Abstract
Postoperative recurrence of Crohn’s disease is common and requires a multidisciplinary approach between surgeons and gastroenterologists in the perioperative and postoperative period to improve outcomes in this patient population. Endoscopic recurrence precedes clinical and surgical recurrence and endoscopic monitoring is crucial to guide postoperative management. Risk stratification of patients is recommended to guide early prophylactic management, and follow-up endoscopic monitoring can guide intensification of therapy. This review summarizes evidence behind postoperative recurrence rates, disease monitoring techniques, nonbiologic and biologic therapies available to prevent and treat postoperative recurrence, risk factors associated with recurrence, and postoperative management strategies guided by endoscopic monitoring.
Keywords: anti-tumor necrosis factor, postoperative Crohn’s disease
Introduction
Approximately 50% of Crohn’s disease (CD) patients require ileocolic resection within 10 years of diagnosis for stricturing or penetrating disease in the prebiologic era.1 In the postbiologic era, there are conflicting reports on whether rates of intestinal resection are declining, potentially due to biologic therapy. However, intestinal resections are still cited to be 20–30% and surgical intervention remains commonplace for CD patients.1–5 Intestinal resection does not provide a cure for CD and ongoing multidisciplinary management between gastroenterologists and surgeons is crucial to reduce postoperative recurrence (POR).
POR of CD is common and typically affects the neoterminal ileum and ileocolonic anastomosis. Clinical recurrence, defined as symptoms attributable to active CD, occurs in 30–60% of patients within 3–5 years of index surgery.6 Approximately 50% of postoperative CD patients subsequently require repeat resection for disease activity or complication, termed surgical recurrence, within 5 years of their first surgery.7,8 Preceding both clinical and surgical recurrence, endoscopic recurrence occurs in 70–90% of patients within 1 year of surgery and histologic recurrence can be seen as early as 1 week after surgery.9–11 Rutgeerts’ et al. found that only 20% of patients became symptomatic despite having endoscopically visible disease activity in 73% of patients 1 year after surgery,1 and these findings were reproduced in prospective clinical studies.12 In patients with clinically silent disease, endoscopic surveillance offers the opportunity to guide management of postoperative therapy.
Monitoring of disease activity postoperatively
Ileocolonoscopy is used to visualize mucosal CD activity in the postoperative period. The Rutgeerts’ score was developed to correlate the severity of endoscopic recurrence to progression of clinical recurrence and outcomes (Table 1).11 The inter- and intra-reliability of the Rutgeerts’ score is considered substantial and has allowed the use of the score as an endpoint in clinical trials (Table 2).13 Low-grade mucosal inflammation is defined by endoscopic scores of i0 and i1 correlating to 10% clinical recurrence in 7 years; intermediate endoscopic activity is defined by i2a (ileocolonic anastomotic disease) and i2b (neo-terminal ileum disease) correlating to 40% clinical recurrence; and severe endoscopic recurrence is defined by scores of i3 and i4 correlating to 60–100% clinical recurrence in 2 years.11 Despite the significance of endoscopic disease in predicting clinical outcomes emphasized by the Rutgeerts’ score, it is important to note that, even in patients with endoscopic remission, late clinical recurrence can occur up in up to 40% of patients.14 Therefore, endoscopic monitoring of disease activity should be ongoing irrespective of the lack of endoscopic evidence of disease in the early postoperative period. The addition of advanced endoscopic techniques, such as confocal laser endomicroscopy, may offer enhanced detection of recurrence early in the postoperative period; however, data is limited and remains under investigation.15
Table 1.
Modified Rutgeerts’ score.
| Score | Endoscopic findings |
|---|---|
| i0 | No lesions in distal ileum |
| i1 | <5 Aphthous lesions |
| i2 | >5 Aphthous lesions with normal mucosa between the lesions, skip areas of large lesions |
| • i2a | • Lesions confined to the ileocolonic anastomosis |
| • i2b | • Lesions in the neoterminal ileum with normal intervening mucosa (with or without anastomotic lesions) |
| i3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| i4 | Diffuse inflation with larger ulcers, nodules, and/or narrowing |
Table 2.
Guideline-recommended risk-group classification for POR of CD.
| Risk group | Risk factors | Risk of clinical recurrence (>18 months after surgery) | Risk of endoscopic recurrence (>18 months after surgery) |
|---|---|---|---|
| Low risk | (1) >50 years old | 20% | 30% |
| (2) Nonsmoker | |||
| (3) 1st surgery for short segment disease (<10 cm) | |||
| (4) Disease duration (>10 years) | |||
| High risk | (1) <30 years old | 50% | 80% |
| (2) Smoker | |||
| (3) ⩾2 Prior surgeries |
CD, Crohn’s disease; POR, postoperative recurrence.
While endoscopic surveillance remains the gold standard for monitoring CD POR, there are inherent risks, cost, and patient inconvenience to this modality. Consequently, noninvasive methods of monitoring disease activity postoperatively are highly attractive. Fecal calprotectin, made and released by neutrophils in response to inflammatory signaling, is a marker that has been shown to correlate with Rutgeerts’ scores on endoscopic evaluation of postoperative recurrence.16–19 Levels >100 µg/g correlated with endoscopic recurrence with 89% sensitivity, 58% specificity, and 91% negative predictive value in one prospective analysis.16 Levels <51 µg/g also suggested endoscopic remission with a negative predictive value (NPV) of 79%. Furthermore, levels correlated to endoscopically visualized response when patients’ therapies were escalated.16 The multifaceted use of fecal calprotectin offers a noninvasive method of monitoring postoperative CD activity. Cytokine profiles may supplement fecal calprotectin in more accurately monitoring disease activity.20 MUC1 expression from neoterminal ileal tissue may serve as a possible biomarker for the severity of postoperative CD recurrence.21 Serum measurements of protein/lipid oxidation and total antioxidant capacity correlate to postoperative CD recurrence and may be pathogenic as well.22 Other serum markers of antibacterial antibodies have been shown to be associated with severe postoperative recurrence as well.23 While noninvasive biomarkers have been shown to be useful in monitoring of POR and assessing treatment response, at the current time they remain adjunctive to endoscopic monitoring.
