Abstract
Objectives:
The neutrophil-to-lymphocyte ratio (NLR) is a prognostic marker in patients with cancer receiving immunotherapy. Recent studies have shown that a high NLR was associated with a poor response and decreased survival. However, there is no intervention to reverse abnormally high NLR and improve clinical outcomes. Astragalus polysaccharide injection (PG2) is an immunomodulatory therapy for cancer-related fatigue. This study aimed to examine whether PG2 might normalize the NLR and affect the overall survival of patients with lung cancer treated with immunotherapy.
Materials and Methods:
We retrospectively examined the medical records of patients with lung cancer treated with immune checkpoint inhibitors (ICIs) between October 1, 2015 and November 30, 2019. All patients received ICI combination chemotherapies, and some similarly received PG2 (Control vs PG2). The NLR was assessed before treatment and 6 weeks after ICI initiation, and the survival data was collected at least 4 years after treatment initiation for the first enrolled patient.
Results:
Fifty-three patients were included. Six weeks after ICI initiation, 91.3% of the patients in the PG2 group exhibited a predefined “Decrease or no change” in the NLR, which was 28% higher than that in the Control group (63.3%) (P = .028). The NLR significantly decreased by 31.60% from baseline in the PG2 group (P = .012), whereas it increased by 5.80% in the Control group (P = .572). Six weeks after ICI treatment initiation, both groups had a median NLR of 3.73, and the overall survival was also similar (PG2 vs Control, 26.1 months vs 25.4 months, respectively); however, the PG2 group had a higher median baseline NLR than the Control group (PG2 vs Control, 4.51 vs 2.81, respectively).
Conclusion:
This study demonstrated that PG2 could normalize the NLR in patients with lung cancer receiving ICI combination treatments.
Keywords: astragalus polysaccharides, lung cancer, immune checkpoint inhibitors, immunotherapy, neutrophil to lymphocyte ratio, overall survival
Introduction
Advances in the standard therapies for lung cancer, including chemotherapy, targeted treatments, and immunotherapy, have been translated into an improvement in the survival of patients with lung cancer over the last 2 decades. The development of immune checkpoint inhibitors (ICIs) is an important breakthrough in cancer treatment, especially when administered in combination with chemotherapy. Many patients with cancer have shown an improvement in survival after receiving ICIs.1,2 However, approximately 50% of patients with cancer do not benefit from ICIs, whereas 10% to 15% experience disease progression.3
The neutrophil-to-lymphocyte ratio (NLR) is a marker of systemic inflammation. Some studies have shown that, among patients with cancer receiving immunotherapy, a higher blood NLR was related to a poor response and shorter survival in those with advanced cancer.4-9 Additionally, several studies have investigated the predictive value of the NLR among patients with lung cancer treated with inhibitors of programmed cell death-1 (PD-1) and its ligand (PD-L1). They have shown that a higher NLR was associated with worse clinical outcomes in various cancers.4,10-12 Although previous studies have demonstrated that blood NLR levels before treatment and approximately 6 weeks after the initiation of ICIs are potential predictive markers of clinical response,13 no intervention that can regulate inflammation to reduce the NLR and improve clinical outcomes in patients with cancer receiving immunotherapy has been established.
A prescription drug, injectable astragalus polysaccharide (PG2) (PhytoHealth Corporation, Taiwan, ROC), is an immunomodulator that has been approved for the alleviation of cancer-related fatigue (CRF).14 PG2 can stimulate medullary hematopoiesis and enhance immune function. Preclinical studies have demonstrated that PG2 has an anti-inflammatory effect by inhibiting LPS-induced TNF-α and IL-1β production in THP-1 cells.15 Additionally, PG2 can promote the proliferation and maturation of bone marrow and spleen precursor cells in mice treated with 5-fluorouracil, mitomycin C, or high-dose radiotherapy, and can promote the recovery of peripheral blood leukocytes, erythrocytes, and platelets.16 Further, PG2 can modulate tumoral M1/M2 macrophage polarization and induce dendritic cell maturation.17
Therefore, we hypothesized that PG2 might normalize the NLR in patients with lung cancer receiving ICI combination treatment. We further explored its association with patient survival.
