Abstract
Background:
Thyrotoxicosis is a clinical syndrome with high amounts of free thyroid hormone levels causing elevated thyroid hormone function in body tissues. Prolonged effects of free thyroid hormones may lead to cardiac complications such as atrial fibrillation (AF) and heart failure (HF).
Case 1:
A 31-year-old female, was admitted due to difficulty in breathing, generalised body swelling and jaundice. She was dyspnoeic with an irregular heart rate, and presented with abnormal vitals, liver and thyroid function tests which were diagnostic for thyroid storm. She was managed over 32 days in-hospital stay with carbimazole, propranolol, hydrocortisone, digoxin and furosemide. Unfortunately, she was readmitted 6 months later with worsened HF symptoms and passed away.
Case 2:
A 57-year-old female, was admitted due to difficulty in breathing, bilateral lower limb swelling and jaundice. She was tachypnoeic with an irregular heart rate, and presented with abnormal liver enzymes and thyroid function tests which were diagnostic for thyrotoxicosis. She was managed with carbimazole, propranolol, digoxin and furosemide, and was discharged on the 6th hospital day.
Conclusion:
Prolonged untreated thyrotoxicosis increases the risk of AF and HF. Early and monitored treatment and follow-up of hyperthyroidism is key to the management of AF and HF in achieving a better outcome.
Keywords: Thyrotoxicosis, hyperthyroidism, heart failure, atrial fibrillation
Introduction
Thyrotoxicosis affects many organ systems including bone, skin, gastrointestinal, nervous and cardiovascular system (CVS). The CVS complications are the most common and life-threatening causing hospitalisation and death.1 These manifestations are characterised by inappropriate high thyroid hormone function in tissues due to a high amount of free thyroid hormone levels.2
Due to the high levels of circulating thyroid hormone, there is an increase in blood volume, improved diastolic function and triiodothyronine (T3) relaxation effect on the peripheral vessels.3,4 This leads to an increase in cardiac preload and reduced peripheral vascular resistance. Furthermore, the prolonged effect of the high thyroid hormone levels may lead to an increase in left ventricular mass, arterial stiffening, left atrium enlargement, raised pulmonary pressure and diastolic dysfunction.4,5
Elevated thyroid hormones alter the receptors in the heart specifically the β1-adrenergic and M2-muscarinic receptors resulting in increased sympathetic function, increased heart rate and decreased atrial refractory period.6 Due to the shorter action potential in the refractory period of repolarisation, there is enhanced ectopic activity from the left atrium to the whole atrium causing atrial fibrillation.5 Achieving euthyroid state may resolve the effects on the CVS otherwise a hyperthyroid state may lead to cardiovascular complications such as atrial fibrillation (AF), stroke, myocardial infarction, heart failure (HF) and sudden death.
Thyrotoxic cardiomyopathy is defined as myocardial damage caused by the toxic effects of excessive thyroid hormone, resulting in altered myocyte contractile function.7 The first complaint is exercise intolerance and exertional dyspnoea due to inadequate increase in cardiac output, and this can be interpreted as the initial sign of HF. The loss of sinus rhythm and myocardial contractility worsen the CVS leading to congestion.3
Managing HF in the presence of thyrotoxicosis can be challenging because of either reversible or irreversible cardiomyopathy. Treatment will eventually relieve features of HF with partial or complete resolution of cardiomyopathy and improvement in left ventricular function.8 Management of thyrotoxicosis is done using either of the 3 modalities; radioactive iodine, anti-thyroid drugs (ATDs) or surgery.9 But in a low resource setting where radioactive iodine is unavailable, the only options are ATDs, as shown in Table 19,10 and surgery. The dosage of the ATDs depend on the free thyroxine (FT4) levels, as the higher the FT4 level, the larger the dosage required.