Radiographic methods of noninvasively monitoring POR include small intestine contrast ultrasonography (SICUS), computed tomography (CT) enterography, and magnetic resonance (MR) enterography. SICUS findings of increased bowel wall thickness and altered vascularity have allowed for the detection of POR when compared with endoscopy, with sensitivities and specificities approaching 100%.24–26 CT and MR enterography have been shown to be sensitive and specific methods of identifying POR,27–29 and further offer the opportunity to detect small-bowel and perianal disease. Capsule endoscopy had a sensitivity of 100% in detecting POR and had capsule retention in only 2.1% of patients.27
Nonbiologic therapies for postoperative Crohn’s disease
Given the high rate of endoscopic, clinical, and surgical recurrence after intestinal resection for CD, there is a clear need to identify mitigating and treatment strategies to reduce the disease burden after index surgery. There has been a plethora of nonbiologic therapies trialed to prevent POR of CD. These include antibiotics, immunomodulators, aminosalicylates, budesonide, probiotics, curcumin, and vitamin D supplementation. While some have demonstrated efficacy, others have not.
Thiopurines
Thiopurine monotherapy, including the purine analogs azathioprine (AZA) and 6-mercaptopurine (6-MP), has been shown to be effective in preventing both clinical and endoscopic postoperative recurrence in CD.30,31 A meta-analysis indicated that purine analogs reduced clinical recurrence [mean difference 13%, confidence interval (CI) 1.8–25%, p = 0.025, number needed to treat (NNT) = 7] and endoscopic recurrence (mean difference 25%, CI 9–37%, p = 0.0016, NNT = 4).30 A Cochrane analysis concluded with moderate certainty that azathioprine and 6-MP are superior to placebo [relative risk (RR) 0.79; 95% CI 0.67–0.92] for maintenance of surgically induced remission of CD.32 Another Cochrane analysis identified that azathioprine/6-MP was associated with significantly reduced risk of clinical recurrence (RR 0.59; 95% CI 0.38–0.92, NNT = 7), and severe endoscopic recurrence (RR 0.64; 95% CI 0.44–0.92, NNT = 4), when compared with placebo.33 For treatment of identified endoscopic recurrence, azathioprine (2–2.5 mg/kg/day) has been shown to reduce subsequent Rutgeerts’ scores.34 Thus, thiopurines appear to be effective in reducing recurrence of postoperative CD with modest efficacy.
Antibiotics
With recent emerging data on intestinal microbiome dysbiosis in the colonized neo-terminal ileum affecting POR, it has been postulated that modulation with antibiotics or probiotics may have a role in the management of postoperative CD.35–37 Of these, nitroimidazoles have been well studied and demonstrated benefit. Compared with placebo, metronidazole (20 mg/kg) reduces endoscopic recurrence at 3 months after surgery (13% versus 43%, p = 0.02) and clinical recurrence at 1 year (4% versus 25%, p = 0.04).38 The known side effects of metronidazole prompted a study to evaluate ornidazole, a nitroimidazole with theoretically lower side effects, in the prevention of POR. Ornidazole (1 g/day) compared with placebo reduced endoscopic recurrence at 1 year (OR 0.31, 95% CI 0.10–0.94, p = 0.037,) and clinical recurrence at 1 year (OR 0.14, CI 0.037–0.0546, p = 0.005). However, importantly, a significant portion of patients dropped out of the study due to side effects, primarily neuropathies and dysgeusia.39 These side effects, along with gastrointestinal distress, are encountered commonly in clinical practice and limit the feasibility of this dosing approach. A more recent study evaluated the use of low-dose metronidazole (250 mg three times daily) and achieved reducing endoscopic recurrence compared with placebo (p = 0.0058), but still had 22.9% of patients develop side effects and a discontinuation rate of 8.6%.40 Consequently, a meta-analysis showed that nitroimidazoles were associated with a higher risk of adverse events (RR 2.39, 95% CI 1.5–3.7).41 However, using metronidazole as an adjunct therapy seems to be even more effective in patients who can tolerate therapy in the first 3 months after surgery as a bridge to other prophylactic therapy. For example, metronidazole with azathioprine reduced endoscopic recurrence compared with metronidazole therapy alone (p = 0.048) in a randomized control study.42 However, another study showed that, in patients treated with azathioprine versus azathioprine plus metronidazole, there was no difference in endoscopic recurrence (p = 0.15) at 1 year postoperatively.43 Other antibiotic classes have been investigated with limited success. For example, ciprofloxacin does not appear to be effective in preventing POR.44 Studies are underway to evaluate the role and impact of newer antibiotics such as the nonabsorbable rifaximin in POR. Additionally, several active studies are evaluating the role and impact of novel selective microbial agents in preventing and treating POR.
Probiotics
Probiotics to modulate the microbiome in efforts to prevent POR have been largely unsuccessful. Compared with placebo, Lactobacillus johnsonii LA1 showed similar rates of endoscopic recurrence at 6 months (64% versus 49%, p = 0.15).45 Lactobacillus GG had similar results (p = 0.297).46 Given that single probiotic formulations were ineffective, a probiotic VSL#3 – a formulation of eight different probiotic species – was studied. Endoscopic recurrence was similar in patients treated with VSL#3 compared with placebo (p = 0.19).47 Ongoing studies of the characterization and manipulation of the neoterminal ileum and anastomotic microbiome are being conducted.
