Abstract
Long non-coding RNA (lncRNA) X inactive specific transcript (XIST) is reported to play an oncogenic role in non-small cell lung cancer (NSCLC). However, the role of XIST in regulating the radiosensitivity of NSCLC cells remains unclear. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the expressions of XIST and miR-16-5p in NSCLC in tissues and cells, and Western blot was used to assess the expression of WEE1 G2 checkpoint kinase (WEE1). Cell counting kit-8 (CCK-8), colony formation and flow cytometry assays were used to determine cell viability and apoptosis after NSCLC cells were exposed to different doses of X-rays. The interaction between XIST and miR-16-5p was confirmed by StarBase database, qRT-PCR and dual-luciferase reporter gene assays. TargetScan database was used to predict WEE1 as a target of miR-16-5p, and their targeting relationship was further validated by Western blot, qRT-PCR and dual-luciferase reporter gene assays. XIST was highly expressed in both NSCLC tissue and cell lines, and knockdown of XIST repressed NSCLC cell viability and cell survival, and facilitated apoptosis under the irradiation. MiR-16-5p was a target of XIST, and rescue experiments demonstrated that miR-16-5p inhibitors could reverse the role of XIST knockdown on radiosensitivity in NSCLC cells. WEE1 was validated as a target gene of miR-16-5p, and WEE1 could be negatively regulated by XIST. XIST promotes the radioresistance of NSCLC cells by regulating the expressions of miR-16-5p and WEE1, which can be a novel target for NSCLC therapy.
Keywords: MiR-16-5p, NSCLC, radiosensitivity, WEE1, XIST
Introduction
Lung cancer is one of the leading causes of cancer-related deaths in the world, and non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases.1–3 Radiation therapy is an important treatment strategy for advanced NSCLC patients.4,5 However, radiation resistance has become a serious obstacle to the effects of radiotherapy.6–8 To improve the clinical outcomes of NSCLC therapy, it is urgent to further explore the mechanisms by which NSCLC cells develop radioresistance.
Long non-coding RNAs (lncRNAs) are recognized as a new set of clinical biomarkers and potential tumor therapeutic targets.9 LncRNAs are a class of non-protein-coding transcripts with a length of longer than 200 nucleotides.10,11 They are involved in the regulation of various biological processes including cell proliferation, migration and apoptosis, as well as the progression of cancer.12–14 Abnormally expressed lncRNAs participate in the progression of NSCLC. For example, LINC01296 promotes the progression of NSCLC by sponging miR-5095.15 LncRNA AK027294 facilitates NSCLC progression by up-regulating the expression of STAT3.16 In addition, lncRNA X inactive specific transcript (XIST) is proved to promote the development and progression of NSCLC.17,18 In terms of mechanism, XIST is a molecular sponge of miR-141, miR-367 and miR-137, and up-regulated expression of XIST results in decreased expression of these microRNAs (miRNAs) and in turn promotes the proliferation and metastasis of cancer cells.19,20 It is reported that overexpression of XIST in nasopharyngeal carcinoma cells can down-regulate the expression of miR-29c and inhibit the radiosensitivity of tumor cells.21 However, little is known about the role of XIST in NSCLC radiosensitivity.
MiRNAs are a class of small non-coding RNAs containing 18–25 nucleotides, leading to translational inhibition or mRNA degradation by specifically binding to the 3’-untranslated region (3’-UTR) of the target mRNA.22 The abnormal expression of miRNAs is closely related to carcinogenesis, playing a critical role in various biological processes such as cell differentiation, stress response, proliferation, apoptosis and so on.23–27 As one of the miRNAs associated with human malignancies, miR-16-5p promotes tumor cell radiosensitivity by modulating the Cyclin D1/E1–pRb–E2F1 signaling in prostate cancer cells.28 However, whether miR-16-5p promotes the radiosensitivity of NSCLC cells and its underlying mechanisms remain unknown.
WEE1 G2 checkpoint kinase (WEE1) is a tyrosine kinase that exhibits the regulatory function in the G2 checkpoint in response to DNA damage.29 Previous studies confirm that WEE1 is a potential target for tumor therapy, such as gastric cancer, acute myelocytic leukemia, head and neck squamous cell carcinosarcoma and melanoma.30–33 WEE1 expression is up-regulated in these malignancies and is closely associated with the adverse prognosis of the patients.30–34 In addition, inhibition of WEE1 expression is reported to enhance the radiosensitivity of osteosarcoma and pancreatic cancer.35,36 A recent study indicates that the WEE1 inhibitor, AZD1755, sensitizes KRAS mutant NSCLC cells to radiotherapy.37 However, the role of WEE1 in NSCLC radioresistance and its upstream regulatory mechanism have not been fully elucidated.
In this study, bioinformatics analysis indicated that XIST had a potential binding site with miR-16-5p and WEE1 was a downstream target of miR-16-5p. We investigated the role of XIST in the radioresistance of NSCLC cells in vitro and its function of regulating WEE1 by sponging miR-16-5p, providing a theoretical basis for the treatment of NSCLC.
Materials and methods
Clinical samples
All patients enrolled signed informed consent in this study, and our research was endorsed by the Ethics Committee of Qilu Hospital (Approval number: 201705006). 31 cases of NSCLC tissues (13 squamous cell carcinomas and 18 adenocarcinomas) and adjacent normal tissues were taken from the Department of Pathology, Qilu Hospital. All patients were diagnosed as NSCLC by histopathology and had never received preoperative chemotherapy or radiation therapy before this study.
Cell lines and cell culture
Human lung cancer cell lines (H157, HCC827, A549 and H838) and normal bronchial epithelial cell lines (16HBE) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Carlsbad, CA, USA) in an incubator at 37°C in 5% CO2.
