Abstract
The present study aimed to investigate the effect and possible mechanism of recombinant human thrombopoietin (rhTPO) on mouse 32D cells (a mouse myeloid progenitor cell line) treated with serum from patients with aplastic anemia and to elucidate the potential mechanism of rhTPO in the treatment of aplastic anemia. After treatment with aplastic anemia serum, the apoptotic rate of 32D cells was increased and the proliferation of 32D cells was significantly inhibited. rhTPO reduced the apoptotic rate and promoted the proliferation of 32D cells, while rhTPO failed to restore the cell proliferation of 32D cells from aplastic anemia serum group to the normal level as compared to that from the normal serum group. The phosphorylation level of STAT3 protein was higher, and the phosphorylation level of STAT5 protein was lower in 32D cells from aplastic anemia serum group than that in normal serum group. After rhTPO treatment, the phosphorylation level of STAT3 protein in aplastic anemia serum group was decreased and the phosphorylation level of STAT5 protein was increased. The expression levels of Survivin and Bcl-2 were significantly decreased in 32D cells from aplastic anemia serum group, which were significantly increased after rhTPO treatment. The expression level of Bax protein in 32D cells from the normal serum group after rhTPO treatment was significantly decreased; while the mRNA expression level of Bax was not affected by rhTPO. The expression levels of Bax mRNA and protein were significantly up-regulated in 32D cells from aplastic anemia serum group, which was significantly decreased by rhTPO treatment. In conclusion, our results indicated that aplastic anemia serum impaired proliferative potential and enhanced apoptosis of 32D cells. Further mechanistic studies revealed that rhTPO promoted cell proliferation and attenuated apoptosis of aplastic anemia serum-treated 32D cells via activating STAT3/STAT5 signaling pathway and modulating apoptosis-related mediators.
Keywords: 32D cells, rhTPO, aplastic anemia, proliferation, apoptosis
Introduction
Aplastic anemia is a typical representative of human bone marrow failure syndrome. Aplastic anemia is a T-cell-mediated autoimmune disease, mainly due to the inhibition of interferon-γ (IFN-γ), however, the exact pathological mechanism is not clear. The main clinical manifestations of aplastic anemia are bleeding, infection, anemia1–3. If aplastic anemia is not properly treated, it will be life threatening. Allogeneic hematopoietic stem cell transplantation is the standard treatment for aplastic anemia4, while it requires appropriate donors and has the risk of developing the graft-versus-host disease5. Standard immunotherapy is not only expensive, but also is easy to relapse in 30–50% patient6,7. Therefore, it is very important to find new and effective methods to improve the treatment efficacy of refractory aplastic anemia.
Thrombopoietin (TPO) is a hematopoietic growth factor secreted by the liver that specifically regulates platelet production. By binding to TPO receptor (MPL), TPO can induce hematopoietic stem cells to differentiate into megakaryocytes, stimulate the proliferation and differentiation of megakaryocytes, and promote production and release of the platelets8. Recombinant human TPO (rhTPO) is the first synthesized in China9. It can promote the proliferation, differentiation and maturation of megakaryocytes by binding to hematopoietic cytokine receptor c-Mpl10,11. C-Mpl is mainly expressed in hematopoietic cells, especially in megakaryocytes and pre-megakaryocytes12. The interaction of rhTPO and c-Mpl can promote the proliferation, differentiation and platelet production of megakaryocytes13. It can also promote the proliferation of hematopoietic stem cells14,15. Zhang et al., analyzed 40 cases of aplastic anemia patients who received immunosuppressive therapy and/or rhTPO16. The results showed that immunosuppressive therapy plus rhTPO significantly improved hematological response and separated from platelet transfusion earlier as compared to immunosuppressive therapy alone. Shao et al., reported that rhTPO can improve the hematological response and bone marrow recovery of aplastic anemia patients who received immunosuppressive therapy17. It also promoted the production of red blood cells and reduced the need for blood transfusion, which suggested that rhTPO has auxiliary effects on aplastic anemia. Recently, studies also showed that the mechanism of eltrombopa in the treatment of aplastic anemia is to relieve the inhibition of IFN-γ on the self-renewal and proliferation of CD34+ cells, while increasing the concentration of rhTPO in vitro does not relieve the inhibition of IFN-γ on the self-renewal and proliferation of CD34+ cells18.
In order to further clarify the mechanism of rhTPO in the treatment of aplastic anemia, we used mouse 32D cells (a mouse myeloid progenitor cell line) to observe the effect of rhTPO on cell proliferation and apoptosis of 32D cells treated with aplastic anemia serum, and to explore the possible mechanism of rhTPO in the treatment of aplastic anemia.