Methods
We collected the medical records of patients with lung cancer treated with ICIs at the Chung Shan Medical University Hospital in Taiwan between October 1, 2015 and November 30, 2019. The trial protocol was approved by the Institutional Review Board of the Chung Shan Medical University Hospital (IRB No.: CS2-20009, Clinicaltrials.gov: NCT04352335). Informed consent was waived due to the retrospective nature of this study. All included patients had adequate bone marrow, renal, and hepatic function and had received at least 1 dose of ICI treatment, including nivolumab, pembrolizumab, or atezolizumab, combined with chemotherapy. PG2 at a dose of 500 mg was administered to relieve CRF either before and/or after ICI initiation. Data of the conducted hematological investigations were collected before ICI treatment (within 3 days) and in the sixth week after initiation (±2 weeks). The primary endpoint was the NLR change 6 weeks after treatment initiation between patients treated with PG2 (PG2 group) and those who were not (Control group). The NLR was defined as the absolute number of neutrophils divided by the absolute number of lymphocytes. The changes in the NLR were classified as “Increase (bad outcome),” which was defined as an increase of 25% or more from the baseline, and “Decrease or no change (good outcome),” which was defined as a decrease, no change, or an increase of less than 25% from the baseline. The data of all the patients and subgroups (baseline NLR ≥5 and <5) were analyzed in this study.4,5 In addition, the overall survival (OS) was compared between the groups, and the data were collected at least 4 years after the day of ICI treatment initiation for the first enrolled patient.
Statistical Analysis
When inferential analyses were required, the Wilcoxon rank-sum test was used for quantitative variables. In contingency tables for categorical variables, the Fisher’s or chi-square tests were used. OS was analyzed using the Kaplan–Meier method and the log-rank test. The significance of the NLR for within-group differences in baseline versus the sixth week was determined using the Wilcoxon signed-rank test, and the significance for differences between the PG2 and Control groups was determined using the Wilcoxon rank-sum test. Data from the sixth week minus baseline were used to compare the significance of between-group differences using the Wilcoxon rank-sum test. Covariance from the NLR at baseline and the sixth week was controlled using ANCOVA to avoid a correlation affecting between-group differences. Values of P < .05 were considered statistically significant. Statistical analyses were performed using SAS v9.4 (SAS Institute, Inc, Cary, NC) and R v3.6.2 (R Core Team (2019), R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Demographics and Disease Characteristics
Fifty-three patients, including 23 treated with ICIs and PG2 (PG2 group) and 30 treated with ICIs alone (Control group), were enrolled and analyzed in the study. The baseline patient characteristics are listed in Table 1. The median age of all the patients was 63 (range, 44-89) years. Most patients were men, current or former smokers, had an Eastern Cooperative Oncology Group performance status score of 1, and were diagnosed with adenocarcinoma, Stage IV, with tumor metastasis to the bone. All patients had received one previous line of immunotherapy, and the baseline characteristics were balanced between the 2 groups.
Table 1.
Baseline Demographic and Disease Characteristics.