Cell transfection
Small interference RNA (siRNA) control (si-con), siRNAs against XIST (si-XIST-1 and si-XIST-2), pcDNA3.1 vector (vector), pcDNA3.1-XIST, miRNA control (miR-con), miR-16-5p mimics (miR-16-5p), and miR-16-5p inhibitors (anti-miR-16-5p) were available from GenePharma Co., Ltd. (Shanghai, China). H838 and A549 cells were seeded in 6-well cell culture plates at a density of 1 × 105 /mL and transfected with the siRNAs (50 nmol), mimics (20 nmol), or inhibitors (20nmol) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the supplier’s instructions. Transfection efficiency was measured by quantitative real-time polymerase chain reaction (qRT-PCR).
Ionizing radiation treatment
Transfected NSCLC cells were irradiated with a linear accelerator (Varian Medical Systems, USA) at room temperature with different doses (0, 2, 4, 6, and 8 Gy, dose rate: 1 Gy/min). After 24–96 h, the cells were used for further analyses.
qRT-PCR
Total RNA from tissues and cells was extracted using TRIzol reagent (Invitrogen, Shanghai, China). 1 μg of total RNA was reversely transcribed into complementary DNA (cDNA) using SuperScript First-Strand Synthesis System (Invitrogen, Shanghai, China). Then qRT-PCR was performed with SYBR Green Master Mix (Takara, Dalian, China). The relative expressions of XIST and miR-16-5p were calculated employing the 2-ΔΔCT method. Additionally, to determine the subcellular fractionation location of lncRNA, Cytoplasmic & Nuclear RNA Purification kit (Biosharp, Hefei, China) was used to obtain the cytoplasmic and nuclear RNA of the cells, respectively. The primers used were as follows: XIST: 5’-GCATAACTCGGCTTAGGGCT-3’ (forward) and 5’-TCCTCTGCCTGACCTGCTAT-3’ (reverse); miR-16-5p, 5’-TAGCAGCACGTAAATATTGGCG-3’ (forward) and 5’-TGCGTGTCGTGGAGTC-3’ (reverse); WEE1: 5’-GCTGCCTCTGAAGAAGGAGA-3’ (forward); 5’-ACATACCACTGTGAGGGCAA-3’ (reverse); U6, 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse); β-actin, 5’-CGTGAAAAGATGACCCAGATCA-3’ (forward) and 5’-CAGCCTGGATGGCTACGTACA-3’ (reverse)
Western blot
The cells were lysed with RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). The supernatant was collected after centrifugation. After the protein was quantified by Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China), SDS-PAGE was performed, and then the protein was transferred onto the PVDF membrane (Life Technologies, Gaithersburg, MD, USA). Then WEE1 antibody (Abcam, ab137377, 1:1000) and internal reference β-actin antibody (Abcam, ab20272, 1:1000) were added, with which the membranes were incubated overnight at 4°C. After the PVDF membrane was washed with TBST solution, they were incubated with the horseradish peroxidase-labeled secondary antibody (Hubei Biossci Biotechnology Co. Ltd., 1:2000) for 1 h at room temperature. After the membranes were rinsed with TBST solution again, the hypersensitive ECL (Hubei Biossci Biotechnology Co., Ltd.) was used for chemiluminescence, and the protein bands were developed.
Cell viability assay
H838 and A549 cells in the logarithmic growth phase were selected and then trypsinized. In brief, 100 μL cell suspension containing 2 × 103 cells was added into each well of the 96-well plates. Then the 96-well plates were placed in an incubator to continue the culture. After 24 h, 10 μL of cell counting kit 8 (CCK-8) solution (Biossci, Wuhan, China) was added to each well and incubated for another hour in the incubator. After the end of the culture, the 96-well plates were placed in a microplate reader, and the absorbance (optical density, OD value) of each well at a wavelength of 450 nm was measured. Thereafter, the absorbance of the cells was measured at 48, 72, and 96 h, respectively.
Colony formation experiment
Transfected NSCLC cells were seeded into 6-well plates (1 × 103 / well) and irradiated with the indicated single dose (0, 2, 4, 6 or 8 Gy). After 2 weeks of culture, cells were fixed with 100% methanol and stained with 0.1% crystal violet for 15 min. The number of colony in each group was then counted and recorded with naked eyes.
Flow cytometry
Cells in each group were treated with or without 4 Gy irradiation, and the percentage of the apoptotic cells was examined by a FACScalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA) using Annexin V-FITC / propidium iodide (PI) double staining kit (Invitrogen, Shanghai, China).
Luciferase reporter assay
The wild type (WT) 3’UTR of WEE1 (WEE1-WT-3’UTR) or the sequence of XIST containing the predicted miR-16-5p targeting site (XIST -WT) was amplified, and then inserted into pGL3 basic vector (Promega, Madison, WI, USA). Similarly, the sequence of mutant (MUT) WEE1 3’UTR or XIST was inserted into the luciferase reporter vector to obtain WEE1-MUT-3’UTR and XIST-MUT reporter plasmids. The cells were seeded in 24-well plates at 5000 cells per well, and cultured for 24 h. After that, the cells were co-transfected with the reporter plasmids with miR-16-5p mimics or miR-con. 48 h later, the relative luciferase activity of each group was determined using dual-luciferase reporter gene assay system (Promega, Madison, WI, USA).
Statistical analysis
All experiments were repeated at least three times independently and the results were shown as mean ± standard deviation (SD). Statistical differences between the two groups were assessed using Student’s t-test. One-way analysis of variance followed by Tukey’s post hoc test was used to compare differences among multiple groups. P < 0.05 was considered statistically significant.
