Abstract
Background:
Angiotensin-converting enzyme II or ACE2 is an integral membrane protein present on many types of cells, including vascular endothelial cells and lung alveolar epithelial cells. This receptor serves as the entry point for SARS-coronaviruses (SARS-CoVs), including a novel coronavirus 2019-nCoV. Limited availability of these receptors can thwart cellular entry of this virus.
Methods:
We tested the effects of ascorbic acid (vitamin C) on cellular expression of ACE2 at the protein and RNA levels in human small alveolar epithelial cells and microvascular endothelial cells. In addition, we investigated whether combinations of ascorbic acid with other natural compounds can affect ACE2 expression.
Results:
The results show that ascorbic acid itself has moderate but consistent lowering effects on ACE2 expression at the cellular, protein, and RNA levels. Some natural compounds were effective in lowering ACE2 cellular expression, with the highest inhibitory effects observed for baicalin (75%) and theaflavin (50%). Significantly, combinations of these and other test compounds with ascorbic acid further decreased ACE2 expression. The highest impact of ascorbate on ACE2 expression was noted when combined with theaflavin (decrease from 50% to 87%), zinc (decrease from 22% to 62%), and with 10-undecenoic acid (from 18% to 53%). Ascorbic acid showed moderate additional benefits in decreasing ACE2 expression when combined with N-acetylcysteine and baicalin.
Conclusion:
Our study provides valuable experimental confirmation of the efficacy of micronutrients in controlling ACE2 expression—the coronavirus cellular “entry” point. It further validates the importance of nutrient interactions in various aspects of cellular metabolism and in considering potential therapeutic applications of nutrient-based approaches. The study shows that ascorbic acid and its combination with some natural compounds could be included in developing preventive and therapeutic approaches toward the current pandemic.
Keywords: Ascorbic acid, coronavirus, ACE2, natural compounds, COVID-19
Introduction
Angiotensin-converting enzyme II (ACE2) is an integral membrane protein present on many cells throughout the human body with its strong expression in the heart, vascular system, gastrointestinal system, kidneys, as well as in type II alveolar cells in the lungs.1 This protein, known as an integral part of RAAS system,2 has attracted much attention since it has been reported that it is the entry point for SARS-CoV-2.3-6
ACE2 receptors on vascular cells are also compromised by the SARS-CoV-2 which results in enhanced vascular permeability, increased lung edema, and worsened lung function. Recent clinical findings show that ACE2 is expressed in blood platelets, which facilitate formation of thrombus and anti-inflammatory response in COVID-19 patients.7 It appears that ACE2 expression is negatively correlated with COVID-19 fatality at both human population and molecular levels.8
Recently there were publications reporting clinical benefits of intravenous ascorbate supplementation in critically ill patients with COVID-19.9,10 However, the mechanisms involved are poorly understood.
The goal of our study was to investigate whether modulation of ACE2 cellular expression can be involved in ascorbate dependent beneficial effects. We applied experimental models of cultured human microvascular endothelial cells and human alveolar epithelial cells to study the effects of ascorbate and its combinations with other natural compounds on ACE2.
Materials and Methods
Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) if not indicated otherwise. Quercetin dihydrate and zinc aspartate were from Powder City (York, PA, USA). Ginkgo biloba extract was from Monterey Bay Spice Company (Monterrey, CA, USA), and Theaflavins from Cayman Chemicals (Ann Arbor, MI, USA).
Cultured cells
Human Small Airways Epithelial Cells (SAEC), purchased from ATCC, (Manassas, VA, USA) were cultured in Airways Epithelial Cells growth medium (ATCC) in plastic flasks at 37°C and 5% CO2. For the experiments, the cells (passage 5-7) were plated to collagen-covered 96-well plates in 100 μL/well growth medium and were grown to confluent layer for 4 to 7 days.
Human Microvascular Endothelial Cells isolated from lung (HMEC), purchased from Lonza, (Hayward, CA, USA) were cultured in EGM-2 MV growth medium (Lonza) in plastic flasks at 37°C and 5% CO2. For the experiments, cells at 5 to 7 passages were plated to collagen-covered 96-well plates in 100 μL/well EGM-2 MV medium and were grown for 3 to 5 days to reach confluent layer.