Table 1.
| Drugs | Standard dose | Thyroid storm |
|---|---|---|
| Methimazole | 5 mg-40 mg once/day (FT4 level dose dependent) | 60 mg-80 mg once/day or 10 mg-20 mg every 4 h |
| Carbimazole | 10 mg-70 mg once/day (FT4 level dose dependent) | 100 mg-130 mg once/day or 20 mg every 4 h |
| Propylthiouracil | 100 mg-800 mg daily in divided dose (FT4 level dose dependent) | 500 mg-1000 mg loading then 200 mg-250 mg every 4 h |
| Propranolol | 10 mg-40 mg 3-4 times/day | 60 mg-80 mg every 4 h |
| Potassium iodide | 38 mg/day (especially combined with methimazole 15 mg/day) | 5 drops (0.25 mL or 250 mg) every 6 h (Lugol’s – 10 drops every 8 h) |
| Hydrocortisone | Not necessary as a standard dose medication | 300 mg i.v. loading then 100 mg every 8 h |
Case Report
Case 1
A 31-year-old female was diagnosed to have toxic goitre 2 years ago but did not proceed to get treatment. She currently presented with a 6-month history of progressive difficulty in breathing, generalised body swelling and jaundice. Her symptoms were associated with a dry cough but no chest pain. She did not report a history of heart disease or diabetes. On examination, she had exophthalmos, conjunctival pallor, jaundice, dyspnoea with oxygen saturation of 84%, tachypnoeic with a respiratory rate of 28 breaths/min, sacral and bilateral lower limb oedema. An audible bruit was heard over her thyroid gland. She had an irregular heart rate ranging between 90 and 120 beats/min, a raised jugular venous pressure (JVP) with a systolic murmur grade V over the mitral area.
The investigations showed her to have a moderate anaemia of 9.2 g/dL. Her biochemical results showed an elevated alkaline phosphatase (ALP) and γ-glutamyl aminotransferase (GGT) of 300.7 U/L (normal 35-104 U/L) and 204 U/L (normal 0-50 U/L). The serum total protein and albumin were reduced to 51.4 mmol/L (normal 60.0-80.0 mmol/L) and 18.6 mmol/L (normal 35.0-55.0 mmol/L) respectively. Aspartate transaminase (AST) and alanine transaminase (ALT) were 43.2 U/L (normal < 35.0 U/L) and 39.4 U/L (normal < 45.0 U/L) respectively. Her international normalised ratio (INR) was 1.3. Her thyroid function tests revealed thyroid-stimulating hormone (TSH) 0.07 uIU/mL (normal 0.27- 4.20 uIU/mL), free T3 (FT3) 14.2 ng/mL (normal 0.69-2.15 ng/mL) and FT4 59.1 ng/mL (normal 52.0-127.0 ng/mL), as shown in Table 2. With the FT3 and FT4 levels and her vitals, she was diagnosed to have thyroid storm with a score of 60 on the Burch-Wartofsky Point Scale. Table 2 shows the reference ranges and follow-up of her thyroid and liver function over 6 months. The electrocardiogram (ECG) showed an irregular rhythm with absent p wave and t wave inversion on the lateral chest leads (Figure 1). A chest radiograph showed pulmonary oedema, blunted right costo-phrenic angle and cephalisation (Figure 2). The echocardiogram showed biventricular failure with severe mitral regurgitation (MR), mild tricuspid regurgitation and an ejection fraction (EF) of 46%. The neck ultrasound revealed a highly vascularised enlarged thyroid gland.
Table 2.
Follow-up laboratory results of thyroid and liver function for case 1.
| Lab tests | Reference range | Prior treatment | 1-month follow-up | 6-months follow-up |
|---|---|---|---|---|
| TSH (uIU/mL) | 0.27-4.20 | 0.07 | <0.01 | 0.08 |
| FT3 (ng/mL) | 0.69-2.15 | 14.2 | 11.7 | 10.0 |
| FT4 (ng/mL) | 52.0-127.0 | 59.1 | 63.3 | 188 |
| Total protein (g/L) | 60.0-80.0 | 51.4 | 50.9 | Not tested |
| Serum albumin (g/L) | 35.0-55.0 | 18.6 | 17.4 | Not tested |
| Total bilirubin (Umol/L) | <20.0 | 5.7 | 94.5 | 425.2 |
| Direct bilirubin (Umol/L) | <5.0 | 4.0 | 88.6 | 417.6 |
| AST (U/L) | <35.0 | 43.2 | 44.0 | 74.2 |
| ALT (U/L) | <45.0 | 39.4 | 34.8 | 53.1 |
| ALP (U/L) | 35-104 | 300.7 | Not tested | Not tested |
| GGT (U/L) | 0-50 | 204 | Not tested | Not tested |
| INR | 0.8-1.2 | 1.4 | Not tested | 1.9 |
Figure 1.
