No improvement was seen, and the decision was made to withdraw active treatment and start palliative sedation. The patient passed away several hours later. No autopsy was performed.
Discussion
This case provides an example of the high risk of thrombosis in patients with a COVID-19 infection with several novel features. It shows the extent of thrombosis with the presence of a large thrombus in the right ventricular apex in conjunction with bilateral sub-segment pulmonary embolism. Notably, the thrombotic event occurred after apparent initial recovery and despite prophylactic anticoagulation. Furthermore, the medical history reported the presence of polycythaemia vera, a MPN that is associated with an increased risk of thrombosis (2). There are several known risk factors in patients with polycythaemia vera, such as erythrocytosis, leucocytosis, and inflammation, that further increase the risk of thrombosis (2). It has been postulated that these patients are likely to be at increased risk of thrombosis if also infected with COVID-19, although there is no data at present to support this fact (3). If a patient with COVID-19 and a MPN deteriorates despite adequate treatment, underlying thromboembolism should be considered.
It has become clear that COVID-19 is associated with an increased risk of thrombosis. One case report revealed the presence of pulmonary embolism in two critically ill patients with COVID-19 infections (4). A recent study assessed the incidence of thrombotic complications in 184 patients with COVID-19 who had been admitted to the intensive care. The thrombotic complications included acute pulmonary embolism, deep-vein thrombosis, ischaemic stroke, myocardial infarction, or systemic arterial embolism. At the time of inclusion, all patients were being treated with thrombosis prophylaxis. The incidence of thrombosis was high (31%) and the majority of these patients (81%) had an acute pulmonary embolism (5). Further research has described significant changes in haemostatic function in COVID-19 patients compared to healthy controls (1). An elevated D-dimer was seen in 46–68% of COVID-19 patients and has been associated with a poor prognosis (6, 7).
Right heart thrombi are seen in less than 4% of patients with pulmonary embolism according to the 2019 ESC Guideline Acute Pulmonary Embolism (8). Further data with regard to right ventricular thrombi in conjunction with pulmonary embolism revealed that only 2.9% of 103 patients with proven pulmonary embolism had a right ventricular thrombus (9). Additionally, two case reports describing the presence of right ventricular thrombus combined with pulmonary embolism were found (10, 11). And one case report was found in which a patient with polycythaemia vera had a right atrial thrombus and pulmonary embolism with resolution of the thrombus after 2 weeks of anticoagulant therapy (12).
Follow-up echocardiography performed after 48 h demonstrated that the thrombus was no longer present in the right ventricular apex, suggesting resolution of the thrombus. Migration of the thrombus into the pulmonary vasculature cannot be excluded, although this seems less likely given the size of the thrombus (26 mm × 36 mm). No data could be found regarding the effect of therapeutic anticoagulation on the resolution of right ventricular thrombi and the time needed for resolution of the thrombus.
Conclusion
This case is the first to report the presence of a thrombus in the right ventricular apex in conjunction with bilateral sub-segmental pulmonary embolism in relation to COVID-19. The patient in this case had polycythaemia vera which is known to have an increased thrombosis risk, especially if erythrocytosis, leucocytosis, and/or inflammation are present.
Lead author biography

Fabienne Vervaat is currently cardiologist in training at Catharina Hospital in the Netherlands. Her area of interests are refractory angina pectoris, coronary artery disease, and interventional cardiology.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient's next of kin in line with COPE guidelines.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Han H, Ynag L, Liu R, Liu F, Wu K, Li J. et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med, 2020;58:1116–1120. [DOI] [PubMed] [Google Scholar]
- 2. Kroll MH, Michaelis LC, Verstovsek S.. Mechanism of thrombogenesis in polycythemia vera. Blood Rev 2015;29:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mesa R, Alvarez-Larran A, Harrison C, Kiladjian JJ, Rambaldi A, Tefferi A. et al. COVID-19 and myeloproliferative neoplasms: frequently asked questions (Version 3.0; last updated May 4, 2020). https://www.hematology.org/covid-19/covid-19-and-myeloproliferative-neoplasms (7 May 2020).
- 4. Xie Y, Wang X, Yang P, Zhang S.. COVID-19 complicated by acute pulmonary embolism. Radiology 2020;2:e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok FA, Kruip MJHA, Vd Meer NJM, Stals MAM, Huisman MV, Endeman H.. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J et al. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konstantinides SV, Meyer G, Becattini Bueno H, Geersing G, Harjola V, Huisman MV; ESC Scientific Document Group et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism. Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 9. Kasper W, Meinertz T, Henkel B, Eissner D, Hahn K, Hofmann T etal.. Echocardiographic findings in patients with proved pulmonary embolism. Am Heart J 1986;112:1284–1290. [DOI] [PubMed] [Google Scholar]
- 10. Mujer M, Saleh Y, Abro C, Kandola SK.. Pulmonary embolism with right ventricular thrombus: a management dilemma. BMJ Case Rep 2019;12:e229184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nawale JM, Chaurasia AS, Nalawade DD, Borikar NA.. A case of mobile right ventricular thrombus with massive pulmonary thromboembolism. J Pract Cardiovasc Sci 2017;3:178–180. [Google Scholar]
- 12. Gangadharamurthy D, Shih H.. Intracardiac thrombosis in polycythemia vera. BMJ Case Rep 2013; doi: 10.1136/bcr-2012-008214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.