ACE2 ELISA assay
For the experiments, cells were supplemented with indicated doses of tested compounds in 100 μL/well of cell growth medium for 6 days with fresh media supplementations every 2 to 3 days. Control cell growth media did not contain ascorbic acid. Culture plate wells were washed twice with phosphate buffered saline (PBS) and fixed with 3% formaldehyde/0.5% Triton X-100/PBS solution for 1 hour at 4°C, then washed 4 times with PBS. Two-hundred microliter of 1% bovine serum albumin-BSA (Sigma-Aldrich, St. Louis, MO, USA) in PBS was added and the plate was incubated at 4°C overnight. Rabbit polyclonal anti-ACE-2 antibodies (Sigma Aldrich, St. Louis, MO, USA) were added in 100 μL/well 1% BSA/PBS solution for 1.5 hours incubation at room temperature (RT). After 3 wash cycles with 0.1% BSA/PBS wells were supplied with 100 μL/well of anti-rabbit IgG antibodies conjugated with horse radish peroxidase (HRP, Sigma, St. Louis, MO, USA) for 1 hour at RT. After 3 wash cycles with 0.1% BSA/PBS the HRP activity retained was determined by incubation with 100 μL/well TMB substrate solution (Sigma-Aldrich, St. Louis, MO, USA) for 20 minutes. at RT, followed by the addition of 50 μL/well of 1N H2SO4 and optical density measurement at 450 nm with microplate reader (Molecular Devices, San Jose, CA, USA). Results are expressed as a percentage of experimental addition-free control (mean ± SD, n = 6). Non-specific control (wells incubated without anti-ACE2 antibodies) mean value (n = 6) was subtracted from all sample values.
Western blot
SAEC and HMEC were plated on 6-well plates and supplemented with 0.3, 0.6, 0.8, and 1 mM ascorbic acid, respectively. After 6 days whole SAEC and HMEC lysates, respectively, were prepared using lysis buffer (50 mM Tris-HCl [pH = 7.4], 1% TritonX-100, 150 mM NaCl, 1 mM EDTA, 2 mM Na3VO4, and 1X Complete protease inhibitors [Roche Applied Science, Indianapolis, IN, USA]). The protein concentration was measured by the Dc protein assay (Bio-Rad, Hercules, CA, USA). Fifty microgram per well of protein was separated on 8% to 16% gradient SDS-PAGE gels (ie, Tris-based electrophoresis using standard Laemmle’s method without β-ME addition to sample buffer) and transferred to a PVDF membrane. Proteins were detected with commercially available anti-ACE-2 monoclonal antibody diluted 1:250 (R&D, Minneapolis, MN, USA) and anti-β-actin antibody as a loading control (Cell Signaling, Danvers, MA, USA).
qRT-PCR
SAEC and HMEC were plated on 6-well plates and stimulated with 0, 0.3, 0.6, 0.8, and 1.0 mM of ascorbic acid, respectively. After 6 days, total RNA was isolated using RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA). Next, 2500 ng of each isolate was transcribed in cDNA using RT² HT First Strand Kit (Qiagen, Germantown, MD, USA) and subjected to qRT-PCR using specific primers for ACE2 (forward primer, 5′-TGGGCAAACTCTATGCTG-3′; and reverse primer, 5′-TTCATTGGCTCCGTTTCTTA-3) and β-actin (forward primer, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′; and reverse primer, 5′-ATGGAGCCACCGATCCACA-3′). The PCR was performed with a QuantiTect SYBR® Green PCR Kit as the source of 2.5 mM Mg2+, deoxynucleotides and HotStarTaq DNA polymerase, in addition to each primer used at a concentration of 0.3 μM in a 50 μL qPCR mixture (Qiagen, Germantown, MD, USA) in a BioRad CFX instrument (BioRad, Hercules, CA, USA). The qPCR cycler conditions were programmed according to the manufacturer’s recommendations and were as follows: an activation step at 95°C for 15 minutes, 40 cycles of: denaturation at 94°C for 15 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. To verify the specificity and identity of the qPCR product, melting curve analyses were performed. The amount of β-actin cDNA was used to normalize the sample amounts used for determinations. All samples were tested in duplicate PCR reactions and the mean of the reactions was used for calculating the expression levels.