Results
XIST expression was up-regulated in NSCLC tissues and cell lines, and associated with the radioresistance of NSCLC cells
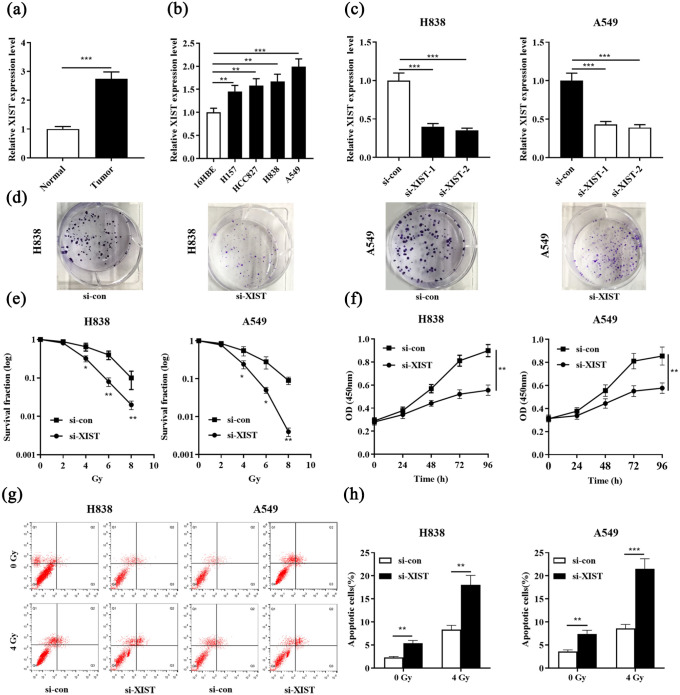
First of all, qRT-PCR was used to examine the expression of XIST in 31 cases of NSCLC tissues and adjacent tissues. It was found that XIST was significantly highly expressed in NSCLC tissues compared with in adjacent normal tissues (Figure 1(a)). Subsequently, the expression of XIST in four different NSCLC cell lines was detected by qRT-PCR. Compared with in the normal bronchial epithelial cell line 16HBE, XIST was significantly highly expressed in all of the four NSCLC cell lines including H157, HCC827, H838, and A549 (Figure 1(b)). To further delve into the role of XIST in the development of radiotherapy resistance in NSCLC, we transfected siRNAs targeting XIST into H838 and A549 cells, and qRT-PCR results confirmed that we successfully constructed cell models with low expression of XIST (Figure 1(c)). Under different doses of radiation, we detected the colony formation of H838 and A549 cells employing colony formation assay. The results suggested that XIST knockdown markedly reduced the number of colonies (Figure 1(d) and (e)). CCK-8 assay confirmed knockdown XIST significantly inhibited the viability of NSCLC cell compared with the control group (Figure 1(f)). Knockdown of XIST also promoted NSCLC cell apoptosis in the absence or presence of radiation (4 Gy) (Figure 1(g) and (h)). Collectively, these data indicated that XIST knockdown enhanced the radiosensitivity of NSCLC cells.
Figure 1.
XIST expression was up-regulated in NSCLC tissues and cell lines, and correlated with the radioresistance. (a) Expression of XIST in NSCLC tissues and adjacent lung tissues was examined by qRT-PCR. (b) Expression of XIST was evaluated by qRT-PCR in NSCLC cell lines and normal bronchial epithelial cell line (16HBE). (c) H838 and A549 cells were transfected with si-XIST-1 and si-XIST-2, and transfection efficiency was detected by qRT-PCR. (d) Representative images of colony formation assays (under 4 Gy irradiation). (e) Colony formation assay was used to measure colony survival rate 2 weeks after H838 and A549 cells transfected with si-XIST or si-con were exposed to the indicated single doses of irradiation (0, 2, 4, 6, or 8 Gy). (f) CCK-8 assay was performed to determine cell proliferation of H838 and A549 cells. (g) and (h) The apoptosis of H838 and A549 cells was evaluated by flow cytometry.
*P < 0.05, **P < 0.01 and ***P < 0.001. si-con vs si-XIST.
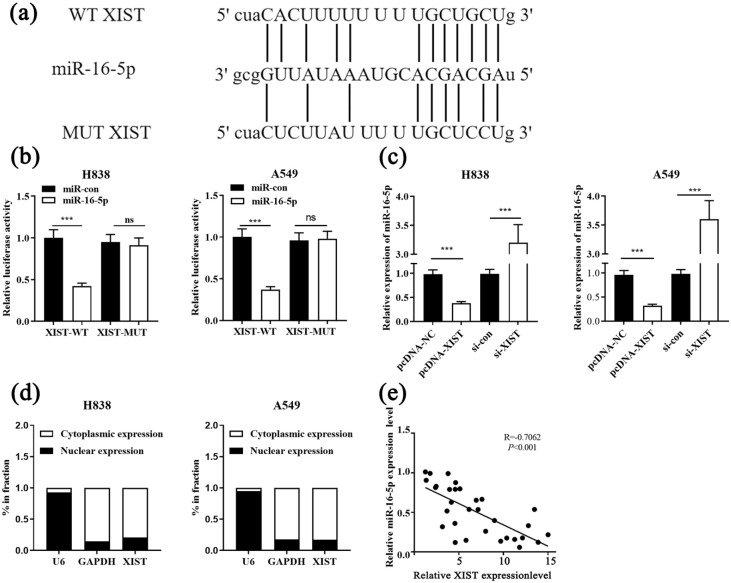
XIST functioned as competing endogenous RNA and sponged miR-16-5p in NSCLC
The online bioinformatics database StarBase was used to search for potential miRNAs capable of pairing with XIST. It was found that XIST had a putative binding site for the seed sequence of miR-16-5p (Figure 2(a)). To further validate whether XIST could sequester miR-16-5p, dual-luciferase reporter assays were performed, and the results of which revealed that miR-16-5p mimics inhibited the luciferase activity of the XIST-WT reporter, but didn’t affect that of the XIST-MUT reporter (Figure 2(b)). Additionally, XIST overexpression decreased miR-16-5p expression while XIST knockdown worked oppositely in NSCLC cells (Figure 2(c)). Besides, XIST was preferentially localized in the cytoplasm of NSCLC cells, which was determined by subcellular fractionation assay, and this suggested that XIST could probably function as a competing endogenous RNA (ceRNA) (Figure 2(d)). Moreover, Pearson’s correlation analysis unmasked a negative correlation between miR-16-5p and XIST expressions in NSCLC tissues (Figure 2(e)). Taken together, we concluded that miR-16-5p was a downstream target of XIST and its expression level was negatively regulated by XIST.