The ECG for case 1 shows an irregular rhythm with absent p wave and t wave inversion in the lateral chest leads.
Figure 2.
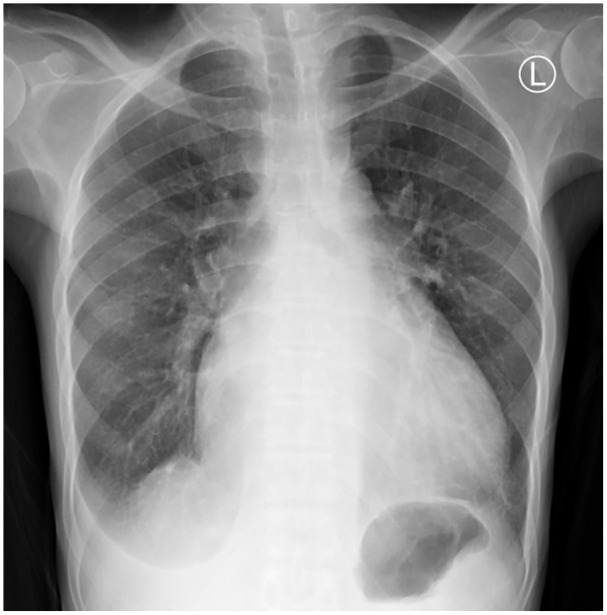
The chest x-ray for case 1 shows pulmonary oedema, blunted right costo-phrenic angle and cephalisation.
For her treatment, she received oxygen and furosemide 80 mg intravenously (i.v.) with little to no effect in the initial stage of her treatment. Lisinopril 2.5 mg once daily, digoxin 0.25 mg once daily and soluble aspirin 75 mg once daily were added to relieve the features of HF. Her condition started to stabilise but the symptoms were not resolving. She was initiated on carbimazole 15 mg thrice daily, propranolol 20 mg twice daily and hydrocortisone 100 mg i.v. thrice daily. A few days later, she started to get symptom relief but still required oxygen. The option of thyroidectomy was raised but she was deemed unfit and was advised to do so once stable. Getting her thyrotoxicosis under control was challenging but managed as she was discharged fairly well on the 32nd hospital day. She was followed up in the clinic 1 month after discharge and she showed signs of improvement and was advised to continue with her medication. She did not show up for her next clinic appointment and follow-up was unsuccessful after contact. Six months later, she was readmitted with symptoms of HF as she was not adherent to her medication due to financial constraints and unfortunately, she passed away.
Case 2
A 57-year-old female was diagnosed to have goitre 8 years’ prior accompanied with jaundice but did not proceed to get treatment. She currently presented with a 2-week history of increasing lower limb swelling and cough, but no chest pain. She did not report a history of heart disease or diabetes. On examination, she had exophthalmos, jaundice, tachypnoeic with a respiratory rate of 24 breaths/min and lower limb oedema, with an irregular heart rate ranging between 65 and 100 beats/min.
The investigations showed her to have a mild anaemia of 11.3 g/dL. Her biochemical results showed her to have an elevated AST of 84.1 U/L and ALT of 235.2 U/L. With total protein of 73.4 mmol/L and reduced albumin of 24.2 mmol/L. Her thyroid function tests revealed TSH 0.08 uIU/mL, FT3 2.9 ng/mL, FT4 131.4 ng/mL (Table 3). Table 3 shows the follow-up of her thyroid and liver function after 1 month only. The ECG showed an irregular rhythm with absent p wave (Figure 3). A chest radiograph showed pulmonary oedema, blunted left costo-phrenic angle and cephalisation (Figure 4). The echocardiogram showed dilated left atrium with a reduced EF of 40%. The neck ultrasound revealed a vascularised enlarged thyroid gland.
Table 3.