Results
Effects of ascorbic acid on ACE2 receptor expression at the protein level
The effects of ascorbic acid on ACE2 at the protein level are presented in Figure 1. The Figure 1A shows the results of Western blot analysis of ACE2 protein expression in human small alveolar epithelial cells and human lung micro-vascular cells incubated in the presence of various concentrations of ascorbic acid for 6 days.
Figure 1.
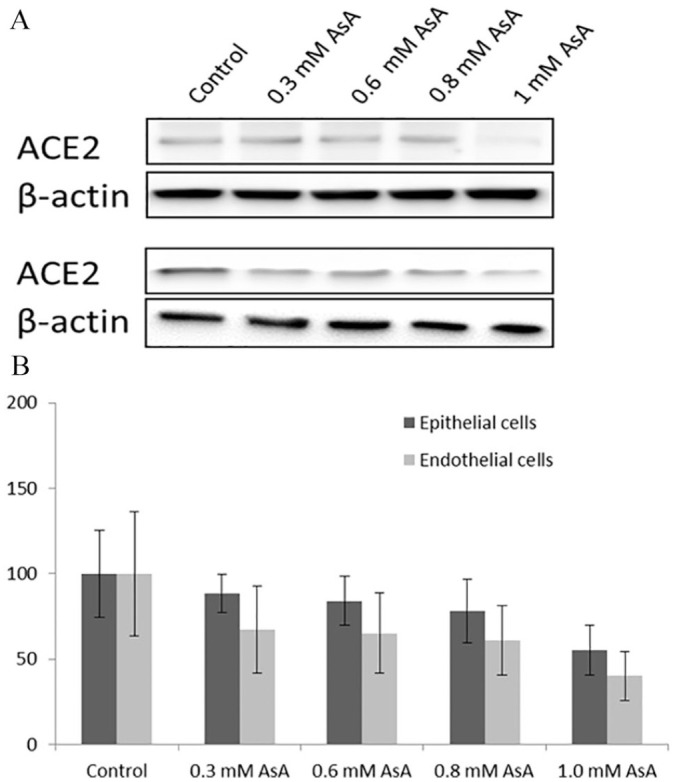
Effects of ascorbic acid on ACE2 receptor expression at the protein level. Western blot analysis of ACE2 protein expression in human small alveolar epithelial cells and human lung micro-vascular cells incubated in the presence of various concentrations of ascorbic acid (A). Quantitative analysis of the effects of ascorbic acid (AsA) on ACE2 protein expression identified by Western blot (B).
Quantitative analysis (Figure 1B) further confirms dose-dependent trend in the reduction of ACE2 protein, starting from 0.3 mM ascorbic acid. Statistically significant reduction of this protein by 55% and 60% was observed at 1.0 mM ascorbic acid in SAEC and HMEC cell lines, respectively.
Effects of ascorbic acid on mRNA expression of ACE2 receptor protein
In order to assess further whether ascorbic acid can inhibit ACE2 expression at the RNA level we performed qRT-PCR analysis. The results in Figure 2 show that in the presence of 1.0 mM ascorbic acid the ACE2 gene expression in SAEC and HMEC decreased by 0.61- and 0.54-fold, respectively. The down-regulating trend by ascorbic acid was evident in both types of cells, although it did not cross a standard cut-off value (ie, 0.5-fold of change).
Figure 2.