Materials and Methods
Cell Line and Cell Culture
The 32D cell line was a generous gift from the Toledo University (Ohio, USA). The 32D cells were cultured with RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10 ng/ml of interleukin-3 (Sigma, St. Louis, MO, USA) and 10% fetal bovine serum (Thermo Fisher Scientific) and were kept in a humidified incubator with 5% CO2 at 37°C. The cell culture medium was changed every 3–4 days.
Lentivirus Infection and Serum and TPO Treatment
The TPO receptor overexpressing vector was packaged into the lentivirus (Shanghai Biotechnology Co, Ltd., Shanghai, China). The 32D cells were infected with the constructed lentivirus according to the manufacturer’s protocol (Shanghai Biotechnology Co, Ltd.). For the serum treatment, the normal serum was obtained from healthy volunteers from Affiliated Hospital of Nantong University. The aplastic anemia serum was obtained from patients with aplastic anemia in the Affiliated Hospital of Nantong University between January 2016 and August 2017. The procedures were approved the Ethics Committee of Affiliated Hospital of Nantong University and each patient signed the informed consent. The 32D cells were treated with 10% serum from healthy subjects or 10% serum from aplastic anemia patients. For the TPO (Sigma) treatment, different concentrations of TPO (50, 100, 150, and 200 U/ml) were used to treat 32D cells for 24 h before further experimental assays.
Analysis of 32D Cell Number by Trypan Blue Counting
The 32D cells with different treatments were seeded with 8,000 cells/well in the 96-well plates. At 24, 48, and 72 h, trypan blue staining was used to assess the number of living cells and the cell survival rate. The experiments were repeated at least for three times.
Determination of 32D Cell Proliferation Using Cell Counting Kit-8 (CCK-8) Assay
The cell proliferation of 32D cells was determined by the CCK-8 assay kit (Beyotime, Beijing, China). The 32D cells with different treatments were seeded onto the 96-well plate at a density of 2,000 cells/well. After culturing for indicated time duration, the cells were incubated with 10 µL of CCK-8 well at 37 °C for 3 h. The proliferation of 32D cells was determined by measuring absorbance at 450 nm.
Flow Cytometry to Detect Apoptosis of 32D Cells
Annexin V-FITC/PI apoptosis detection kit (Thermo Fisher Scientific) was used to detect the apoptosis of 32D cells. The adherent cells after different treatments were collected, washed, and re-suspended in ice-cold binding buffer and diluted to a final concentration of 5 × 105 cells/mL. Aliquots of 1 × 105 cells were incubated with 0.5 µL of Annexin-V-FITC and 10 µL of PI per tube for 15 min at room temperature. After that, 400 µL of binding buffer was added before the flow cytometric analysis. For each sample, 1 × 104 cells were analyzed on a FACS II flow cytometer (BD Biosciences, San Jose, CA, USA). CellQuestTM Pro software (BD Biosciences) was used to perform the flow cytometric analysis.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Total RNA was reversely transcribed into cDNA using the cDNA Synthesis Kit (Sigma). The real-time PCR was carried out on the ABI 7900 PCR detection system (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq II kit (Takara, Dalian, China). The relative expression levels of these genes were analyzed using comparative Ct method. GAPDH was used as the internal control for gene expression. The primer sequences for the qRT-PCR were summarized in Table 1.
Table 1.
The Primer Sequences for qRT-PCR.
| Genes | Primer sequences | Fragments (bp) |
|---|---|---|
| Survivin | Forward: 5′-CAACCGGACGAATGCTTTT-3′ Reverse: 5′-AAGAACTGGCCCTTCTTGGA-3′ |
124 |
| Bcl-2 | Forward: 5′-GCTACCGTCGTGACTTCGC-3′ Reverse: 5′-CCCCACCGAACTCAAAGAAGG-3′ |
147 |
| Bax | Forward: 5′-AGACAGGGGCCTTTTTGCTAC-3′ Reverse: 5′-AATTCGCCGGAGACACTCG-3′ |
137 |
| Bcl-xl | Forward:5′- ACATCCCAGCTTCACATAACCC-3′ Reverse: 5′-CCATCCCGAAAGAGTTCATTCAC-3′ |
77 |
| c-myc | Forward: 5′- GGCTCCTGGCAAAAGGTCA-3′ Reverse: 5′-CTGCGTAGTTGTGCTGATGT-3′ |
119 |
| GAPDH | Forward: 5′- GGAGCGAGATCCCTCCAAAAT-3′ Reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
197 |
Western Blot Assay
The 32D cells with different treatments were washed and and the protein sample from the cells were extracted using radio-immunoprecipitation assay buffer containing proteinase inhibitor (Roche, Basel, Switzerland). The protein levels were determined using a BCA kit (Thermo Fisher Scientific). The proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by transferring to polyvinylidene difluoride (Millipore, Bedford) membranes. The membranes were then blocked with 5% bovine serum albumin and incubated overnight with antibodies against TPO (1:1000; #ab180728), p-STAT3 (1:1000; #ab32143), t-STAT3 (1:1500; #ab119352), p-STAT5 (1:800; #ab32364), t-STAT5 (1:1000; #ab32364), Bcl-2 (1:1500; #ab32124), survivin (1:2000; #ab649), Bax (1:2000; #ab32503), c-myc (1:1500; #32072), Bcl-xL (1:1500; #32370), and GAPDH (1:1000; Abcam, Cambridge, UK) at dilutions specified by the manufacturer’s instructions. After incubating with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h, an ECL chemiluminescence detection system (Pierce Biotechnology Inc, Rockford, IL, USA) were used according to the manufacturer’s instructions to detect and semi-quantitatively analyze the proteins.