Corticosteroids
Budesonide is ineffective compared with placebo in reducing endoscopic and clinical recurrence in those with surgery for fibrostenotic disease, but may have some effectiveness for those patients undergoing surgery for inflammatory activity.48,49 There is limited data on the use of systemic steroids to prevent postoperative recurrence.50 Despite the lack of evidence of corticosteroids in the postoperative period, one study found that one-third of patients in a United States (US) national claims database received postoperative corticosteroids (systemic or enteric).51
Aminosalicylates
Given the early data for mesalamine in managing CD and relatively favorably safety profile, there has been interest in utilizing aminosalicylates to prevent POR. Unfortunately, mesalamine was shown to be ineffective in reducing clinical (RR 0.76, 95% CI0.62–0.94) or endoscopic (RR 0.50, 95% CI 0.29–0.84) recurrence,33 a finding supported in subsequently meta-analysis including aminosalicylates and sulfasalazine.52 For treatment of POR, studies have demonstrated variable efficacy for aminosalicylates. Mesalamine was shown to improve in Rutgeerts’ scores in a minority (11/32, 34%) of patients, with significantly lower rates of improvement compared with azathioprine (19/30, 63.3%), but was better tolerated.34 Together, these data suggest that mesalamine is minimally effective for preventing or treating endoscopic POR and that alternative therapies should be considered.
Complementary medications, vitamins, and supplements
Turmeric and its chief active ingredient, curcumin, have long been recognized for anti-inflammatory properties. Curcumin was evaluated by a prospective, double-blind, placebo-controlled trial evaluating AZA with or without 3 g curcumin or placebo daily. At 6 months, endoscopic POR rates were similar (58% AZA + curcumin versus 68% AZA + placebo, p = 0.60) and severe POR was significantly more common in AZA + curcumin compared with AZA + placebo (55% versus 26%, p = 0.03).53 No significant differences in adverse events were seen, but the study was discontinued after interim analysis due to futility.
Similarly, vitamin D has received great interest for its immune modifying capabilities and vitamin D deficiency is common in CD. High-dose vitamin D (25,000 IU oral weekly dosing) was evaluated in a prospective randomized trial versus placebo. Despite significant increases in serum 25-hydroxy vitamin D, there were no significant differences in endoscopic (p = 0.37) or clinical recurrence (p = 0.91) at 6 months,54 diminishing hopes for vitamin D supplementation to prevent POR.
Biologic therapies for postoperative CD recurrence
Anti-tumor necrosis factor agents
Infliximab
Compared with nonbiologic agents, anti-tumor necrosis factor (TNF) therapy, thus far, appears to be the most effective treatment in preventing POR. The first pilot trial that examined infliximab (IFX) (5 mg/kg every 8 weeks for 200 weeks) versus placebo in 24 subjects following ileocecal resection showed efficacy in reducing endoscopic (p = 0.006) and histologic recurrence (p = 0.01) at 1 year, but not clinical recurrence (p = 0.38).55 These findings were replicated in similar studies.56 The benefit of IFX persists out to 5 years as well when compared with placebo in preventing recurrence (p < 0.001).57 In a small pilot study, compared with thiopurines, IFX was shown to reduce histologic recurrence (p = 0.008), but not endoscopic or clinical recurrence.58 A major limitation to these investigations was the relatively small cohorts studied.
The largest study to date evaluating the efficacy of anti-TNFs to prevent POR, the PREVENT trial, evaluated the efficacy of IFX in patients at high risk for recurrence. This study was a double-blind, placebo-controlled trial comparing infliximab 5 mg/kg every 8 weeks without a 3-dose induction to placebo in adult patients with ileocolonic resection and anastomosis. Inclusion criteria for patients were a baseline CD activity index (CDAI) score of <200 and a high-risk feature defined as having at least one of the following: qualifying surgery that was the patient’s second resection within 10 years, third or more resection, resection for penetration CD, perianal disease, or active smoking status. The primary outcome was clinical recurrence defined as a CDAI score ⩾200 and a 70 point or more increase from baseline, and the secondary outcome was endoscopic recurrence defined as a Rutgeerts’ score of ⩾i2 at 18 months. Overall endoscopic recurrence (Rutgeerts’ ⩾i2) was reduced significantly in the IFX group compared with the placebo group (22.4% versus 51.3%, p < 0.001 respectively), rates similar to prior IFX studies mentioned previously. Furthermore, severe recurrence (Rutgeerts’ i3 or i4) was decreased dramatically from 71.6% in placebo arm to 16.9% in IFX. However, the primary endpoint of clinical recurrence at 76 weeks postoperatively was not met (20.0% placebo versus 12.9% IFX, p = 0.097).59 For treatment of identified endoscopic POR, infliximab initiation reduces endoscopic inflammation in the majority of patients and prevented future clinical recurrence compared with either azathioprine or mesalamine (p = 0.006).60 Thus, infliximab is quite efficacious in preventing and treating POR.
Adalimumab
Anti-TNF’s reduction in endoscopic POR appears to be a class effect as studies found similar results from adalimumab and infliximab.61,62 In a large randomized controlled trial, adalimumab reduced endoscopic recurrence when compared with azathioprine (OR = 0.036, 95% CI 0.004–0.347) and to mesalamine (OR = 0.013, 95% CI 0.001–0.143). Additionally, adalimumab was found to reduce clinical recurrence compared with azathioprine (OR = 0.078, 95% CI 0.013–0.464) and mesalamine (OR = 0.143, 95% CI 0.025–0.819).63 However, one phase III, multicenter randomized superiority study contradicted this when comparing adalimumab to azathioprine coupled with metronidazole. The findings of the study revealed similar rates of endoscopic recurrence in the adalimumab group versus azathioprine group (33.3% versus 29.7%, p = 0.76), but the study showed significantly better tolerance to adalimumab over azathioprine.64
The use of anti-TNFs postoperatively is safe and has similar adverse events compared with placebo and nonbiologic agents. In a meta-analysis comparing IFX with nonbiologic agents, there was no significant difference in adverse events (p = 0.69).65 Even when comparing IFX with placebo, there appears to be no difference in adverse events.66 In the largest randomized control trial comparing adalimumab with azathioprine and mesalamine, there were fewer adverse events reported in the adalimumab group.63 While limited-to-no data exist for other anti-TNFs in this setting, overall, anti-TNFs as a class appear to be safe and very effective in the management and prevention of postoperative recurrence of CD.