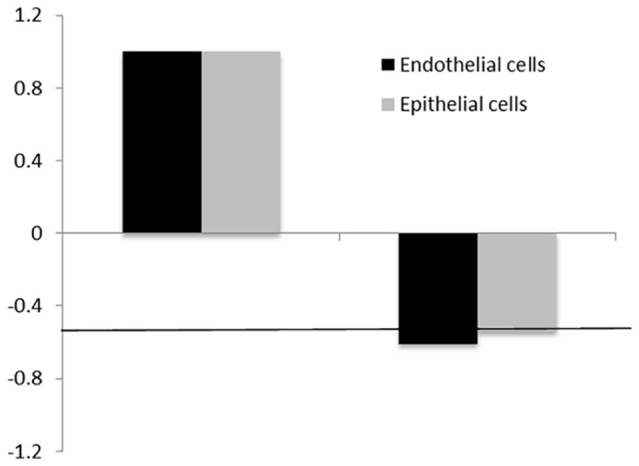
Effects of ascorbic acid on mRNA expression of ACE2 receptor protein. ACE2 expression at mRNA level in SAEC and HMEC in the presence of 1 mM ascorbic acid (AsA). The horizontal line—marks change by 0.5-fold.
Effects of ascorbic acid on cellular expression of ACE2 receptors in the presence of natural compounds
Although earlier studies using molecular modeling technology suggested several natural compounds as potential candidates against coronavirus, the experimental proof of their efficacy, in particular their direct effects on cellular ACE2 receptor targeted by SARS-CoV-2, were not evaluated. Understanding these aspects is critical since many of these natural compounds are available in our diet or can be taken as dietary supplements for different health aspects, including as preventive measures against viral infections.
In the presence of ascorbic acid there was dose dependent decrease of ACE2 expression with about 18% decrease at 0.6 mM and 27% at 1.0 mM concentration (Figure 3A). As presented on Figure 3B, exposure of these epithelial cells to ascorbyl palmitate resulted in 50% inhibition of ACE2 expression, already at much lower concentrations compared to ascorbic acid of 75 µM. At higher concentrations (>100 µM) ascorbyl palmitate showed cytotoxic effects.
Figure 3.
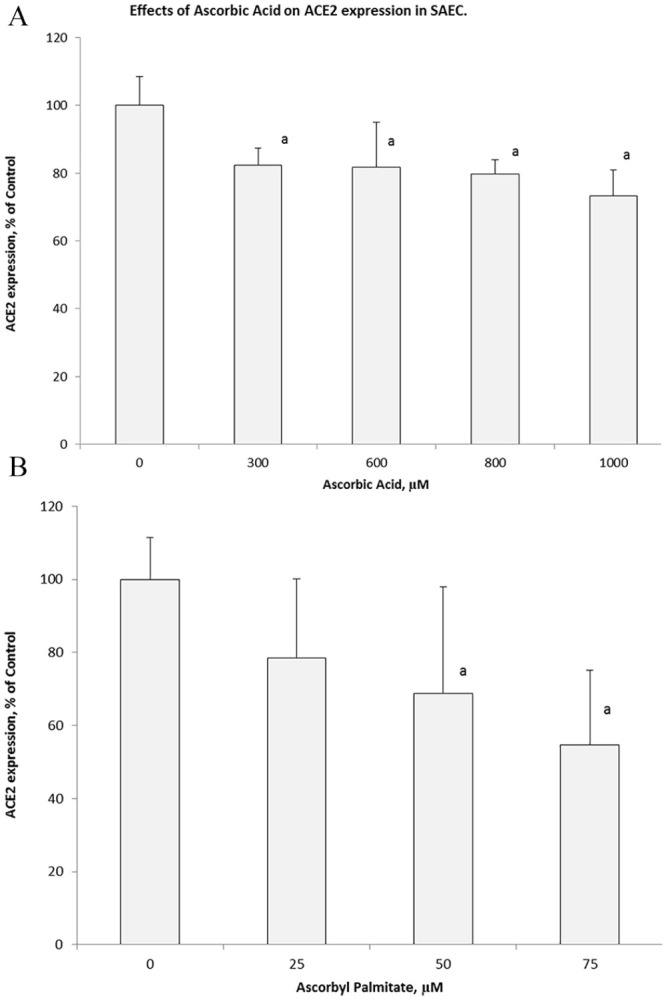
Changes in cellular expression of ACE2 on SAEC in presence of ascorbic acid and ascorbyl palmitate. Changes in ACE-2 cellular expression on SAEC measured by a specific antibody affinity directed toward this receptor in the presence of various concentrations of ascorbic acid (A) and its lipid-soluble derivative, ascorbyl palmitate (B).