Figure 2.
XIST acted as the sponge of miR-16-5p. (a) The potential binding site between XIST and miR-16-5p was predicted by bioinformatics. (b) Dual luciferase reporter assay showed that XIST could bind with miR-16-5p. (c) After transfecting H838 and A549 cells with pcDNA-NC, pcDNA-XIST, si-con and si-XIST, the expression of miR-16-5p was detected by qRT-PCR. (d) The enrichment of XIST in nucleus and cytoplasm was measured by qRT-PCR. (e) A negative correlation between the expressions of XIST and miR-16-5p was observed in NSCLC tissues.
***P < 0.001. ns, not significant.
XIST and miR-16-5p showed opposite changes in NSCLC under irradiation
Subsequently, our data showed that miR-16-5p expression was down-regulated in NSCLC tissues and cell lines compared to in adjacent tissues and 16HBE cell line (Figure 3(a) and (b)). To further investigate whether radiation exposure would cause changes in the expressions of XIST and miR-16-5p in H838 and A549 cells, their expressions in H838 and A549 cells were detected by qRT-PCR at the interval of 3 h after the cells were treated with 4 Gy radiation. It was authenticated that XIST expression in H838 and A549 cells was significantly increased compared with that of the control group (0 Gy) (Figure 3(c)); and as expected, miR-16-5p expression was significantly inhibited with the same treatment (Figure 3(d)). The above results indicated that XIST and miR-16-5p showed opposite changes under irradiation, and changes in the expressions of XIST and miR-16-5p might affect the radiosensitivity of NSCLC cells.
Figure 3.
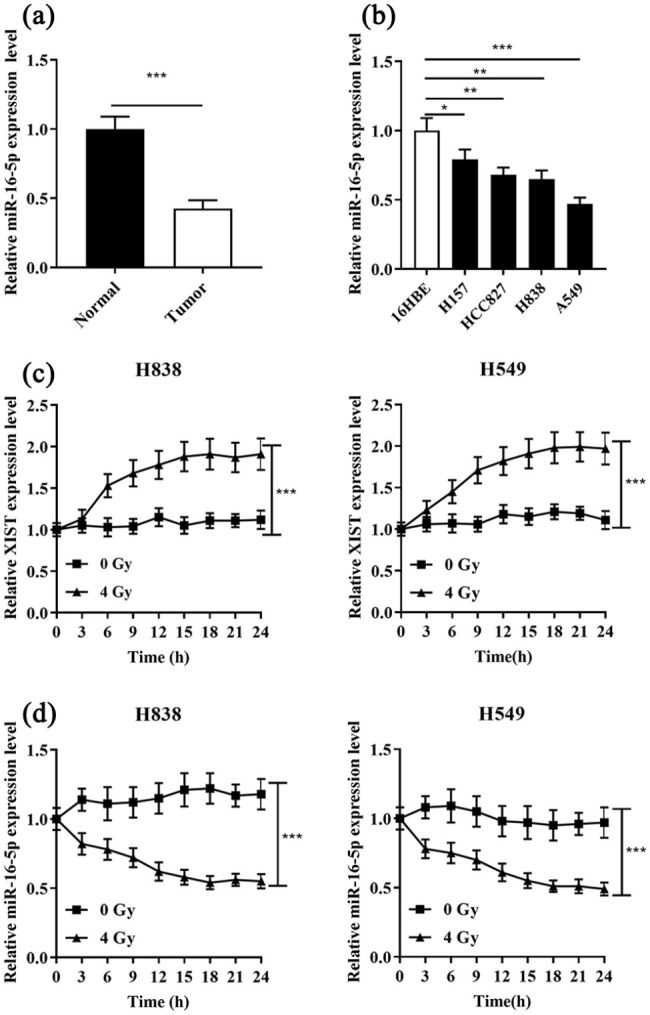
Opposite changes in the expressions of XIST and miR-16-5p in NSCLC cells under irradiation. (a) and (b) qRT-PCR was used to detect the expression of miR-16-5p in four NSCLC tissue and cell lines (16HBE). (c) XIST expression in H838 and A549 cells was detected under X-ray irradiation at a dose of 4 Gy every 3 h. (d) MiR-16-5p expression in H838 and A549 cells was detected under X-ray irradiation at a dose of 4 Gy every 3 h.
*P < 0.05, **P < 0.01 and ***P < 0.001.
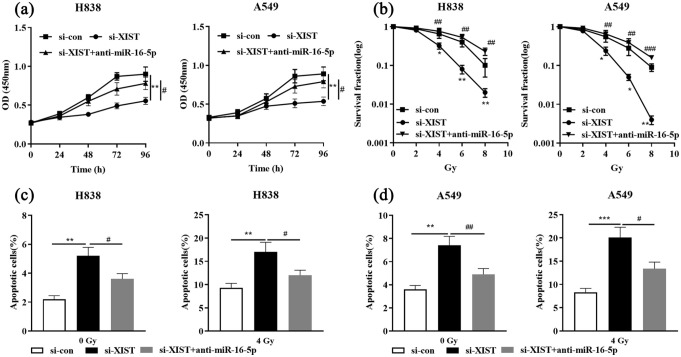
Inhibition of miR-16-5p reversed the effects of XIST knockdown on NSCLC cells
To further elaborate on the underlying mechanism by which XIST induced radioresistance of NSCLC cells, we transfected si-con, si-XIST or si-XIST+anti-miR-16-5p into H838 and A549 cells, respectively. qRT-PCR showed that the transfection was successful (Supplementary Figure 1). The results of CCK-8 assay suggested that the inhibitory effect on cell viability induced by XIST knockdown was attenuated by anti-miR-16-5p (Figure 4(a)). In addition, we treated these cells with different doses of X-rays, and as shown, XIST knockdown sensitized NSCLC cells to irradiation, increasing the apoptosis and reducing the colony formation, while anti-miR-16-5p attenuated these effects (Figure 4(b)–(d)).