| PG2 (N = 23) | Control (N = 30) | P value* | |
|---|---|---|---|
| Age at immunotherapy (y) | |||
| Median (range) | 66 (44-81) | 61.5 (48-89) | .148 |
| Sex (N, %) | .747 | ||
| Male | 18 (78.3%) | 22 (73.3%) | |
| Female | 5 (21.7%) | 8 (26.7%) | |
| Smoking status (N, %) | .387 | ||
| Never-smoker | 9 (39.1%) | 8 (26.7%) | |
| Current or ex-smoker | 14 (60.9%) | 22 (73.3%) | |
| ECOG performance status (N, %) | .431 | ||
| 1 | 14 (60.9%) | 22 (73.3%) | |
| 2 | 8 (34.8%) | 8 (26.7%) | |
| 3 | 1 (4.4%) | 0 (0.00%) | |
| Histology (N, %) | .352 | ||
| Adenocarcinoma | 13 (56.5%) | 19 (63.3%) | |
| Squamous cell | 5 (21.7%) | 9 (30.0%) | |
| Others (Small cell, LELC) | 5 (21.7%) | 2 (6.7%) | |
| Stage (N, %) | .845 | ||
| II | 0 (0.0%) | 1 (3.3%) | |
| III | 4 (17.4%) | 7 (23.3%) | |
| IV | 19 (82.6%) | 22 (73.3%) | |
| Previous lines of systemic treatment (N, %) | .769 | ||
| 1 | 9 (39.1%) | 10 (33.3%) | |
| ≥2 | 14 (60.9%) | 20 (66.7%) | |
| PD-L1 positivity (N, %) | .751 | ||
| <1, Negative | 7 (30.4%) | 7 (23.3%) | |
| 1-49% | 5 (21.7%) | 4 (13.3%) | |
| ≥50% | 4 (17.4%) | 7 (23.3%) | |
| Unknown | 7 (30.4%) | 12 (40.0%) | |
| Metastasis | |||
| Liver | 4 (17.4%) | 2 (6.7%) | |
| Brain | 11 (47.8%) | 5 (16.7%) | |
| Bone | 15 (65.2%) | 12 (40.0%) | |
| Other: lymph node, adrenal gland, pleura | 9 (39.1%) | 19 (63.3%) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LELC, Lymphoepithelioma-like carcinoma; PD-L1, programmed death ligand 1; PG2, Astragalus polysaccharide injection.
Wilcoxon rank-sum test, Chi-square test, or Fisher’s exact test.
Changes in the NLR
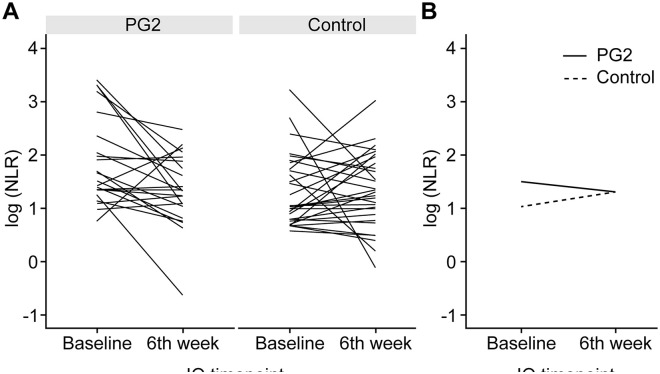
In the PG2 group, 91.3% of the patients exhibited a predefined “Decrease or no change” in the NLR 6 weeks after ICI initiation, which was 28% higher than that in the Control group (63.3%) (P = 0.028; Table 2). The changes in the NLR before and 6 weeks (±2 weeks) after ICI initiation in individual patients and all patients’ NLR median scores are shown in Figure 1a and b, respectively. The NLR level in the PG2 group 6 weeks after ICI initiation significantly decreased from baseline (−1.92, −31.60%; P = .012), whereas that in the Control group slightly increased from baseline (0.13, 5.80%; P = .572). A significant difference in the NLR change was observed between the PG2 and control groups (P = .014). However, the median baseline NLR was significantly higher in the PG2 group than in the Control group (P = .009). After adjusting for the baseline NLR, no difference in the change in the NLR was observed between the 2 groups (P = .573) (Figure 2a, Supplemental Table S1). In the PG2 group, the proportion of patients with an NLR ≥ 5 decreased in week 6 by 17.4% compared to that before treatment, whereas no change was observed in the Control group (Figure 3, Supplemental Table S2).
Table 2.