Non-anti-TNF biologics
Vedolizumab
Given the efficacy of anti-TNFs, the increasing portion of the CD population exposed to anti-TNFs prior to surgery, and relative safety profiles, there has been interest in other non-anti-TNF biologics in the prevention of POR. One retrospective study evaluating 22 CD patients that received vedolizumab for postoperative prophylaxis compared with 58 who received anti-TNF found that vedolizumab was associated with increased risk of endoscopic recurrence (75% vedolizumab versus 34.2% anti-TNF; OR 5.77; 95% CI 1.71–19.4, p = 0.005).67 However, there were a multitude of confounding factors and limitations to this analysis including key population differences.68 There is limited data assessing the efficacy of vedolizumab in treating POR.
Ustekinumab
A recent study, presented in abstract form, compared ustekinumab-treated postoperative patients with a cohort of azathioprine-treated subjects as part of the previously mentioned azathioprine with or without curcumin study.69 In a propensity-matched analysis, endoscopic POR at 6 months was significantly lower in ustekinumab compared with azathioprine (28% versus 54.5%, p = 0.03); however, this was driven largely by moderate (Rutgeerts’ i2) disease as no significance difference was observed when limited to Rutgeerts’ ⩾i3 (16.9% versus 27.9%, p = 0.24). The 6-month endoscopic POR rates for ustekinumab are similar to anti-TNFs, which may suggest comparable efficacy in this setting, but additional studies are needed.69 There are limited data assessing the efficacy of ustekinumab in treating POR.
Enteral nutrition for postoperative Crohn’s disease
Enteral nutrition in the prevention of postoperative Crohn’s disease has also been evaluated in several small studies. One trial of 40 Japanese patients all receiving mesalamine in the postoperative period assessed nocturnal self-intubation and infusion of elemental enteral feeding, and found that high-volume enteral nutrition (>1200 kcal/day) significantly reduces postoperative endoscopic recurrence compared with low- or no-volume enteral nutrition (<1200 kcal/day) (p = 0.02).70 A similar nonrandomized study of 40 Japanese patients found that enteral nutrition significantly reduces endoscopic recurrence at 12 months compared with no therapy (30% versus 70%, p = 0.027).71 In regards to surgical recurrence, another study found that enteral nutrition compared with placebo reduced recurrence but without statistical significance (p = 0.08). The placebo group in this study had a significantly higher cumulative recurrence rate requiring infliximab (p = 0.03), suggesting that enteral nutrition may play a role in supplementing or replacing pharmacologic prophylaxis.72 Limitations to these studies include small and highly motivated adult populations willing to self-intubate nasogastric apparatuses nightly and infuse enteric formulas for indefinite time periods, thus limiting generalizability. Future large randomized control trials assessing enteral nutrition as a nonpharmacologic therapy are necessary to determine its role in preventing and treating postoperative CD recurrence.
Therapeutic drug monitoring
Given the effectiveness of anti-TNFs in the maintenance of endoscopic remission, studies have assessed the role of drug-monitoring to optimize therapy in postsurgical patients. A retrospective analysis found that lower serum trough infliximab levels at 15 months after surgery and 8 months from treatment onset, [2.4 µg/ml (0.45–4.1) versus 1.1 µg/ml (0–0.6), p = 0.008] and the presence of antidrug antibodies were associated significantly with endoscopic recurrence.73 Another study assessing adalimumab found that drug levels did not differ significantly between patients in endoscopic remission versus recurrence, with average serum concentrations in both cohorts in the therapeutic range (9.98 µg/ml versus 8.43 µg/ml, p = 0.39).74 It is the authors’ opinion that, in patients who have had resection of all gross disease and are started on anti-TNF prophylaxis in a timely manner (within 2–4 weeks surgery and anastomosis), the role of therapeutic drug monitoring may be more limited due to the lack of drug clearance from inflammatory burden consumption. Periodic proactive dosing optimization in maintenance, akin to the TAXIT trial, may optimize long-term outcomes, but no data exist in this setting.75 Similarly, reactive drug-monitoring may have an important role when determining anti-TNF ineffectiveness in the postoperative management of CD, but further studies are required. No data yet exists on therapeutic drug monitoring for non-anti-TNF agents in the postoperative setting.
Risk factors and stratification for postoperative recurrence
Risk factors for POR have been assessed extensively in attempts to identify patients at highest risk of recurrence and thus may gleam most benefit from prophylactic or close monitoring strategies. Categories of risk factors studied include modifiable and nonmodifiable patient related risk factors (Table 3).
Table 3.
Risk factors for POR of CD.
| Risk factors |
| • Age • Gender • IBD family history • Smoking • Disease-related risks (duration prior to first surgery, location, behavior, perianal disease) • Disease-treatment modifiers (perioperative steroid use, anti-inflammatory use) • Surgical risk factors (anastomosis, margins of resection, laparoscopic versus open, strictureplasty) • Postoperative complications • Histology (myenteric plexitis, granulomas, lymphatic vessel density, and transmural activity) • Genetics • Microbiome |
CD, Crohn’s disease; IBD, inflammatory bowel disease; POR, postoperative recurrence.
The AGA guideline on the management of POR CD outlines significant risk factors to be younger age (<30), smoking, and ⩾2 prior surgeries for penetrating disease (Table 2).2,76–79 These high-risk factors have proposed clinical and endoscopic recurrence rates of 50% and 80%, respectively.3 Other society guidelines such as the European Crohn’s and Colitis Organization (ECCO) inflammatory bowel disease (IBD) guidelines incorporate additional risk factors, including extensive small bowel resection (>50 cm), perianal disease, and histologic evidence of granulomas or myenteric plexitis on resected specimens.80 In the few prospective, randomized, clinical trials assessing POR management strategies, risk factors included smoking, perforating disease (abscess, fistula, or free perforation), or previous resection.57,59,81 There is an ongoing need to validate the proposed risk factors and risk classification recommended by guidelines.