Figure 4.
Down-regulation of miR-16-5p expression impairs the effects on NSCLC cell proliferation and radiosensitivity induced by XIST knockdown. si-con, si-XIST or si-XIST+anti-miR-16-5p was transfected into H838 and A549 cells, respectively. (a) Cell viability of NSCLC cells was measured by CCK-8 assay. (b) The survival rate of NSCLC cells was detected by colony formation assays 2 weeks after being exposed to different doses of X-ray irradiation (0, 2, 4, 6 or 8 Gy). (c) and (d) The apoptosis of H838 and A549 cells was evaluated by flow cytometry.
*P < 0.05, **P < 0.01 and ***P < 0.001, si-con vs si-XIST. #P < 0.05, ##P < 0.01 and ###P < 0.001, si-XIST vs si-XIST+anti-miR-16-5p.
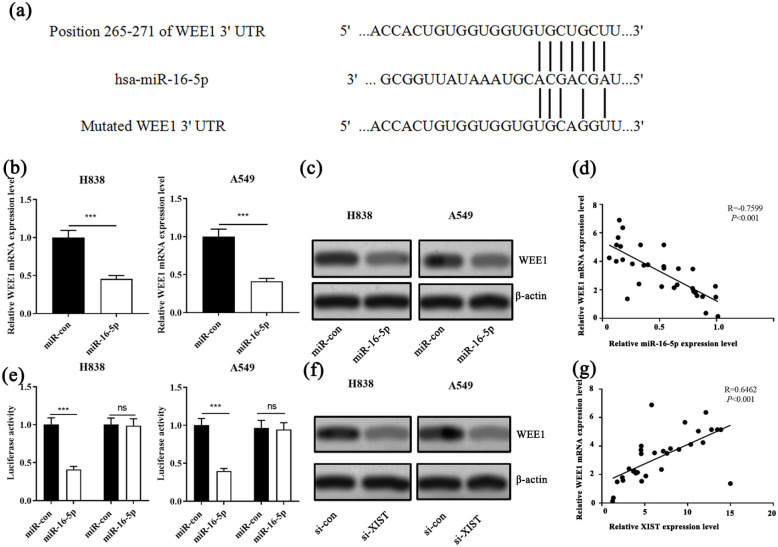
MiR-16-5p directly targeted the 3’ UTR of WEE1
Next, we predicted the target gene of miR-16-5p with TargetScan database and found that WEE1 was one of the candidate target genes of miR-16-5p (Figure 5(a)). qRT-PCR analysis unearthed that miR-16-5p mimics was successfully transferred into NSCLC cells (Supplementary Figure 2). qRT-PCR and Western blot showed that WEE1 mRNA and protein were significantly decreased after the transfection of miR-16-5p into H838 and A549 cells (Figure 5(b) and (c)). Moreover, Pearson’s correlation analysis revealed a significant negative correlation between miR-16-5p expression and WEE1 mRNA expression in NSCLC tissues (Figure 5(d)). Subsequently, dual luciferase reporter assay demonstrated that miR-16-5p specifically bound to the 3’UTR of WEE1 (Figure 5(e)). Additionally, Western blot showed that WEE1 expression was significantly decreased after the transfection of si-XIST (Figure 5(f)). Besides, we found a significant positive correlation between XIST and WEE1 mRNA expressions in NSCLC tissues (Figure 5(g)). These results indicated that WEE1 was a downstream gene of miR-16-5p, and its expression level was negatively regulated by the latter, and XIST could positively regulate the expression level of WEE1 indirectly.
Figure 5.
miR-16-5p and XIST regulated the expression of WEE1 in NSCLC cells. (a) The binding site between miR-16-5p and WEE1 was predicted by bioinformatics. (b) The expression of WEE1 mRNA was detected by qRT-PCR after the transfection of miR-con and miR-16-5p mimics into H838 and A549 cells. (c) The expression of WEE1 was detected by Western blot after the transfection of miR-con and miR-16-5p into H838 and A549 cells. (d) The correlation between miR-16-5p expression and WEE1 expression in NSCLC samples. (e) WEE1-WT-3’UTR or WEE1-MUT-3’UTR plasmid was co-transfected into H838 and A549 cells with miR-16-5p or miR-con. The luciferase activity was determined at 48 h after transfection. (f) The expression of WEE1 was detected by Western blot after transfection of si-XIST into H838 and A549 cells. (g) The correlation between XIST expression and WEE1 expression in NSCLC samples.
***P < 0.001, si-XIST vs si-XIST+anti-miR-16-5p.
Discussion
Previous studies indicate that XIST, miR-16-5p, WEE1 figure prominently in regulating the development and progression of NSCLC.17,18,38,39 It is widely accepted that radiotherapy is currently one of the most effective methods for the treatment of a variety of advanced malignancies, including NSCLC.2,40 However, the effect of radiotherapy is not satisfactory due to the reduced sensitivity of NSCLC cells to X-rays.41 In this study, we validate that XIST was up-regulated in NSCLC tissues and cell lines, while miR-16-5p was down-regulated. Additionally, we proved that XIST knockdown sensitized NSCLC cells to irradiation, and XIST could positively regulate the expression of WEE1, probably via repressing miR-16-5p. Our work provided a novel ceRNA network compose of XIST, miR-16-5p and WEE1, which participated in the radioresistance of NSCLC cells.