Statistical Analysis
All the data analysis was performed using the GraphPad Prism V5.0 (GraphPad Software, La Jolla, CA, USA). All the experimental data were presented as mean ± standard deviation. Significant differences between treatment groups were assessed using unpaired Student’s t-test, one-way ANOVA or two-way ANOVA followed by Bonferroni’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Effects of TPO on the Cell Proliferation of in 32D Cells with rhTPO Receptor Overexpression
Firstly, the 32D cells were infected with the lentivirus vector with TPO receptor overexpression, and the western blot results showed that the protein levels of TPO receptor was significantly up-regulated in the 32D cells infected with TPO receptor-overexpressing vector (Fig. 1A). Furthermore, CCK-8 assay was performed to determine the effects of rhTPO on the proliferation of the 32D cells with TPO receptor overexpression. As shown in Fig. 1B, rhTPO concentration dependently promoted the cell proliferation, and the maximal effects were seen at the concentration of 100 U/ml. Thus, 100 U/ml of rhTPO was selected for subsequent studies.
Figure. 1.
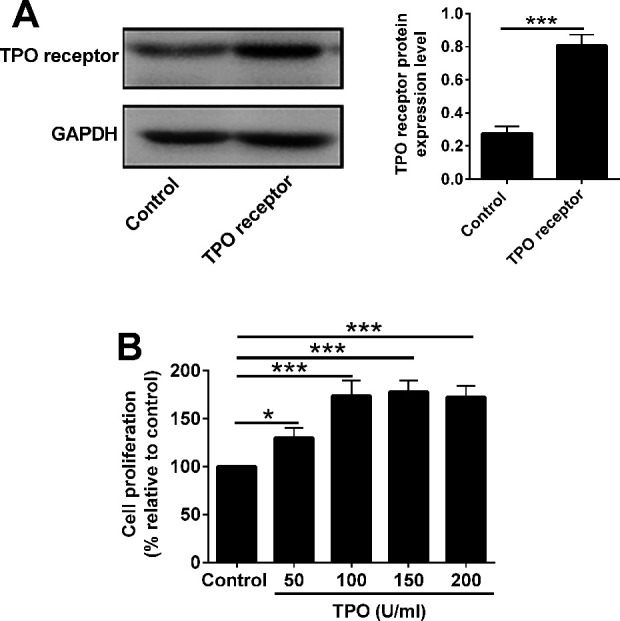
Effects of rhTPO on the cell proliferation of in 32Dcells with TPO receptor overexpression. (A) Western blot analysis of TPO receptor in 32D cells transfected with control lentivirus or lentivirus with TPO receptor overexpression. (B) 32D cells with rhTPO receptor overexpression were treated with different concentrations of TPO. CCK-8 assay was used to analyze 32D cell viability. N = 3; *P < 0.05 and ***P < 0.001.