Follow-up laboratory results of thyroid and liver function for case 2.
| Lab tests | Reference range | Prior treatment | 1-month follow-up |
|---|---|---|---|
| TSH (uIU/mL) | 0.27-4.20 | 0.08 | 0.11 |
| FT3 (ng/mL) | 0.69-2.15 | 2.9 | 2.1 |
| FT4 (ng/mL) | 52.0-127.0 | 131.4 | 86.5 |
| Total protein (mmol/L) | 60.0-80.0 | 73.4 | 75.7 |
| Serum albumin (mmol/L) | 35.0-55.0 | 24.2 | 25.5 |
| Total bilirubin | <20.0 | 199.3 | 180.5 |
| Direct bilirubin | <5.0 | 140.3 | 113.7 |
| AST (U/L) | <35.0 | 84.1 | 922.7 |
| ALT (U/L) | <45.0 | 235.2 | 27.6 |
| ALP | 35-104 | 162 | Not tested |
| GGT | 0-50 | 108 | Not tested |
| INR | 0.8-1.2 | 1.3 | Not tested |
Figure 3.
The ECG for case 2 shows an irregular rhythm with absent p wave.
Figure 4.

The chest x-ray of case 2 shows pulmonary oedema, blunted left costo-phrenic angle and cephalisation.
For her treatment, she received oxygen and furosemide 40 mg i.v. twice daily proving to be useful in her treatment. Later added digoxin 0.125 mg once daily and soluble aspirin 75 mg once daily. She was initiated on carbimazole 5 mg twice daily and propranolol 20 mg twice daily. A few days later, she felt better and got symptom relief. She was discharged, fairly well, on the 6th hospital day. Unfortunately, she was lost to follow up after 1 month, as she was contacted without success and further workup was not done.
Discussion
Both patients were below the age of 60 years and developed thyrotoxic heart disease. In the first case, the patient developed thyroid storm with HF, and the second case was much stable and progressed well on her treatment. The first case was diagnosed with thyrotoxicosis and developed features of heart failure much sooner than the latter patient due to the severity of the thyrotoxicosis.
Early detection and treatment of thyrotoxicosis are vital to prevent disease progression and death. Diagnosing and treating hyperthyroidism early helps to negate the potential negative effects of the free thyroid hormones. Both the cases were diagnosed with thyrotoxicosis before they developed HF but did not proceed to get treated. In our setting, availability of diagnostics, treatment and follow-up are a challenge which was likely the reason both cases did not proceed to get adequate care upon diagnosis.
After the development of AF and HF, early treatment has the potential to reverse the complications as cardiac function improves. In the case of thyrotoxic HF or AF, most will be asymptomatic for HF within 6 months,11 and over half of the patients will return to sinus rhythm within 6 months.12 But in some cases, there may be partial resolution or persistent symptoms even though there is improved cardiac function and euthyroidism has been achieved.8,13,14
Development of HF in patients with thyrotoxicosis occurs due to poor oxygen supply and changes in the myosin isoform expression in the cardiomyocytes affecting contractility. In addition, there is blood volume expansion and increase in end-diastolic pressure and cardiac workload.15 Around half of patients with thyrotoxicosis are admitted due to cardiovascular complications.16 Studies have shown the prevalence of HF in up to 68%17 and incidence of 14%18 of patients with thyrotoxicosis. On the other hand, AF is fairly common in patients with thyrotoxicosis in up to 90% of cases.19
The management of AF is always rate control regardless of the cause. Beta-blockers are the drugs of choice.19 Non-dihydropyridine calcium channel blockers can be used for ventricular rate response but may be contraindicated due to the negative inotropic effects. Digoxin can be used in patients with HF though due to the increase in renal clearance, it is not as effective.20 Both our cases were given digoxin and they showed improvement. Digoxin should still be considered in patients with thyrotoxic HF and AF in centres with limited drug resources. Amiodarone is an alternative anti-arrhythmic medication which inhibits T4 to T3 conversion and inhibits thyroid hormone synthesis and secretion. But it may cause thyroid dysfunction in patients with thyroid disease. Amiodarone is over one-third iodine by weight, has a prolonged half-life due to storage in adipose tissue and while it may acutely lower T3 levels over the longer term, it may result in worsening of thyroid function which can be very resistant to treatment.21 Adding amiodarone to cases where thyroid surgery is not available could be life-threatening.