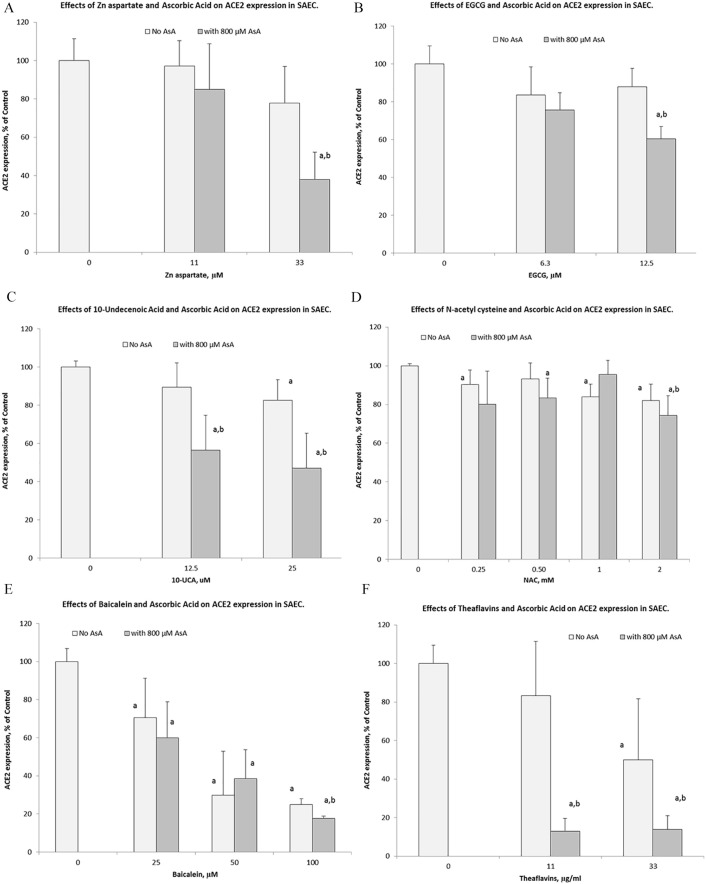
Subsequently, we evaluated changes in ACE2 expression in SAEC in the presence of select natural components applied individually and in combination with ascorbic acid. The results are presented in Figure 4A to F.
Figure 4.
Effects of ascorbic acid (800 µM) on ACE2 cellular expression on human SAEC in the presence of various concentrations of natural compounds: (A) zinc aspartate, (B) epigallocatechin gallate (EGCG), (C) 10-undecenoic acid (UCA), (D) N-acetylcysteine (NAC), (E) baicalein, and (F) theaflavins.
The results show that all these natural compounds had inhibitory effects on ACE2 expression in SAEC, but to different degrees. Of all test ingredients, baicalein was the most effective and at 25 µM concentration was able to inhibit ACE2 expression by 75% compared to no ascorbate control (Figure 4E). Theaflavin used individually inhibited ACE2 expression by about 50% at concentrations of 33 µg/mL and 50 µM respectively (Figure 4F). Combinations of these compounds with ascorbic acid further enhanced their inhibitory effects on ACE2 expression from 50% to 87%, in case of theaflavin and from 75% to 82% with baicalein. Figure 4A shows that a combination of ascorbate with zinc aspartate increased zinc’s inhibitory effect by an additional 40%, resulting in ACE2 expression inhibition by 62% by 2 compounds together Ascorbate combination with 10-undecanoic acid also decreased ACE2 expression from 18% ( individual effect) to 53%, when combined with AA resulting in an additional 35% inhibitory effect (Figure 4C). NAC had the smallest effects on ACE2 expression in SAEC compared to other compounds (18% inhibition at 2 mM). However, NAC’s combination with ascorbic acid further lowered ACE2 expression resulting in 26% inhibition (Figure 4D).
Discussion
This study shows that vitamin C used individually and in combination with select natural bioactive compounds can inhibit expression of ACE2 receptor protein, which has attracted wide scientific interests as the primary cellular entry route for SARS-CoV-2 causing the current COVID-19 pandemic.