rhTPO Promoted Proliferative Potential of 32D Cells With TPO Receptor Overexpression via Regulating STAT3/STAT5 Expression
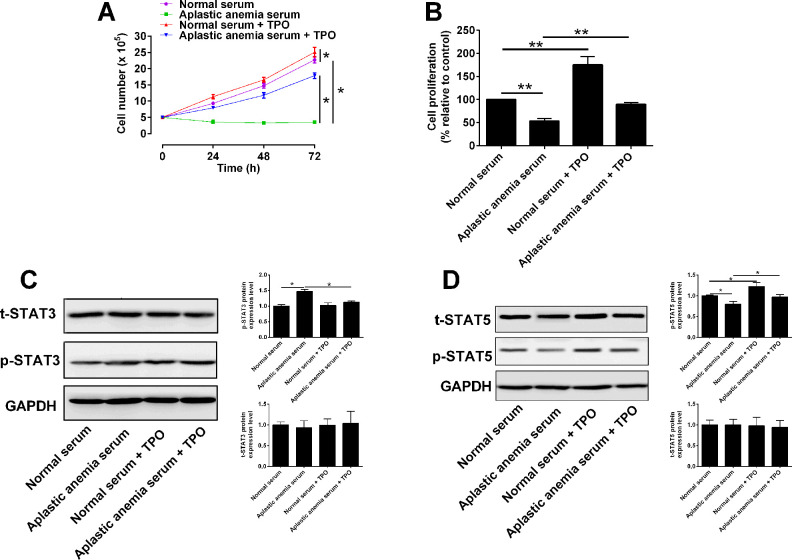
Next, the effects of normal serum and aplastic anemia serum on the proliferation of 32D cells were studied. 32D cells with TPO receptor overexpression received following treatments: (1) normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO. The trypan blue assay results showed that 32D cells in aplastic anemia serum did not proliferate at 24, 48, and 72 h, and the proliferative potential of 32D cells in the aplastic anemia serum was significantly lower than that in the normal serum (Fig. 2A). Compared with aplastic anemia serum group, rhTPO increased the number of 32D cells, but rhTPO treatment could not restore the number of 32D cells to the level in the normal serum group (Fig. 2A). The CCK-8 results showed that aplastic anemia serum significantly inhibited the proliferation of 32D cells compared with normal serum group (Fig. 2B). Under the condition of normal serum and aplastic anemia serum culture, rhTPO promoted the proliferation of 32D cells, but rhTPO failed to restore the proliferation level of cells in aplastic anemia serum group to that in normal serum group (Fig. 2B)
Figure 2.
rhTPO promoted proliferative potential of 32D cells with TPO receptor overexpression. 32D cells with TPO receptor overexpression received following treatments: (1) Normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO, and (A) number of viable cells was analyzed by Tryplan blue staining; (B) cell proliferation of 32D cells was analyzed by CCK-8 assay; (C) protein levels of t-STAT3 and p-STAT3 were analyzed by western blot; (D) protein levels of t-STAT5 and p-STAT5 were analyzed by western blot. N = 3; *P < 0.05 and **P < 0.01.
Our previous study found that the increased phosphorylation of STAT3 protein and decreased phosphorylation of STAT5 protein were involved in the signal transduction process of 32D cell apoptosis induced by aplastic anemia serum14,15. The present study determined whether rhTPO can reverse the phosphorylation changes of STAT3 and STAT5 in 32D cells treated by aplastic anemia serum. The western blot results showed that the phosphorylation level of STAT3 protein in 32D cells of aplastic anemia serum group was higher than that of normal serum group (Fig. 2C), and the phosphorylation level of STAT5 protein in the aplastic anemia serum group was significantly lower than that of normal serum group (Fig. 2D). Further study found that rhTPO treatment can decreased the phosphorylation level of STAT3 protein and increased the phosphorylation level of STAT5 protein in aplastic anemia serum group (Fig. 2C, D). The results suggested that rhTPO-mediated increase in the proliferation of 32D cells is closely related to the activation of STAT3 and STAT5.
rhTPO Attenuated Apoptosis of 32D Cells with TPO Receptor Overexpression
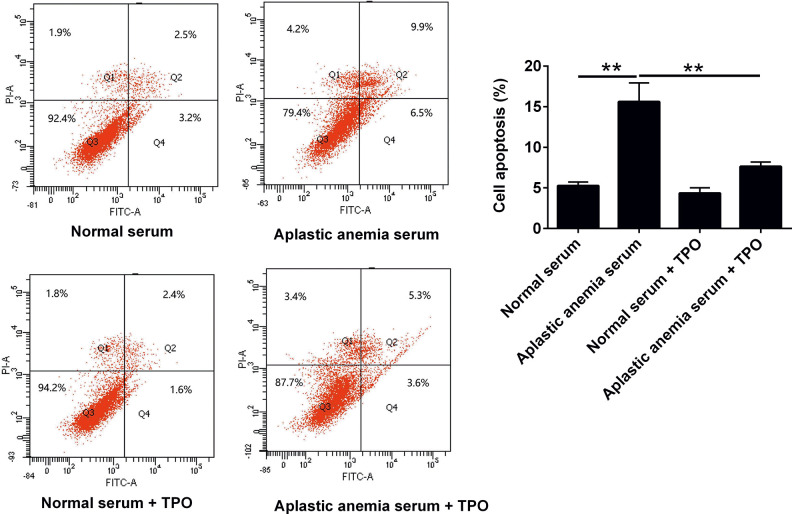
To further explore the relationship between rhTPO and 32D cell apoptosis, flow cytometry was used to detect cell apoptosis. The results showed that aplastic anemia serum could significantly induce the apoptosis of 32D cells compared with normal serum culture group (Fig. 3). Under the condition of aplastic anemia serum culture, rhTPO attenuated the apoptosis of 32D cells, but rhTPO can not restore apoptotic level of 32D cells in aplastic anemia serum group to that in normal serum group (Fig. 3).