More recent risk factor data in predicting and influencing POR include novel anastomotic techniques, histologic characteristics, microbiome signatures, and other “-omic” approaches. The Kono-S anastomosis (antimesenteric functional end-to-end anastomosis) demonstrates a promising surgical approach that has been shown in retrospective and prospective studies to reduce both endoscopic and clinical recurrence.82,83 Similarly, a wide mesenteric excision approach was evaluated in a retrospective study and shown to associate with reduced surgical recurrence.84 Prospective studies evaluating this approach are underway. Histologic risk factors, including positive margins of resection, plexitis, lymphatic vessel density, and morphologic analysis of Paneth cells, may predict POR.85,86 Finally, microbiome dysbiosis is being recognized as a risk factor with recolonization of microbiota, including Proteobacteria, Akkermansia spp., Fusobacteriaceae and a depletion of Streptococcaceae, Actinomycineae, Faecalibacterium.35–37 Interestingly, active smoking was associated with elevated levels of Proteus,37 and thus these risk factors may be interactive. The role of other “-omics”, including ileal tissue transcriptomics, blood transcriptomics, and urinary metabolomics, is being evaluated.87,88 These emerging risk factors will need additional study and validation prior to routine implementation.
Postoperative management strategies
Ultimately, the goal of postoperative management is to identify patients at highest risk for recurrence and implement strategies to minimize clinical and surgical recurrence and CD-related complications. The 2017 American Gastroenterological Association guidelines for postoperative Crohn’s management recommend using preoperative risk factor stratification to determine POR strategy, with the decision to utilize early and sustained therapy with anti-inflammatory monoclonal antibodies for those who are high risk (termed prophylaxis) or performing endoscopic disease monitoring to guide treatment in those who are low risk. These risk groups are identified in Table 2.2 The role of ileocolonoscopy is to detect endoscopic recurrence that may precede clinical or surgical recurrence.
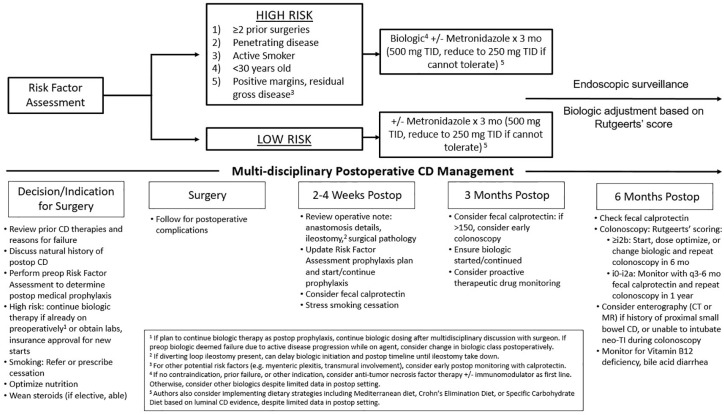
Active endoscopic surveillance of disease modifies treatment strategy and reduces endoscopic recurrence postoperatively (Figure 1). The landmark POCER trial showed that algorithmic step up treatment for endoscopic recurrence was superior to clinical observation up until 18 months postoperatively. In the POCER trial, patients were randomized to either an active or standard care arm. The active arm had patients undergo endoscopic surveillance at 6 months, which allowed for the opportunity to step up therapy if endoscopic recurrence (Rutgeerts’ i⩾2) was present. The standard arm had patients undergo endoscopic surveillance at 18 months. The initial treatment within each arm was dependent on whether patients were low (nonsmoker, first surgery, absence of penetrating disease) or high (smoking, penetrating disease, or previous resection) risk. Low-risk patients received metronidazole for 3 months postoperatively if tolerated, while high risk patients received azathioprine or 6-MP for 18 months. If patients were intolerant, they received adalimumab for 18 months. If the 6 months colonoscopy demonstrated endoscopic recurrence in the active arm, then medical therapy was escalated. At 18 months, the patients in the active arm had significantly lower endoscopic recurrence (p = 0.03) compared with those in the standard arm.81 In a POCER subanalysis, adalimumab was superior to thiopurines in preventing endoscopic recurrence (p = 0.02), in line with prior studies.89 Based on these findings, a 6-month colonoscopy can guide intensification or altering of treatment regardless of the medical strategy chosen. While early endoscopic assessment is critical in detecting early CD recurrence, ongoing surveillance is needed. Poullon and colleagues found that up to 40% of patients have late recurrence despite initial endoscopic remission.14
Figure 1.
Proposed management strategies for prevention of POR of CD.
CD, Crohn’s disease; POR, postoperative recurrence.
While remaining the gold standard, endoscopic surveillance is invasive, costly, and with inherent risks. Thus, as previously mentioned, there is a growing body of evidence evaluating noninvasive biomarkers for POR including fecal calprotectin, serum derived biomarker profiles, and radiographic studies, including small bowel ultrasound and cross-sectional imaging. Currently, it is the authors’ opinion that these tests can serve an adjunctive monitoring role and help guide timing and frequency of endoscopic evaluation, but do not yet have the body of evidence to supplant endoscopic surveillance. Prospective studies implementing these biomarkers into treatment algorithms are needed.
The future of POR of CD
Despite significant advancements in understanding the natural history, pathogenesis, risk factors, and mitigation strategies for POR, there remain many unanswered questions and avenues for investigation. These include personalized risk factor assessment and prediction, evaluating risk factor interaction, prospective validation of microbiome and other “-omic” risk factors, integration of the various clinical and molecular risk profiles to guide care, understanding the impact of newer approved therapies on preventing POR, identifying the optimal timing of prophylaxis initiation, the ideal biologic strategy in biologic-exposed individuals, evaluation of novel therapies such as selective antimicrobials to prevent POR, combining therapeutic strategies, innovating technologies to monitor postoperative disease, and implementing biomarker and noninvasive surveillance into monitoring algorithms to name a few. The evidence-based preventative and therapeutic options are outlined in Table 4 and Figure 1. Postoperative recurrence remains a frequent clinical dilemma and much work remains.
Table 4.