In HF, the correction of thyroid dysfunction should be the first management, as ATDs may correct thyroid dysfunction and total thyroidectomy may rapidly restore euthyroidism.3 It is important to note that ATDs take time to have an effect. Some patients remain thyrotoxic despite high doses and that side effects may limit dosing. Total thyroidectomy can rapidly restore the patient to a euthyroid state but consideration needs to be given to the anaesthetic risk and the need to improve biochemical status prior to surgery whenever possible, such as the use of Lugol’s iodine. In patients with congestive HF, treatment with beta-blockers and diuretics can improve the congestive symptoms.19
Elevated thyroid hormones affect several coagulation and fibrinolytic factors irrespective of the underlying disease. With an elevated thyroid hormone, there is a hypercoagulable and hypofibrinolytic state, with a rise in factors VIII and IX, fibrinogen, von Willebrand factor and plasminogen activator inhibitor-1.22 Also, AF is associated with increased risk of ischaemic stroke, and risk stratification is done using the CHA₂DS₂-VASc score.23 Since the AF caused is reversible, there would be no benefit in implementing long term anticoagulation, and both our cases were initiated on low dose aspirin. Though both cases should have been initiated on anticoagulation. It is advised that having a moderate-high risk score of ⩾2 to be on anticoagulation, especially warfarin due to the change in cardiovascular hemodynamic.23
Both of the cases had jaundice and hypoalbuminemia. Metabolism of thyroid hormones occurs in the liver. There has been reported nonspecific changes on the liver from cases with thyrotoxicosis including intrahepatic cholestasis, lobular inflammation and Kupffer cell hyperplasia.24 Cholestatic liver injury in patients with thyrotoxicosis has been associated with autoimmune hepatitis or primary biliary cirrhosis. It may occur due to liver congestion from HF, side effects of ATDs or hepatic necrosis from systemic embolisation from AF.25,26 Studies have reported a decrease in serum albumin, and increase in serum bilirubin, AST, ALT, γ-glutamyl aminotransferase and alkaline phosphatase levels, in untreated thyrotoxic patients. Most of these cases normalising following adequate treatment.24
Follow up is crucial in all patients with hyperthyroidism, mainly because of treatment options and complications.2 Life-threatening thyrotoxicosis or thyroid storm characterised by multisystem involvement has mortality rates between 8% and 25%.9,16,18 In our case, the options available at our centre were ATDs and surgery. The management of thyrotoxicosis with ATDs is 12 to 18 months.10 Even though the first case was followed up, she was halfway through her therapy and developed HF symptoms that led to her death. And the second case was lost to follow up. Thorough precautions should have been in place to avoid such outcomes.
Conclusion
These cases highlight the need for an early evaluation of causes leading to AF and HF especially in patients with thyrotoxicosis. Prolonged untreated or undertreated thyrotoxicosis increases the risk of AF and HF but this may be influenced by thyrotoxicosis severity combined with patient age and comorbidities. Hyperthyroidism is underdiagnosed and inadequately managed due to limited access to clinical and diagnostic resources which are necessary for care. Patients should be subjected to close clinical follow-up so as to ascertain minimal complications thus subjecting patients to a good prognosis. Early, effective and monitored treatment of hyperthyroidism is key to the management of AF and HF in achieving a better outcome.
Acknowledgments
The authors are grateful to the patients’ participation and acknowledge the support provided by the department.
Footnotes
Funding:The author(s) received no financial support for the research, authorship and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author Contributions: AS and NC wrote the manuscript and provided the case information and images.
Patient Consent: We received written consent to publish findings of these cases from both the patients.