Figure 3.
TPO attenuated apoptosis of 32D cells with TPO receptor overexpression. 32D cells with TPO receptor overexpression received following treatments: (1) Normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO, and cell apoptosis was analyzed by flow cytometry.
Effects of rhTPO Treatment on the Expression of Bcl-2 and Survivin in 32D Cells with TPO Receptor Overexpression
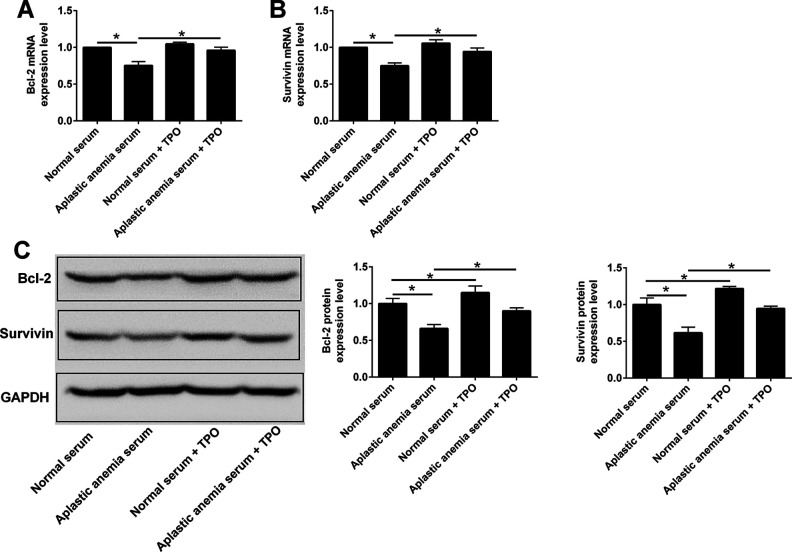
Survivin is a member of apoptosis inhibitory protein family, which plays an important role in regulating cell proliferation and apoptosis19. Bcl-2 protein is a mitochondrial membrane protein, which can inhibit apoptosis20. In order to further explore the molecular mechanism of rhTPO in promoting the proliferation of 32D cells and inhibiting apoptosis, the mRNA and protein expression levels of survivin and Bcl-2 were detected. Compared with the normal serum group, the protein expression levels of survivin and Bcl-2 in 32D cells of the normal serum group after rhTPO treatment were up-regulated, but the mRNA level was not affected (Fig. 4). Further results showed that compared with the normal serum group, the mRNA and protein levels of survivin and Bcl-2 in 32D cells from aplastic anemia serum group were significantly down-regulated, and the mRNA and protein levels of survivin and Bcl-2 in 32D cells from aplastic anemia serum group were significantly up-regulated after rhTPO treatment (Fig. 4).
Figure 4.
Effects of rhTPO treatment on the expression of Bcl-2 and survivin in 32D cells with TPO receptor overexpression. 32D cells with TPO receptor overexpression received following treatments: (1) Normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO, and (A) Blc-2 mRNA and (B) survivin mRNA expression levels were determined by qRT-PCR. (C) Bcl-2 and survivin protein levels were determined by western blot assay. N = 3; *P < 0.05.
Effects of rhTPO Treatment on the Expression of Bax in 32D Cells with TPO Receptor Overexpression
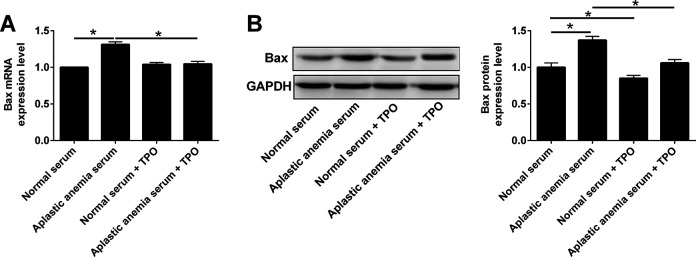
Bax is a member of Bcl-2 protein family, which forms heterodimer with Bcl-2 and functions to promote cell apoptosis21,22. In order to investigate whether rhTPO can regulate the expression of Bax of 32D cells induced by aplastic anemia serum, qRT-PCR and western blot were used to detect the mRNA and protein levels of Bax. Compared with the normal serum group, the protein expression level of Bax in 32D cells from the normal serum group after rhTPO treatment was significantly decreased (Fig. 5); while the mRNA level was not affected (Fig. 5). Further results showed that compared with the normal serum group, Bax mRNA and protein levels of 32D cells in aplastic anemia serum group were significantly increased (Fig. 5); while Bax mRNA and protein levels of 32D cells in aplastic anemia serum group were significantly decreased after rhTPO treatment (Fig. 5).