It is worth noting that in recent years, more and more studies confirm that non-coding RNA plays an important regulatory role in the occurrence and development of various malignant tumors.7,42–45 For example, XIST expression level is abnormally up-regulated in NSCLC, and associated with shorter survival time and worse prognosis.46 In terms of mechanism, XIST promotes the occurrence and development of NSCLC through inhibiting KLF2 expression by binding to EZH2.46 Moreover, XIST is confirmed to be an oncogene in nasopharyngeal carcinoma, and it promotes cancer progression through up-regulating E2F3 expression by sponging miR-34a-5p.47 It is also indicated that XIST knockdown inhibits proliferation of nasopharyngeal carcinoma cells and increases radiosensitivity by inhibiting DNA damage repair.21 In this study, we found XIST expression was markedly upregulated in NSCLC tissue and cell lines. The expression level of XIST in NSCLC cells was observably increased under the irradiation. Besides, we successfully constructed NSCLC cell models with lowly expressed XIST. Knockdown of XIST significantly inhibited cell proliferation and survival, and promoted apoptosis, suggesting that XIST was a promising therapeutic target to sensitize NSCLC cells to radiotherapy.
MiRNAs are also important regulators of radioresistance in human tumor cells.26,48 For example, miR-16-5p targets Cyclin D1/E1 3’-UTR in prostate cancer and induces cell cycle arrest in the G0/G1 phase by modulating the Cyclin D1/Cyclin E1/pRb/E2F1 pathway; overexpression of miR-16-5p inhibits cell viability and promotes apoptosis to enhance the sensitivity to X-rays.28 In addition, miR-16-5p expression is reported to be significantly down-regulated in the serum of NSCLC patients.38 Therefore, we were curious about the role of miR-16-5p in the development of radioresistance in NSCLC cells and its regulatory mechanisms. In the present study, we found that miR-16-5p expression was observably down-regulated in NSCLC tissues compared with that in adjacent normal tissues. Subsequently, we predicted the presence of binding sites between XIST and miR-16-5p through an online bioinformatics database. Dual luciferase reporter assay verified that XIST could sponge miR-16-5p and negatively regulate the expression of the latter. Functional experiments manifested that the promoting effect of knockdown XIST on the radiosensitivity of NSCLC cells could be reversed by the miR-16-5p inhibitors. Therefore, we concluded that XIST reduced the radiosensitivity of NSCLC cells by modulating the expression of miR-16-5p.
In recent years, the significance of WEE1 in regulating the biological behaviors of NSCLC cells has become increasingly prominent, including its regulatory function on the radiosensitivity of cancer cells.49,50 It is reported that inhibition of WEE1 expression is a potential therapeutic approach to increase the radiosensitivity of NSCLC cells harboring p53 and KRAS mutations.37,51 In the present work, we explored the regulatory mechanism of WEE1 expression in NSCLC cells. We found that WEE1 was a downstream target of miR-16-5p, and its expression level was negatively regulated by the latter. Subsequently, we transfected si-XIST into NSCLC cells and found that the expression of WEE1 was significantly inhibited. Therefore, we concluded that WEE1 was directly and negatively regulated by miR-16-5p and could be positively and indirectly regulated by XIST.
Conclusion
In summary, knockdown of XIST enhances the radiosensitivity of NSCLC cells by modulating the miR-16-5p/WEE1 axis, and it is expected to expand the understanding of the molecular mechanisms of radioresistance of NSCLC. However, this study in which only in vitro experiments were conducted needs to be reconfirmed with animal models.
Supplemental Material
Supplemental material, sj-tif-1-iji-10.1177_2058738420966087 for Knockdown of lncRNA X inactive specific transcript (XIST) radiosensitizes non-small cell lung cancer (NSCLC) cells through regulation of miR-16-5p/WEE1 G2 checkpoint kinase (WEE1) axis by Ran Du, Feng Jiang, Yanhua Yin, Jinfen Xu, Xia Li, Likuan Hu and Xiuyu Wang in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-tif-2-iji-10.1177_2058738420966087 for Knockdown of lncRNA X inactive specific transcript (XIST) radiosensitizes non-small cell lung cancer (NSCLC) cells through regulation of miR-16-5p/WEE1 G2 checkpoint kinase (WEE1) axis by Ran Du, Feng Jiang, Yanhua Yin, Jinfen Xu, Xia Li, Likuan Hu and Xiuyu Wang in International Journal of Immunopathology and Pharmacology
Acknowledgments
None.
Footnotes
Authors’ Contribution: Conceived and designed the experiments: JFX, LKH, XYW;
Performed the experiments: JFX, XL, LKH, RD, FJ, YHY;
Analyzed statistic: JFX;
Wrote the paper: JFX, LKH;
Performed the revision: RD, FJ, YHY.
All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from Ethnic Committee of Qilu Hospital (APPROVAL NUMBER: 201705006)*.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Not applicable.