We observed that ascorbic acid by itself can decrease cellular expression of ACE2 receptors in lung small airway epithelial cells (SAEC) and human microvascular endothelial cells (HMEC) at the protein and the RNA levels. The inhibitory effect of ascorbic acid on ACE2 expression was seen at its higher concentrations (between 0.8 and 1 mM) and after 6 days of cell exposure. This finding may be helpful in understanding some of the clinical aspects of the efficacy of ascorbic acid applied at high doses, for example, by intravenous application in COVID-19 patients. The intravenous delivery route enables achieving much higher than 1 mM plasma concentrations of ascorbic acid compared to its dietary intake and would among other possible mechanisms result in a decrease of ACE2 receptors. Ascorbic acid concentration in blood plasma is rapidly declining after its intravenous infusion due to kidney clearance. However, they allow sustainable intracellular ascorbic acid accumulation up to 10 mM level.11 Similar effects occur in cell culture medium. Ascorbic acid content in medium in cell culture medium is rapidly declining within hours after cell supplementation due to ascorbic acid oxidation. However, even with fresh cell culture medium supplementations every 2 or 3 days there is a sustainable intracellular ascorbic acid accumulation.12 Therefore, the cell culture model could be regarded as a valid representation of the corresponding in vivo conditions. Interpretation of the results could be less straightforward with lung epithelial cells as the changes in ascorbic content in alveolar epithelium after its intravenous injections have not been yet characterized.
Investigation of molecular mechanisms of ascorbic acid effects and its physiological implications for RAAS and viral infectivity was beyond the scope of this study and it should be a focus of further investigations. However, in our assumption the observed cellular effects of ascorbic acid on ACE2 protein expression were due rather its intracellular than extracellular action.
Our experimental results indicate that longer exposure to ascorbic acid would be needed to obtain measurable inhibitory effects. Ascorbyl palmitate was more effective than ascorbic acid in lowering ACE2 and at much lower concentrations since this lipid-soluble compound can easily cross cellular membranes and is more stable than ascorbic acid.
Another novel aspect of our study includes experimental proof of the efficacy of various natural compounds in inhibiting expression of ACE2 receptors, which can be further enhanced by their interactions with ascorbic acid. While some molecular modeling studies indicated the potential of individual natural compounds against coronavirus,13 they did neither include experimental testing nor evaluate the eventual synergistic effects of their combinations. Experimental evidence of the efficacy of natural compounds in addressing specific mechanisms associated with coronavirus infection is scarce, and to our knowledge does not address ACE2 expression.
All compounds selected in our study demonstrated cooperative effects with ascorbic acid, albeit to various degrees. For instance, its combination with N-acetylcysteine (NAC) had a smaller effect on ACE2 inhibition than with theaflavins, zinc, or 10-undecenoic acid (10-UCA). The mechanistic aspects of these differences need to be further evaluated.
Several review studies suggested a possible therapeutic use of vitamin C and other natural compounds in COVID-19 patients as an adjunct to pharmacological treatments. Except of vitamin C which has been used clinically in COVID-19 patients,9,10 these recommendations are generally based on reviewing available data on general anti-viral and immune enhancing effects of natural compounds.14,15
The present study, however, is—to the best of our knowledge—the first systematic experimental approach to evaluate natural compounds that work in synergy with ascorbate to impede key mechanisms of coronavirus infection. Baicalein and theaflavins used individually and in combination with ascorbic acid could be beneficial in coping with the COVID-19 pandemic, by among other significantly decreasing cellular ACE2 receptors expression in SAEC. Theaflavin combined with ascorbate showed the strongest 87% inhibition of ACE2 expression.