Figure 5.
Effects of rhTPO treatment on the expression of Bax in 32D cells with TPO receptor overexpression. 32D cells with TPO receptor overexpression received following treatments: (1) Normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO, and (A) Bax mRNA and (B) Bax protein expression levels were determined by qRT-PCR and western blot, respectively. N = 3; *P < 0.05.
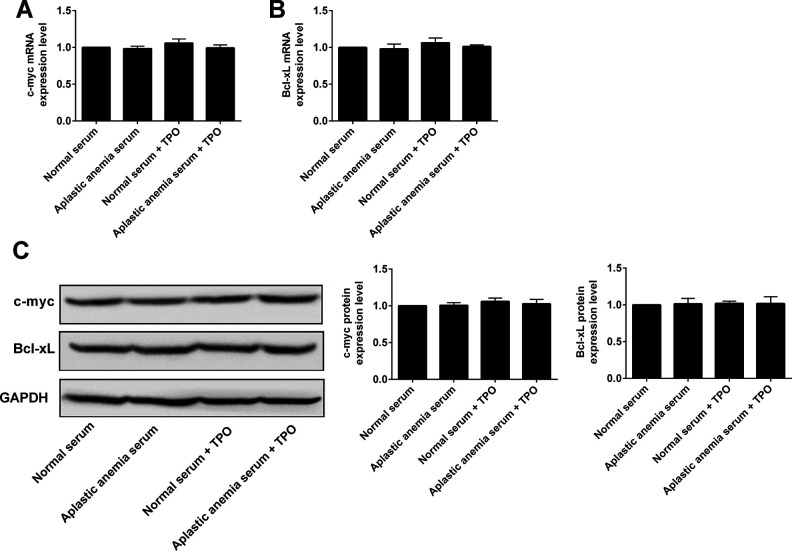
Effects of rhTPO Treatment on the Expression of c-myc and Bcl-xL in 32D Cells with TPO Receptor Overexpression
Furthermore, the results showed that the mRNA and protein expression level of Bcl-xL and c-myc had no significant changes among normal serum, aplastic anemia serum, normal serum + rhTPO, and aplastic anemia serum + rhTPO groups (Fig. 6). These results suggest that rhTPO may not play a role in modulating the Bcl-xl and c-myc signaling pathway.
Figure 6.
Effects of rhTPO treatment on the expression of c-myc and Bcl-xL in 32D cells with TPO receptor overexpression. 32D cells with TPO receptor overexpression received following treatments: (1) Normal serum, (2) aplastic anemia serum, (3) normal serum + rhTPO, (4) aplastic anemia serum + rhTPO, and (A) c-myc mRNA and (B) Bcl-xL mRNA expression levels were determined by qRT-PCR. (C) c-myc and Bcl-xL protein levels were determined by western blot assay. N = 3.
Discussion
Aplastic anemia is generally considered as a heterogeneous disease, and abnormal activation of T lymphocytes and hyperfunction of bone marrow play a major role in the pathogenesis of acquired aplastic anemia23. Allogeneic hematopoietic stem cell transplantation and immunosuppressant are the first-line treatment for aplastic anemia, but some patients still have poor efficacy. The treatment of eltrombopag and rhTPO provides a new choice for patients with relapsed and refractory aplastic anemia. Studies have shown that rhTPO can improve the immunosuppressive therapy-based hematological response rate of aplastic anemia and accelerate platelet recovery16. It is suggested that exogenous rhTPO can improve the hematopoiesis of aplastic anemia patients on the basis of the increased endogenous rhTPO level. However, Alvarado et al., suggest that increasing rhTPO concentration in vitro does not relieve the inhibition of IFN-γ on the self-renewal and proliferation of CD34 + cells18. Therefore, it is necessary to further elucidate the mechanism of rhTPO in the treatment of aplastic anemia.