Distribution of the Change in the NLR 6 weeks (±2 weeks) After ICI Initiation.
| Population | Classification | PG2 |
Control |
P value‡ |
|---|---|---|---|---|
| N (%) | N (%) | |||
| All Patients (N = 53) | Decrease or no change* | 21 (91.3) | 19 (63.3) | .028 |
| Increase† | 2 (8.7) | 11 (36.7) | ||
| Baseline NLR ≥5 (N = 21) | Decrease or no change* | 11 (100) | 8 (80.0) | .214 |
| Increase† | 0 (0) | 2 (20.0) | ||
| Baseline NLR <5 (N = 32) | Decrease or no change* | 10 (83.3) | 12 (60.0) | .139 |
| Increase† | 2 (16.7) | 8 (40.0) |
Abbreviations: ICI, immune checkpoint inhibitors; NLR, neutrophil-to-lymphocyte ratio; PG2, Astragalus polysaccharide injection.
Decrease or no change: The NLR decreased or increased by <25% from baseline.
Increase: The NLR increased by ≥25% from baseline.
Chi-square test or Fisher’s exact test.
Figure 1.
Change in the NLR before and 6 weeks after (±2 weeks) ICI initiation among all patients. (A) Each line represents the data for an individual patient. (B) The median of the 2 groups.
Abbreviations: ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio.
Figure 2.
NLR at baseline and 6 weeks (±2 weeks) after ICI initiation. (A) All patients. (B) Patients with a baseline NLR ≥ 5. (C) Patients with a baseline NLR <5.
Mann–Whitney tests: *P < .05.
Abbreviations: ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio.
Figure 3.
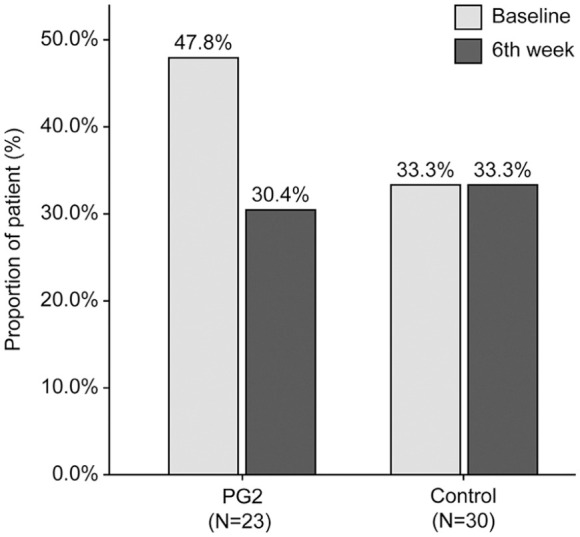
The proportion of patients with an NLR ≥ 5 at baseline and 6 weeks (±2 weeks) after initiation of ICIs.
Abbreviations: ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio.
In patients with a baseline NLR ≥ 5, 100% of the patients in the PG2 group exhibited a predefined “Decrease or no change” in the NLR; however, only 80% of these patients in the Control group demonstrated a “Decrease or no change” in the NLR (Table 2). The NLR decreased significantly in the PG2 group (−4.80, −52.40%; P = .005); however, no significant change was observed in the Control group 6 weeks after ICI initiation (Figure 2b, Supplemental Table S1).
Among patients with a baseline NLR < 5, 83.3% and 60.0% of the patients in the PG2 and the Control group, respectively, exhibited a predefined “Decrease or no change” in the NLR (Table 2). The NLRs had slightly increased 6 weeks after treatment initiation in the PG2 group (0.11, 2.72%, P = .507); however, they significantly increased by 0.52 (19.00%, P = .009) in the Control group. Nevertheless, the baseline NLR in the PG2 group was significantly higher than that of the Control group (P = .003) (Figure 2c, Supplemental Table S1).
Survival Outcomes
The median OS was 6 months in patients with an NLR ≥ 5 at 6 weeks, compared with 25.4 months in those with an NLR < 5 at 6 weeks (P = .006, Figure 4a). We performed survival analysis to examine the survival in patients with advanced lung cancer receiving ICIs and PG2. Six weeks after ICI treatment initiation, both groups had a median NLR of 3.73. The median OS was 26.1 months in the PG2 group (95% confidence interval [CI]: 6-not applicable) and 25.4 months in the Control group (95% CI: 10.7-29.9). No significant difference was observed between the 2 groups (P = 0.76) (Figure 4b).