ORCID iD: Likuan Hu  https://orcid.org/0000-0002-6450-4633
https://orcid.org/0000-0002-6450-4633
Data Availability Statement: The data used to support the findings of this study are available from the corresponding author upon request.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Jiang W, Jin G, Cai F, et al. (2019) Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Experimental and Molecular Medicine 51(2): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu D, Li Y, Zhang H, et al. (2017) Knockdown of Lncrna PVT1 enhances radiosensitivity in non-small cell lung cancer by sponging Mir-195. Cellular Physiology and Biochemistry 42(6): 2453–2466. [DOI] [PubMed] [Google Scholar]
- 3. Sheng J, Wang L, Han Y, et al. (2018) Dual roles of protein as a template and a sulfur provider: A general approach to metalsulfides for efficient photothermal therapy of cancer. Small 14(1): 1702529. [DOI] [PubMed] [Google Scholar]
- 4. Zheng L, Wang Y, Xu Z, et al. (2019) Concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV non-small cell lung cancer harboring EGFR active mutations. Oncologist 24(8): 1031–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voong KR, Hazell SZ, Fu W, et al. (2019) Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clinical Lung Cancer 20(4): e470–e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin J, Zhao J, Hu W, et al. (2017) Disturbance of the let-7/LIN28 double-negative feedback loop is associated with radio- and chemo-resistance in non-small cell lung cancer. PLoS One 12(2): e0172787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou YL, Li Y, Luo DM, et al. (2015) Microstructures, mechanical and corrosion properties and biocompatibility of as extruded Mg-Mn-Zn-Nd alloys for biomedical applications. Materials Science and Engineering C: Materials for Biological Applications 49: 93–100. [DOI] [PubMed] [Google Scholar]
- 8. Tang L, Wei F, Wu Y, et al. (2018) Role of metabolism in cancer cell radioresistance and radiosensitization methods. Journal of Experimental and Clinical Cancer Research 37(1): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talebi A, Akbari A, Mobini GR, et al. (2019) Biological and clinical relevance of long non-coding RNA PCAT-1 in cancer, a systematic review and meta-analysis. Asian Pacific Journal of Cancer Prevention 20(3): 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang S, Zhang S, He Y, et al. (2019) HOXA11-AS regulates JAK-STAT pathway by miR-15a-3p/STAT3 axis to promote the growth and metastasis in liver cancer. Journal of Cellular Biochemistry 120(9): 15941–15951. [DOI] [PubMed] [Google Scholar]
- 11. Bhan A, Soleimani M, Mandal SS. (2017) Long noncoding RNA and cancer: A new paradigm. Cancer Research 77(15): 3965–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang KC, Yang YW, Liu B, et al. (2011) A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472(7341): 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou J, Wang C, Gong W, et al. (2018) uc.454 Inhibited growth by targeting heat shock protein family a member 12B in non-small-cell lung cancer. Molecular Therapy: Nucleic Acids 12: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Hu J, Li J, et al. (2019) Long noncoding RNA LINC-PINT inhibits non-small cell lung cancer progression through sponging miR-218-5p/PDCD4. Artificial Cells, Nanomedicine, and Biotechnology 47(1): 1595–1602. [DOI] [PubMed] [Google Scholar]
- 15. Hu X, Duan L, Liu H, et al. (2019) Long noncoding RNA LINC01296 induces non-small cell lung cancer growth and progression through sponging miR-5095. American Journal of Translational Research 11(2): 895–903. [PMC free article] [PubMed] [Google Scholar]
- 16. Chen B, Ling CH. (2019) Long noncoding RNA AK027294 acts as an oncogene in non-small cell lung cancer by up-regulating STAT3. European Review for Medical and Pharmacological Sciences 23(3): 1102–1107. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Zhang G, Cheng Z, et al. (2018) Knockdown of LncRNA-XIST suppresses proliferation and TGF-β1-induced EMT in NSCLC through the Notch-1 pathway by regulation of miR-137. Genetic Testing and Molecular Biomarkers 22(6): 333–342. [DOI] [PubMed] [Google Scholar]
- 18. Xu Z, Xu J, Lu H, et al. (2017) LARP1 is regulated by the XIST/miR-374a axis and functions as an oncogene in non-small cell lung carcinoma. Oncology Reports 38(6): 3659–3667. [DOI] [PubMed] [Google Scholar]
- 19. Li C, Wan L, Liu Z, et al. (2018) Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Letters 418: 185–195. [DOI] [PubMed] [Google Scholar]
- 20. Jiang H, Zhang H, Hu X, et al. (2018) Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. International Journal of Biological Macromolecules 111:623–631. [DOI] [PubMed] [Google Scholar]
- 21. XHan Q, Li L, Liang H, et al. (2017) Downregulation of lncRNA X inactive specific transcript (XIST) suppresses cell proliferation and enhances radiosensitivity by upregulating mir-29c in nasopharyngeal carcinoma cells. Medical Science Monitor 23: 4798–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valdmanis PN, Kim HK, Chu K, et al. (2018) miR-122 removal in the liver activates imprinted microRNAs and enables more effective microRNA-mediated gene repression. Nature Communications 9: 5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tam C, Wong JH, Tsui SKW, et al. (2019) LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: Updates in recent years. Applied Microbiology and Biotechnology 103(12): 4649–4677. [DOI] [PubMed] [Google Scholar]
- 24. Braicu C, Zimta AA, Harangus A, et al. (2019) The function of non-coding rnas in lung cancer tumorigenesis. Cancers (Basel) 11(5): 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomasik B, Chałubińska-Fendler J, Chowdhury D, et al. (2018) Potential of serum microRNAs as biomarkers of radiation injury and tools for individualization of radiotherapy. Translational Research 201: 71–83. [DOI] [PubMed] [Google Scholar]