Efficacy of various therapies and knowledge gaps for the prevention and treatment of POR.
| Medication | POR prevention | Treatment of POR |
|---|---|---|
| Curcumin | –53 | ? |
| Enteral nutrition | +70–72 | ? |
| Nitroimidazole/antibiotics | +38,39 | – |
| Mesalamine | –31,63 | –34 |
| Budesonide | –48,49 | ?a |
| Thiopurines | +31,41,42 | +34 |
| Anti-TNF | +++55–59,62–64 | +++60 |
| Vedolizumab | ++?68 | ? |
| Ustekinumab | ++?69 | ? |
Authors opinion. Budesonide may be used for short term induction therapy, but similar to luminal ileal CD, is not likely effective for long-term therapy.
CD, Crohn’s disease; POR, postoperative recurrence; TNF, tumor necrosis factor.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ravi S. Shah  https://orcid.org/0000-0002-5001-860X
https://orcid.org/0000-0002-5001-860X
Contributor Information
Ravi S. Shah, Cleveland Clinic - Internal Medicine, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Benjamin H. Click, Cleveland Clinic - Gastroenterology, Hepatology, and Nutrition, Cleveland, OH, USA
References
- 1. Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalisations and surgery in Crohn’s disease. Gut 2012; 61: 622–629. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011; 141: 90–97. [DOI] [PubMed] [Google Scholar]
- 3. Vind I, Riis L, Jess T, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen city and county, 2003–2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol 2006; 101: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 4. Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut 2010; 59: 1200–1206. [DOI] [PubMed] [Google Scholar]
- 5. Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut 2020; 69: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut 1992; 33: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baldassano RN, Han PD, Jeshion WC, et al. Pediatric Crohn’s disease: risk factors for postoperative recurrence. Am J Gastroenterol 2001; 96: 2169–2176. [DOI] [PubMed] [Google Scholar]
- 8. Bobanga ID, Bai S, Swanson MA, et al. Factors influencing disease recurrence after ileocolic resection in adult and pediatric onset Crohn’s disease. Am J Surg 2014; 208: 591–596. [DOI] [PubMed] [Google Scholar]
- 9. D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998; 114: 262–267. [DOI] [PubMed] [Google Scholar]
- 10. Hirten RP, Mashiana S, Cohen BL, et al. Ileocolic anastomotic inflammation after resection for Crohn’s disease indicates disease recurrence: a histopathologic study. Scand J Gastroenterol 2020; 55: 795–799. [DOI] [PubMed] [Google Scholar]
- 11. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 12. Regueiro M, Kip KE, Schraut W, et al. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis 2011; 17: 118–126. [DOI] [PubMed] [Google Scholar]
- 13. Ma C, Gecse KB, Duijvestein M, et al. Reliability of endoscopic evaluation of postoperative recurrent Crohn’s disease. Clin Gastroenterol Hepatol 2020; 18: 2139–2141e2. [DOI] [PubMed] [Google Scholar]
- 14. Pouillon L, Remen T, Amicone C, et al. Risk of late postoperative recurrence of Crohn’s disease in patients in endoscopic remission after ileocecal resection, over 10 years at multiple centers. Clin Gastroenterol Hepatol. Epub ahead of print 20 May 2020. DOI: 10.1016/j.cgh.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 15. Auzoux J, Boschetti G, Anon B, et al. Usefulness of confocal laser endomicroscopy for predicting postoperative recurrence in patients with Crohn’s disease: a pilot study. Gastrointest Endosc 2019; 90: 151–157. [DOI] [PubMed] [Google Scholar]
- 16. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015; 148: 938–947e1. [DOI] [PubMed] [Google Scholar]
- 17. Qiu Y, Mao R, Chen B, et al. Fecal calprotectin for evaluating postoperative recurrence of Crohn’s disease: a meta-analysis of prospective studies. Inflamm Bowel Dis 2015; 21: 315–322. [DOI] [PubMed] [Google Scholar]
- 18. Lobatón T, López-García A, Rodríguez-Moranta F, et al. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis 2013; 7: e641–e651. [DOI] [PubMed] [Google Scholar]
- 19. Boschetti G, Laidet M, Moussata D, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol 2015; 110: 865–872. [DOI] [PubMed] [Google Scholar]
- 20. Cerrillo E, Moret I, Iborra M, et al. A nomogram combining fecal calprotectin levels and plasma cytokine profiles for individual prediction of postoperative Crohn’s disease recurrence. Inflamm Bowel Dis 2019; 25: 1681–1691. [DOI] [PubMed] [Google Scholar]
- 21. Hashash JG, Beatty PL, Critelli K, et al. Altered expression of the epithelial mucin MUC1 accompanies endoscopic recurrence of postoperative Crohn’s disease. J Clin Gastroenterol 2021; 55: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luceri C, Bigagli E, Agostiniani S, et al. Analysis of oxidative stress-related markers in Crohn’s disease patients at surgery and correlations with clinical findings. Antioxidants (Basel) 2019; 8: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton AL, Kamm MA, De Cruz P, et al. Serologic antibodies in relation to outcome in postoperative Crohn’s disease. J Gastroenterol Hepatol 2017; 32: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 24. Rigazio C, Ercole E, Laudi C, et al. Abdominal bowel ultrasound can predict the risk of surgery in Crohn’s disease: proposal of an ultrasonographic score. Scand J Gastroenterol 2009; 44: 585–593. [DOI] [PubMed] [Google Scholar]
- 25. Calabrese E, Petruzziello C, Onali S, et al. Severity of postoperative recurrence in Crohn’s disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009; 15: 1635–1642. [DOI] [PubMed] [Google Scholar]
- 26. Paredes JM, Ripollés T, Cortés X, et al. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohns Colitis 2013; 7: 192–201. [DOI] [PubMed] [Google Scholar]
- 27. Yung DE, Har-Noy O, Tham YS, et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn’s disease: systematic review and meta-analysis. Inflamm Bowel Dis 2017; 24: 93–100. [DOI] [PubMed] [Google Scholar]
- 28. Mao R, Gao X, Zhu Z, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis 2013; 19: 977–982. [DOI] [PubMed] [Google Scholar]