Figure 4.

Kaplan–Meier analysis of overall survival. (A) Patients with a baseline NLR ≥ 5 and <5. (B) The PG2 and control groups.
Abbreviations: CI, confidence interval; NLR, neutrophil to lymphocyte ratio; NA, not applicable; wk, week.
Discussion
The NLR, a readily obtainable inexpensive biomarker, can predict treatment outcomes and survival in various cancers.18 Recent evidence indicated that a higher NLR was associated with worse clinical outcomes and shorter OS in patients with cancer treated with emerging ICIs. Nevertheless, no drug can reverse abnormal NLR and improve clinical outcomes.
In this study, patients with lung cancer who received combination therapy with PG2 and ICIs had a stable or decreased NLR 6 weeks after treatment initiation (PG2 vs Control: 91.3% vs 63.3%, respectively). This result was independent of a high or normal baseline NLR. Further, a slightly lower proportion (8.3%) of patients with a baseline NLR < 5 had an NLR ≥ 5 at 6 weeks in the PG2 group (PG2 vs Control, 16.7% vs 25.0%, data not shown, respectively). An NLR ≥ 5 or an increase in the NLR by ≥25% after 6 weeks of treatment was associated with a worse OS.19-21 Interestingly, we observed that the median baseline NLR was higher in the PG2 group than in the Control group (4.51 vs 2.81); however, 6 weeks after ICI treatment initiation, the median NLRs in the 2 groups were comparable (3.73). We hypothesized that a high NLR might be associated with CRF, which indicates PG2 administration. No significant difference in the median OS was observed between the 2 groups (PG2 vs Control, 26.1 months vs 25.4 months, respectively); this is notable given the significantly higher baseline NLR in the PG2 group before the ICI and PG2 treatment initiation. Therefore, we propose that in patients receiving ICI combination therapy, PG2 may decrease or stabilize the NLR, and the survival time might be similar to that of patients with low NLR.
This is the first study identifying a drug that may normalize the NLR in patients with lung cancer receiving an ICI combination therapy. PG2, an immunomodulator, might affect the tumor microenvironment by reducing inflammation.19 ICIs are known to enhance the host immune response to cancer by negative regulation to prevent tumor cells from avoiding immune cell-mediated death.20 As PG2 stabilizes or decreases the NLR, alterations in the relative proportions of circulating lymphocytes might promote the antitumor immune effect mediated by immunotherapy. Furthermore, PG2 can prevent bone marrow injury, which is a common adverse effect of most chemotherapies. Hence, more enriched circulating lymphocytes could be beneficial, especially for patients receiving ICI combination therapy. Conversely, cancer-associated chronic inflammation is linked with neutrophilia and accompanied by relative lymphocytopenia.21 Additionally, higher blood neutrophil levels are correlated with fatigue severity after therapy,22 and PG2 significantly improves CRF by regulating chronic inflammation, which is a critical pathway in the etiology of CRF.19,23,24 PG2 treatment may have reduced inflammation and consequently, the NLR among patients in the PG2 group, who had CRF as well as elevated NLR before ICI treatment initiation. In contrast, 20% of the patients in the control group, who had no use of PG2, showed increased NLR after treatment initiation.
This study was a retrospective study with small sample size. Although the baseline NLR was higher in the PG2 group, the results were comparable because no significant difference in the demographics was observed between the 2 groups. On the other hand, the NLR change in patients treated with a combination of immunotherapy and varying chemotherapy regimens remains unknown. In this study, patients in the 2 groups had been treated with similar ICI combination chemotherapy regimens; therefore, this does not affect the NLR results. As mentioned earlier, a similar trend was observed between the 2 groups. However, considering the limitations of this study, a more in-depth randomized controlled trial is needed to confirm the efficacy of PG2 in NLR normalization and patient survival in the future.