ORCID iD: Abid M Sadiq  https://orcid.org/0000-0002-7812-8042
https://orcid.org/0000-0002-7812-8042
References
- 1. Ertek S, Cicero AF. State of the art paper hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci. 2013;5:944-952. doi: 10.5114/aoms.2013.38685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646. doi: 10.1089/thy.2010.0417 [DOI] [PubMed] [Google Scholar]
- 3. Biondi B. Mechanisms in endocrinology: heart failure and thyroid dysfunction. Eur J Endocrinol. 2012;167:609-618. doi: 10.1530/EJE-12-0627 [DOI] [PubMed] [Google Scholar]
- 4. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735. doi: 10.1161/CIRCULATIONAHA.106.678326 [DOI] [PubMed] [Google Scholar]
- 5. Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6:431-443. doi: 10.1038/nrendo.2010.105 [DOI] [PubMed] [Google Scholar]
- 6. Reddy V, Taha W, Kundumadam S, Khan M. Atrial fibrillation and hyperthyroidism: a literature review. Indian Heart J. 2017;69:545-550. doi: 10.1016/j.ihj.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osuna PM, Udovcic M, Sharma MD. Hyperthyroidism and the Heart. Methodist Debakey Cardiovasc J. 2017;13:60-63. doi: 10.14797/mdcj-13-2-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Umpierrez GE, Challapalli S, Patterson C. Congestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidism. Am J Med Sci. 1995;310:99-102. doi: 10.1097/00000441-199531030-00003 [DOI] [PubMed] [Google Scholar]
- 9. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343-1421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 10. Burch HB, Cooper DS. Antithyroid drug therapy: 70 years later. Eur J Endocrinol. 2018;179:R261-R274. doi: 10.1530/EJE-18-0678 [DOI] [PubMed] [Google Scholar]
- 11. Riaz K, Forker AD, Isley WL, Hamburg MS, McCullough PA. Hyperthyroidism: a “curable” cause of congestive heart failure-three case reports and a review of the literature. Congest Hear Fail. 2003;9:40-46. doi: 10.1111/j.1527-5299.2003.01124.x [DOI] [PubMed] [Google Scholar]
- 12. Klein I, Danzi S. Thyroid disease and the heart. Curr Probl Cardiol. 2016;41:65-92. doi: 10.1016/j.cpcardiol.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 13. Ebisawa K, Ikeda U, Murata M, et al. Irreversible cardiomyopathy due to thyrotoxicosis. Cardiology. 1994;84:274-277. doi:10.1159/000176411 [DOI] [PubMed] [Google Scholar]
- 14. Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy. A matched case-control study. J Am Coll Cardiol. 2007;49:71-81. doi: 10.1016/j.jacc.2006.08.042 [DOI] [PubMed] [Google Scholar]
- 15. Jorge AJL, de Martins WA, de Gripp EA, de Almeida BM, Figueroa CCRP, Sabino CL. Severe mitral regurgitation by hyperthyroidism in the absence of left ventricular dilatation. Int J Cardiovasc Sci. Published online 2017. doi: 10.5935/2359-4802.20170089 [DOI] [Google Scholar]
- 16. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Factors associated with mortality of thyroid storm. Medicine (Baltimore). 2016;95:e2848. doi: 10.1097/MD.0000000000002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albakri A. Thyrotoxic heart failure: a review of clinical status and meta-analysis of electrocardiogram diagnosis and medical clinical management methods. Integr Mol Med. 2018;5:1-11. doi: 10.15761/imm.1000350 [DOI] [Google Scholar]
- 18. Selmer C, Olesen JB, Hansen ML, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014;99:2372-2382. doi: 10.1210/jc.2013-4184 [DOI] [PubMed] [Google Scholar]
- 19. Siu C-W, Yeung C-Y, Lau C-P, Kung AWC, Tse H-F. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483-487. doi: 10.1136/hrt.2006.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrow D, Gaffney T, Braunwald E. Studies on digitalis VIII. Effect of autonomic innervation and of myocardial catecholamine stores upon the cardiac action of ouabain. J Pharmacol Exp Ther. 1963;:236-245. [Google Scholar]
- 21. Martin A, Camm AJ. Thyrotoxic atrial fibrillation. Br Med J (Clin Res Ed). 1982;285:1574. doi: 10.1136/bmj.285.6354.1574-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuijver D, van Zaane B, Romualdi E, Brandjes D, Gerdes V, Squizzato A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors. Thromb Haemost. 2012;108:1077-1088. doi: 10.1160/TH12-07-0496 [DOI] [PubMed] [Google Scholar]
- 23. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 24. Evlice M, Aksoz Z. Thyrotoxicosis associated with severe hypoalbuminemia and hyperbilirubinemia. Egypt J Intern Med. 2017;29:196. doi: 10.4103/ejim.ejim_7_17 [DOI] [Google Scholar]
- 25. Khemichian S, Fong T-L. Hepatic dysfunction in hyperthyroidism. Gastroenterol Hepatol (N Y). 2011;7:337-339. [PMC free article] [PubMed] [Google Scholar]
- 26. Abebe A, Eck LM, Holyoak M. Severe cholestatic jaundice associated with Graves’ disease. Clin Case Reports. 2018;6:2240-2245. doi: 10.1002/ccr3.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]