- 29. Choi IY, Park SH, Park SH, et al. CT enterography for surveillance of anastomotic recurrence within 12 months of bowel resection in patients with Crohn’s disease: an observational study using an 8-year registry. Korean J Radiol 2017; 18: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peyrin-Biroulet L, Deltenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn’s disease: a meta-analysis. Am J Gastroenterol 2009; 104: 2089–2096. [DOI] [PubMed] [Google Scholar]
- 31. Hanauer SB, Korelitz BI, Rutgeerts P, et al. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology 2004; 127: 723–729. [DOI] [PubMed] [Google Scholar]
- 32. Gjuladin-Hellon T, Iheozor-Ejiofor Z, Gordon M, et al. Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn’s disease. Cochrane Database Syst Rev 2019; 8: CD010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doherty G, Bennett G, Patil S, et al. Interventions for prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst Rev 2009; 4: CD006873. [DOI] [PubMed] [Google Scholar]
- 34. Reinisch W, Angelberger S, Petritsch W, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut 2010; 59: 752–759. [DOI] [PubMed] [Google Scholar]
- 35. Machiels K, Pozuelo del Río M, Martinez-De la Torre A, et al. Early postoperative endoscopic recurrence in Crohn’s disease is characterised by distinct microbiota recolonisation. J Crohns Colitis 2020; 14: 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sokol H, Brot L, Stefanescu C, et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut 2020; 69: 462–472. [DOI] [PubMed] [Google Scholar]
- 37. Wright EK, Kamm MA, Wagner J, et al. Microbial factors associated with postoperative Crohn’s disease recurrence. J Crohns Colitis 2017; 11: 191–203. [DOI] [PubMed] [Google Scholar]
- 38. Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 1995; 108: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 39. Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2005; 128: 856–861. [DOI] [PubMed] [Google Scholar]
- 40. Glick LR, Sossenheimer PH, Ollech JE, et al. Low-dose metronidazole is associated with a decreased rate of endoscopic recurrence of Crohn’s disease after ileal resection: a retrospective cohort study. J Crohns Colitis 2019; 13: 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doherty GA, Bennett GC, Cheifetz AS, et al. Meta-analysis: targeting the intestinal microbiota in prophylaxis for post-operative Crohn’s disease. Aliment Pharmacol Ther 2010; 31: 802–809. [DOI] [PubMed] [Google Scholar]
- 42. D’Haens GR, Vermeire S, Van Assche G, et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008; 135: 1123–1129. [DOI] [PubMed] [Google Scholar]
- 43. Mañosa M, Cabré E, Bernal I, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Inflamm Bowel Dis 2013; 19: 1889–1895. [DOI] [PubMed] [Google Scholar]
- 44. Herfarth HH, Katz JA, Hanauer SB, et al. Ciprofloxacin for the prevention of postoperative recurrence in patients with Crohn’s disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2013; 19: 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marteau P, Lémann M, Seksik P, et al. Ineffectiveness of lactobacillus Johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut 2006; 55: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prantera C, Scribano ML, Falasco G, et al. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with lactobacillus GG. Gut 2002; 51: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fedorak RN, Feagan BG, Hotte N, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 928–935.e2. [DOI] [PubMed] [Google Scholar]
- 48. Hellers G, Cortot A, Jewell D, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn’s disease. The IOIBD Budesonide study group. Gastroenterology 1999; 116: 294–300. [DOI] [PubMed] [Google Scholar]
- 49. Ewe K, Böttger T, Buhr HJ, et al. Low-dose budesonide treatment for prevention of postoperative recurrence of Crohn’s disease: a multicentre randomized placebo-controlled trial. German Budesonide study group. Eur J Gastroenterol Hepatol 1999; 11: 277–282. [DOI] [PubMed] [Google Scholar]
- 50. Summers RW, Switz DM, Sessions JT, Jr, et al. National cooperative Crohn’s disease study: results of drug treatment. Gastroenterology 1979; 77: 847–869. [PubMed] [Google Scholar]
- 51. Ong M-S, Grand RJ, Mandl KD. Trends in pharmacologic interventions for preventing recurrence of Crohn’s disease after ileocolonic surgery. Inflamm Bowel Dis 2016; 22: 2432–2441. [DOI] [PubMed] [Google Scholar]
- 52. Ford AC, Khan KJ, Talley NJ, et al. 5-aminosalicylates prevent relapse of Crohn’s disease after surgically induced remission: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 413–420. [DOI] [PubMed] [Google Scholar]
- 53. Bommelaer G, Laharie D, Nancey S, et al. Oral curcumin no more effective than placebo in preventing recurrence of Crohn’s disease after surgery in a randomized controlled trial. Clin Gastroenterol Hepatol 2020; 18: 1553–1560.e1. [DOI] [PubMed] [Google Scholar]
- 54. de Bruyn JR, Bossuyt P, Ferrante M, et al. High-dose vitamin d does not prevent postoperative recurrence of Crohn’s disease in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. Epub ahead of print 24 May 2020. DOI: 10.1016/j.cgh.2020.05.037. [DOI] [PubMed] [Google Scholar]
- 55. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009; 136: 441–450.e1; quiz 716. [DOI] [PubMed] [Google Scholar]
- 56. Yoshida K, Fukunaga K, Ikeuchi H, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis 2012; 18: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 57. Regueiro M, Kip KE, Baidoo L, et al. Postoperative therapy with infliximab prevents long-term Crohn’s disease recurrence. Clin Gastroenterol Hepatol 2014; 12: 1494–1502.e1. [DOI] [PubMed] [Google Scholar]
- 58. Armuzzi A, Felice C, Papa A, et al. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn’s disease: an open-label pilot study. J Crohns Colitis 2013; 7: e623–e629. [DOI] [PubMed] [Google Scholar]
- 59. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016; 150: 1568–1578. [DOI] [PubMed] [Google Scholar]