Conclusions
A high NLR at 6 weeks after ICI treatment initiation was associated with a worse OS. We observed that PG2, which is used clinically to alleviate fatigue, could decrease the prognostic indicator, NLR, among patients with advanced lung cancer treated with ICI combination therapies.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_1534735421995256 for Astragalus Polysaccharide Injection (PG2) Normalizes the Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Lung Cancer Receiving Immunotherapy by Shih Ming Tsao, Tz Chin Wu, JiZhen Chen, Feichi Chang and Thomos Tsao in Integrative Cancer Therapies
Supplemental material, sj-docx-2-ict-10.1177_1534735421995256 for Astragalus Polysaccharide Injection (PG2) Normalizes the Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Lung Cancer Receiving Immunotherapy by Shih Ming Tsao, Tz Chin Wu, JiZhen Chen, Feichi Chang and Thomos Tsao in Integrative Cancer Therapies
Acknowledgments
The authors thank all patients who contributed to this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Thomos Tsao  https://orcid.org/0000-0002-6254-0251
https://orcid.org/0000-0002-6254-0251
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. 2018;10:93-105. [DOI] [PubMed] [Google Scholar]
- 2. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-937. [DOI] [PubMed] [Google Scholar]
- 3. Passiglia F, Galvano A, Castiglia M, et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther Adv Med Oncol. 2019;11:1758835919839928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang T, Bai Y, Zhou F, et al. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer. 2019;130:76-83. [DOI] [PubMed] [Google Scholar]
- 5. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7. [DOI] [PubMed] [Google Scholar]
- 6. Cao D, Xu H, Xu X, Guo T, Ge W. A reliable and feasible way to predict the benefits of Nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology. 2018;7:e1507262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176-181. [DOI] [PubMed] [Google Scholar]
- 9. Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren F, Zhao T, Liu B, Pan L. Neutrophil-lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). Onco Targets Ther. 2019;12:4235-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-230. [DOI] [PubMed] [Google Scholar]
- 13. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu CK, Wu PH, Lai HC. Impressive response of advanced hepatocellular carcinoma to cisplatin combined with sorafenib, nivolumab, and PG2 immunomodulatory injection: a case report. J Clin Case Rep. 2018;8:1161. [Google Scholar]
- 15. He X, Shu J, Xu L, Lu C, Lu A. Inhibitory effect of Astragalus polysaccharides on lipopolysaccharide-induced TNF-a and IL-1beta production in THP-1 cells. Molecules. 2012;17:3155-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng HC, Wang LS. New trends in the therapy of cancer-related fatigue. J Pharm. 2014;121:13-20. [Google Scholar]
- 17. Bamodu OA, Kuo KT, Wang CH, et al. Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients. 2019;11:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilen MA, Martini DJ, Liu Y, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125:127-134. [DOI] [PubMed] [Google Scholar]
- 19. Huang WC, Kuo KT, Bamodu OA, et al. Astragalus polysaccharide (PG2) ameliorates cancer symptom clusters, as well as improves quality of life in patients with metastatic disease, through modulation of the inflammatory cascade. Cancers (Basel). 2019;11:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160-167. [DOI] [PubMed] [Google Scholar]
- 23. Chen HW, Lin IH, Chen YJ, et al. A novel infusible botanically-derived drug, PG2, for cancer-related fatigue: a phase II double-blind, randomized placebo-controlled study. Clin Invest Med. 2012;35:E1-E11. [DOI] [PubMed] [Google Scholar]
- 24. Yeh CT, Wang LS. Potential pathophysiological mechanism of cancer-related fatigue and current management. Formos J Surg. 2014;47:10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_1534735421995256 for Astragalus Polysaccharide Injection (PG2) Normalizes the Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Lung Cancer Receiving Immunotherapy by Shih Ming Tsao, Tz Chin Wu, JiZhen Chen, Feichi Chang and Thomos Tsao in Integrative Cancer Therapies
Supplemental material, sj-docx-2-ict-10.1177_1534735421995256 for Astragalus Polysaccharide Injection (PG2) Normalizes the Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Lung Cancer Receiving Immunotherapy by Shih Ming Tsao, Tz Chin Wu, JiZhen Chen, Feichi Chang and Thomos Tsao in Integrative Cancer Therapies