The main component of hemopoietic inhibitors in sera of patients with aplastic anemia is IFN-γ24. IFN-γ can reduce the phosphorylation of rhTPO-mediated signal transducer and activator of transcription STAT, inhibit the proliferation of HSC, and promote cell apoptosis, which may lead to the pathogenesis of aplastic anemia25. To confirm whether rhTPO enhances HSC proliferation by regulating STAT pathway, in this study, 32D cells were stably transfected with the lentivirus overexpressing TPO receptor. The results showed that aplastic anemia serum could significantly inhibit the proliferation of 32D cells compared with normal serum culture. Further study showed that rhTPO could reverse the inhibitory effects of aplastic anemia serum on 32D cell proliferation. Western blot results further confirmed the phosphorylation level of STAT3 and STAT5 in 32D cells of aplastic anemia serum group. Compared with the normal serum group, the phosphorylation level of STAT3 protein in aplastic anemia serum group was significantly higher than that in normal serum group, and the phosphorylation level of STAT5 protein was significantly lower than that in normal serum group. After rhTPO treatment, STAT3 protein phosphorylation was decreased and STAT5 protein phosphorylation was increased. These results suggested that the effect of rhTPO on cell proliferation is closely related to the activation of STAT3 and STAT5 proteins.
STAT3 is purified as an acute phase response factor in the inflammatory process of interleukin-6 (IL-6) in 199426. STAT3 activation can induce the expression of a series of downstream transcription factors closely related to apoptosis, such as survivin, Bcl-2 and Bax. The activation or inhibition of these molecules can regulate the expression of upstream proteins. In order to further explore the downstream molecules of rhTPO to enhance the viability of 32D cells and inhibit apoptosis, the mRNA and protein of Survivin and Bcl-2 were detected. The results showed that the mRNA and protein levels of Survivin and Bcl-2 in 32D cells from aplastic anemia serum group were significantly lower than those of normal serum group. The mRNA and protein expression of Survivin and Bcl-2 in 32D cells of aplastic anemia serum group were significantly increased after rhTPO treatment. Compared with the normal serum group, the mRNA and protein expression level of Survivin and Bcl-2 in 32D cells from the normal serum group after rhTPO treatment were up-regulated, but the mRNA level was not significantly affected. These results suggested that rhTPO may enhance the survival ability and inhibit apoptosis of 32D cells to activate JAK-STAT pathway by up-regulating the expression of Survivin and Bcl-2 and down-regulating the phosphorylation level of STAT3 protein. Interestingly, the mRNA levels of Survivin and Bcl-2 in rhTPO treated 32D cells of normal serum group were not significantly higher than those in aplastic anemia group. We speculated that rhTPO did not affect the Survivin and Bcl-2 at the mRNA expression level in 32D cells, but affected the degradation of Survivin and Bcl-2 proteins in 32D cells. However, the mechanism underlying rhTPO-mediated-degradation of these proteins is still unclear, which needs further investigation.
Bax is a member of Bcl-2 protein family, forms heterodimer with Bcl-2, and can promote cell apoptosis21,22. In order to investigate whether rhTPO can regulate the expression of Bax to inhibit the apoptosis of 32D cells induced by aplastic anemia serum, qRT-PCR and Western blot assays were used to detect the mRNA and protein levels of Bax gene. The results showed that Bax mRNA and protein levels of 32D cells in aplastic anemia serum group were significantly higher than those in normal serum group. After rhTPO treatment, Bax mRNA and protein expression leveles of 32D cells in aplastic anemia serum group were significantly decreased. Compared with the normal serum group, the expression level of Bax protein in 32D cells from the normal serum group was significantly decreased after rhTPO treatment, but the mRNA level was not affected. These results suggested that the inhibition of rhTPO on 32D cells is related to the inhibition of Bax protein degradation pathway. At present, various genes that targeted by STAT3 have been identified, including Bcl-xL and Mcl-1, cyclin D1, c-myc, and angiogenic factors27,28.In this study, the mRNA and protein of Bcl-xl and c-myc were also detected by qRT-PCR and Western blot. The results suggest that rhTPO may not play a role in regulating Bcl-xl and c-myc expression.
The results from the present studies are still subjected to several limitations. The caspase signaling is the key pathway in regulating cell apoptosis, but we have not examined the expression or the activities of the caspases in our experiments, which should be considered in future studies. The current investigation is limited to just one cell line, and further studies may include more in vitro cellular models to confirm our findings. Moreover, the present study lacks the examination of the regulatory effects of rhTPO in the in vivo animal model, which is important to decipher the therapeutic role of rhTPO in aplastic anemia. As rhTPO-mediated signaling pathway may not be limited in the present study, further studies may consider to examine role of rhTPO in other signaling pathways associated with the pathophysiology of aplastic anemia.
Conclusions
In conclusion, our results indicated that aplastic anemia serum impaired proliferative potential and enhanced apoptosis of 32D cells. Further mechanistic studies revealed that rhTPO promoted cell proliferation and attenuated apoptosis of aplastic anemia serum-treated 32D cells via activating STAT3/STAT5 signaling pathway and modulating apoptosis-related mediators.