- 26. Ni J, Bucci J, Chang L, et al. (2017) Targeting microRNAs in prostate cancer radiotherapy. Theranostics 7(13): 3243–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Bezawy R, Tinelli S, Tortoreto M, et al. (2019) miR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCε and ZEB1 inhibition. Journal of Experimental & Clinical Cancer Research 38(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang F, Mao A, Tang J, et al. (2019) MicroRNA-16-5p enhances radiosensitivity through modulating Cyclin D1/E1-pRb-E2F1 pathway in prostate cancer cells. Journal of Cellular Physiology 234(8): 13182–13190. [DOI] [PubMed] [Google Scholar]
- 29. Ghiasi N, Habibagahi M, Rosli R, et al. (2014) Tumour suppressive effects of WEE1 gene silencing in breast cancer cells. Asian Pacific Journal of Cancer Prevention 14(11): 6605–6611. [DOI] [PubMed] [Google Scholar]
- 30. Do K, Doroshow JH, and Kummar S. (2013) Wee1 kinase as a target for cancer therapy. Cell Cycle 12(19): 3159–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HY, Cho Y, Kang H, et al. (2016) Targeting the WEE1 kinase as a molecular targeted therapy for gastric cancer. Oncotarget 7(31): 49902–49916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qi W, Xu X, Wang M, et al. (2019) Inhibition of Wee1 sensitizes AML cells to ATR inhibitor VE-822-induced DNA damage and apoptosis. Biochemical Pharmacology 164: 273–282. [DOI] [PubMed] [Google Scholar]
- 33. Lee JW, Parameswaran J, Sandoval-Schaefer T, et al. (2019) Combined Aurora Kinase A (AURKA) and WEE1 inhibition demonstrates synergistic antitumor effect in squamous cell carcinoma of the head and neck. Clinical Cancer Research 25(11): 3430–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magnussen GI, Holm R, Emilsen E, et al. (2012) High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS One 7(6): e38254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. PosthumaDeBoer J, Würdinger T, Graat HC, et al. (2011) WEE1 inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer 11: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karnak D, Engelke CG, Parsels LA, et al. (2014) Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clinical Cancer Research 20(19): 5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parsels LA, Karnak D, Parsels JD, et al. (2018) PARP1 trapping and DNA replication stress enhance radiosensitization with combined WEE1 and PARP inhibitors. Molecular Cancer Research 16(2): 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan L, Qi H, Teng J, et al. (2016) Identification of serum miRNAs by nano-quantum dots microarray as diagnostic biomarkers for early detection of non-small cell lung cancer. Tumor Biology 37(6): 7777–7784. [DOI] [PubMed] [Google Scholar]
- 39. Lee JH, Sung JY, Choi EK, et al. (2019) C/EBPβ is a transcriptional regulator of Wee1 at the G₂/M phase of the cell cycle. Cells 8(2): 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Zhang DX, Ju T, et al. (2019) The effect of postoperative radiotherapy on the survival of patients with resectable stage III-N2 non-small-cell lung cancer: A systematic review and meta-analysis. Neoplasma 66(5): 717–726. [DOI] [PubMed] [Google Scholar]
- 41. Li Q, Zong Y, Li K, et al. (2019) Involvement of endothelial CK2 in the radiation induced perivascular resistant niche (PVRN) and the induction of radioresistance for non-small cell lung cancer (NSCLC) cells. Biological Research 52(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiong K, Shao LH, Zhang HQ, et al. (2018) MicroRNA-9 functions as a tumor suppressor and enhances radio-sensitivity in radio-resistant A549 cells by targeting neuropilin 1. Oncology Letters 15(3): 2863–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma W, Ma CN, Zhou NN, et al. (2016) Up-regulation of miR-328-3p sensitizes non-small cell lung cancer to radiotherapy. Scientific Reports 6: 31651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang QS, Li B, Xu G, et al. (2019) Long noncoding RNA LINC00483/microRNA-144 regulates radiosensitivity and epithelial-mesenchymal transition in lung adenocarcinoma by interacting with HOXA10. Journal of Cellular Physiology 234(7): 11805–11821. [DOI] [PubMed] [Google Scholar]
- 45. Chen J, Shen Z, Zheng Y, et al. (2015) Radiotherapy induced Lewis lung cancer cell apoptosis via inactivating β-catenin mediated by upregulated HOTAIR. International Journal of Clinical and Experimental Pathology 8(7):7878–7886. [PMC free article] [PubMed] [Google Scholar]
- 46. Fang J, Sun CC, Gong C. (2016) Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochemical and Biophysical Research Communications 478(2): 811–817. [DOI] [PubMed] [Google Scholar]
- 47. Song P, Ye LF, Zhang C, et al. (2016) Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene 592(1): 8–14. [DOI] [PubMed] [Google Scholar]
- 48. Li H, Jin X, Chen B, et al. (2018) Autophagy-regulating microRNAs: Potential targets for improving radiotherapy. Journal of Cancer Research and Clinical Oncology 144(9): 1623–1634. [DOI] [PubMed] [Google Scholar]
- 49. Caiola E, Frapolli R, Tomanelli M, et al. (2018) Wee1 inhibitor MK1775 sensitizes KRAS mutated NSCLC cells to sorafenib. Scientific Reports 8(1): 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoshida T, Tanaka S, Mogi A, et al. (2004) The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Annals of Oncology 15(2): 252–256. [DOI] [PubMed] [Google Scholar]
- 51. Ku BM, Bae YH, Koh J, et al. (2017) Mutational status of TP53 defines the efficacy of Wee1 inhibitor AZD1775 in KRAS-mutant non-small cell lung cancer. Oncotarget 8(40): 67526–67537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-iji-10.1177_2058738420966087 for Knockdown of lncRNA X inactive specific transcript (XIST) radiosensitizes non-small cell lung cancer (NSCLC) cells through regulation of miR-16-5p/WEE1 G2 checkpoint kinase (WEE1) axis by Ran Du, Feng Jiang, Yanhua Yin, Jinfen Xu, Xia Li, Likuan Hu and Xiuyu Wang in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-tif-2-iji-10.1177_2058738420966087 for Knockdown of lncRNA X inactive specific transcript (XIST) radiosensitizes non-small cell lung cancer (NSCLC) cells through regulation of miR-16-5p/WEE1 G2 checkpoint kinase (WEE1) axis by Ran Du, Feng Jiang, Yanhua Yin, Jinfen Xu, Xia Li, Likuan Hu and Xiuyu Wang in International Journal of Immunopathology and Pharmacology