- 60. Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn’s disease: a prospective pilot study. Inflamm Bowel Dis 2009; 15: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 61. Kotze PG, Yamamoto T, Danese S, et al. Direct retrospective comparison of adalimumab and infliximab in preventing early postoperative endoscopic recurrence after ileocaecal resection for Crohn’s disease: results from the MULTIPER database. J Crohns Colitis 2015; 9: 541–547. [DOI] [PubMed] [Google Scholar]
- 62. Papamichael K, Archavlis E, Lariou C, et al. Adalimumab for the prevention and/or treatment of post-operative recurrence of Crohns disease: a prospective, two-year, single center, pilot study. J Crohns Colitis 2012; 6: 924–931. [DOI] [PubMed] [Google Scholar]
- 63. Savarino E, Bodini G, Dulbecco P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: a randomized controlled trial. Am J Gastroenterol 2013; 108: 1731–1742. [DOI] [PubMed] [Google Scholar]
- 64. López-Sanromán A, Vera-Mendoza I, Domènech E, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomised trial. J Crohns Colitis 2017; 11: 1293–1301. [DOI] [PubMed] [Google Scholar]
- 65. Qiu Y, Mao R, Chen B, et al. Systematic review with meta-analysis of prospective studies: anti-tumour necrosis factor for prevention of postoperative Crohn’s disease recurrence. J Crohns Colitis 2015; 9: 918–927. [DOI] [PubMed] [Google Scholar]
- 66. Regueiro M, El-Hachem S, Kip KE, et al. Postoperative infliximab is not associated with an increase in adverse events in Crohn’s disease. Dig Dis Sci 2011; 56: 3610—3615. [DOI] [PubMed] [Google Scholar]
- 67. Yamada A, Komaki Y, Patel N, et al. The use of vedolizumab in preventing postoperative recurrence of Crohn’s disease. Inflamm Bowel Dis 2018; 24: 502–509. [DOI] [PubMed] [Google Scholar]
- 68. Regueiro MD. It’s too soon to count out vedo for postoperative Crohn’s disease. Inflamm Bowel Dis 2018; 24: 296–297. [DOI] [PubMed] [Google Scholar]
- 69. Buisson A, Nancey S, Manlay L, et al. Ustekinumab is more effective than azathioprine to prevent endoscopic postoperative recurrence in Crohn’s disease. United Eur Gastroenterol J 2020; 8(Suppl. 8): 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Esaki M, Matsumoto T, Hizawa K, et al. Preventive effect of nutritional therapy against postoperative recurrence of Crohn disease, with reference to findings determined by intra-operative enteroscopy. Scand J Gastroenterol 2005; 40: 1431–1437. [DOI] [PubMed] [Google Scholar]
- 71. Yamamoto T, Nakahigashi M, Umegae S, et al. Impact of long-term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn’s disease: a prospective, non-randomized, parallel, controlled study. Aliment Pharmacol Ther 2007; 25: 67–72. [DOI] [PubMed] [Google Scholar]
- 72. Yamamoto T, Shiraki M, Nakahigashi M, et al. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: a five-year prospective cohort study. Int J Colorectal Dis 2013; 28: 335–340. [DOI] [PubMed] [Google Scholar]
- 73. Fay S, Ungar B, Paul S, et al. The association between drug levels and endoscopic recurrence in postoperative patients with Crohn’s disease treated with tumor necrosis factor inhibitors. Inflamm Bowel Dis 2017; 23: 1924–1929. [DOI] [PubMed] [Google Scholar]
- 74. Wright EK, Kamm MA, De Cruz P, et al. Anti-TNF therapeutic drug monitoring in postoperative Crohn’s disease. J Crohns Colitis 2018; 12: 653–661. [DOI] [PubMed] [Google Scholar]
- 75. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–1329.e3. [DOI] [PubMed] [Google Scholar]
- 76. Blonski W, Buchner AM, Lichtenstein GR. Clinical predictors of aggressive/disabling disease: ulcerative colitis and Crohn disease. Gastroenterol Clin North Am 2012; 41: 443–462. [DOI] [PubMed] [Google Scholar]
- 77. Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005; 11: 3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol 2008; 103: 196–205. [DOI] [PubMed] [Google Scholar]
- 79. Pascua M, Su C, Lewis JD, et al. Meta-analysis: factors predicting post-operative recurrence with placebo therapy in patients with Crohn’s disease. Aliment Pharmacol Ther 2008; 28: 545–556. [DOI] [PubMed] [Google Scholar]
- 80. Bemelman WA, Warusavitarne J, Sampietro GM, et al. ECCO-ESCP consensus on surgery for Crohn’s disease. J Crohns Colitis 2018; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 81. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015; 385: 1406–1417. [DOI] [PubMed] [Google Scholar]
- 82. Kono T, Ashida T, Ebisawa Y, et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn’s disease. Dis Colon Rectum 201; 54: 586–592. [DOI] [PubMed] [Google Scholar]
- 83. Luglio G, Rispo A, Imperatore N, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD study - a randomized clinical trial. Ann Surg 2020; 272: 210–217. [DOI] [PubMed] [Google Scholar]
- 84. Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis 2018; 12: 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bressenot A, Peyrin-Biroulet L. Histologic features predicting postoperative Crohn’s disease recurrence. Inflamm Bowel Dis 2015; 21: 468–475. [DOI] [PubMed] [Google Scholar]
- 86. Poredska K, Kunovsky L, Marek F, et al. The influence of microscopic inflammation at resection margins on early postoperative endoscopic recurrence after ileocaecal resection for Crohn’s disease. J Crohns Colitis 2020; 14: 361–368. [DOI] [PubMed] [Google Scholar]
- 87. Keshteli AH, Tso R, Dieleman LA, et al. A distinctive urinary metabolomic fingerprint is linked with endoscopic postoperative disease recurrence in Crohn’s disease patients. Inflamm Bowel Dis 2018; 24: 861–870. [DOI] [PubMed] [Google Scholar]
- 88. Borren NZ, Plichta D, Joshi AD, et al. Multi-“-Omics” profiling in patients with quiescent inflammatory bowel disease identifies biomarkers predicting relapse. Inflamm Bowel Dis 2020; 26: 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Cruz P, Kamm MA, Hamilton AL, et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients - a POCER study analysis. Aliment Pharmacol Ther 2015; 42: 867–879. [DOI] [PubMed] [Google Scholar]