Footnotes
Authors Note: Juan Qian, and Xin Cao authors contributed to this work equally and should be regarded as co-first authors.
Authors Contribution: JQ, XY and HL conceived the whole study; JQ, XC and QS performed the experiments; YC and WL analyzed the data; HY collected the serum samples; all the authors approved the manuscript for submission.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval: The procedures were approved the Ethics Committee of Affiliated Hospital of Nantong University.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with guidelines of the Ethics Committee of Affiliated Hospital of Nantong University.
Statement of Informed Consent: Each patient signed the informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Nantong Science and Technology Project (MS12018050); The Spring Bud Plan of the research fund for young and middle-aged students in Sansheng TCP; the Jiangsu Youth Medical Talents Project (QNRC2016683).
ORCID iD: Hong Liu  https://orcid.org/0000-0002-2670-620X
https://orcid.org/0000-0002-2670-620X
References
- 1. Zeng Y, Katsanis E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol. 2015;180(3):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15(3):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436. [DOI] [PubMed] [Google Scholar]
- 5. Bacigalupo A. Alternative donor transplants for severe aplastic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018(1):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101(6):527–535. [DOI] [PubMed] [Google Scholar]
- 7. Peslak SA, Olson T, Babushok DV. Diagnosis and treatment of aplastic anemia. Curr Treat Options Oncol. 2017;18(12):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zang Y, Zhang X, Yuan D, Zhang Y, Zhu J, Lu H, Chang C, Qin J. Expression, purification, and characterization of a novel recombinant fusion protein, rhTPO/SCF, in Escherichia coli. Protein Expr Purif. 2006;47(2):427–433. [DOI] [PubMed] [Google Scholar]
- 10. Peck-Radosavljevic M, Wichlas M, Zacherl J, Stiegler G, Stohlawetz P, Fuchsjäger M, Kreil A, Metz-Schimmerl S, Panzer S, Steininger R, Mühlbacher F, et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95(3):795–801. [PubMed] [Google Scholar]
- 11. Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, Roth GJ, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369(6481):568–571. [DOI] [PubMed] [Google Scholar]
- 12. Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuter DJ. New thrombopoietic growth factors. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S347–S356. [DOI] [PubMed] [Google Scholar]
- 14. Fujiwara T, Harigae H, Kameoka J, Yokoyama H, Takahashi S, Tomiya Y, Yamada M, Ishizawa K, Imaizumi M, Sasaki T. A case of familial thrombocytosis: possible role of altered thrombopoietin production. Am J Hematol. 2004;76(4):395–397. [DOI] [PubMed] [Google Scholar]
- 15. Seita J, Ema H, Ooehara J, Yamazaki S, Tadokoro Y, Yamasaki A, Eto K, Takaki S, Takatsu K, Nakauchi H. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci U S A. 2007;104(7):2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou K, Liu CX, Li Y, Li JP, Fan HH, Zhang L, Jing LP, Peng GX, Ye L, Li Y, Song L, et al. Evaluation of efficacy of immunosuppressive therapy plus recombinant human thrombopoietin for children with severe aplastic anemia [in Chinese]. Zhonghua Er Ke Za Zhi. 2017;55(7):523–528. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Dong Q, Fu R, Qu W, Ruan E, Wang G, Liu H, Wu Y, Song J, Xing L, Guan J, et al. Recombinant human thrombopoietin treatment promotes hematopoiesis recovery in patients with severe aplastic anemia receiving immunosuppressive therapy. Biomed Res Int. 2015;2015:597293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarado LJ, Huntsman HD, Cheng H, Townsley DM, Winkler T, Feng X, Dunbar CE, Young NS, Larochelle A. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood. 2019;133(19):2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003;88:31–52. [DOI] [PubMed] [Google Scholar]
- 20. Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109. [DOI] [PubMed] [Google Scholar]
- 21. Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. Febs J. 2018;285(3):416–431. [DOI] [PubMed] [Google Scholar]
- 23. Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morales-Mantilla DE, King KY. The Role of interferon-gamma in hematopoietic stem cell development, homeostasis, and disease. Curr Stem Cell Rep. 2018;4(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101(9):3730–3740. [DOI] [PubMed] [Google Scholar]
- 26. de Bruin AM, Demirel Ö, Hooibrink B, Brandts CH, Nolte MA. Interferon-γ impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. [DOI] [PubMed] [Google Scholar]
- 27. Furtek SL, Backos DS, Matheson CJ, Reigan P. Strategies and approaches of targeting stat3 for cancer treatment. ACS Chem Biol. 2016;11(2):308–318. [DOI] [PubMed] [Google Scholar]
- 28. Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. [DOI] [PubMed] [Google Scholar]