Our findings further stress the importance of nutrient interactions that have been the foundation of our previous research in addressing many other aspects of cellular metabolism. We have shown earlier that a mixture of ascorbic acid with amino acids (lysine, proline, and arginine), minerals (copper and manganese), and plant compounds (EGCG and quercetin) has a more pronounced inhibitory effect on cellular ACE2 expression than any of these compounds have individually. This inhibitory effect has been particularly pronounced under pro-inflammatory conditions.16 While our study focuses on ACE2 receptors targeted by SARS-CoV-2, it is anticipated that micronutrients can also affect other mechanisms associated with this viral infection, including viral replication and others. Along these lines, we have already shown that a specific combination of natural components and plant extracts can significantly decrease not only ACE2 expression (up to 92%) in human lung epithelial cells, but also inhibit receptor binding domain RBD on the spike surface of the coronavirus to its ACE2 receptor on the surface of human cells site by 97%.17
Numerous studies indicate that various natural compounds could be considered in developing practical approaches to many aspects of immunity. Due to their multiple functions and synergistic interactions in cellular metabolism, they can simultaneously affect different mechanisms involved in immune system function and can directly modulate infectivity of a wide range of pathogens.18-20 Numerous in vitro, in vivo, and human studies conducted by us and others, involving different viruses, for example, HIV, H1N1, H1N9, and bird flu, confirm the universality of ascorbic acid efficacy in various viral infections and pleiotropic effects achieved from its combination with other natural compounds.21-23
Our findings show that vitamin C has a consistent and significant lowering effect on ACE2 expression exercised at different molecular levels in human alveolar epithelial cells, but also in microvascular endothelial cells—the 2 main cell types affected by the SARS-CoV-2. In microvascular endothelial cells, ascorbic acid could inhibit ACE2 expression at the protein (Western blot) and RNA levels.
The results of our study indicate that ascorbic acid—dependent modulation of the expression of ACE2 receptor in SAEC and HVEC cells could be at least 1 of the cellular mechanisms involved in producing beneficial therapeutical effects of intravenous ascorbate supplementation in critically ill patients with COVID-19.9,10 Furthermore, the results suggest that combinations of ascorbic acid with select natural compounds could further enhance its efficacy which should be taken into consideration in the future efforts to research and design preventive and therapeutic health approaches to the current pandemic.
Acknowledgments
The authors thank Dr. Bilwa Bhanap and Ms. Cathy Flowers, for the valuable input during preparation and submission of this manuscript.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization: MR, AN; Data curation: VI, AG, SI; Formal analysis: VI, AG, SI; Investigation: VI, AG, SI; Methodology: VI, AG; Project administration: AN; Supervision: AN; Validation: VI, AG, SI, AN, MR; Visualization: VI, AG; Writing-original draft: AN and MR.
Ethical Approval: This in vitro study is exempt from ethics committee approval.
References
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma RK, Stevens BR, Obukhov AG, et al. ACE2 (Angiotensin-converting enzyme 2) in cardiopulmonary diseases Ramifications for the control of SARS-CoV-2. Hypertension. 2020;76:651-661. doi: 10.1161/HYPERTENSIONAHA.120.15595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li W, Moore M, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA. 2005;102:7988-7993.doi: 10.1073/pnas.0409465102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front Med. 2020;14:185-192. doi: 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S, Liu Y, Yang L, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19:e13168. doi: 10.1111/acel.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang J, Rao X, Li Y, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Preprint. Posted online August 10, 2020. doi: 10.21203/rs.3.rs-52778/v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan HMW, Parikh N, Megala SM, Predeteanu GS. Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C. Am J Case Rep. 2020;21:e925521. doi: 10.12659/AJCR.925521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533-537. [DOI] [PubMed] [Google Scholar]
- 12. Michels AJ, Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 2013;5:5161-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh R, Chacraborty A, Biswas A, Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV02) main potease (pro) inhibitors – an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. Published online June 22, 2020. doi: 10.1080/07391102.2020.1779818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:99-101. doi: 10.1080/14787210.2020.1706483 [DOI] [PubMed] [Google Scholar]
- 15. Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Fronti Immunol. 2020;11:1451. doi: 10.3389/frimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivanov V, Ivanova S, Niedzwiecki A, Rath M. Effective and save global public health strategy to fight the COVID-19 pandemic: specific micronutrient combination inhibits Coronavirus cell-entry receptor (ACE2) expression. J Cell Med & Nat. Health. Published online 2 July 2020. https://www.jcmnh.org/effective-and-safe-global-public-health-strategy-to-fight-the-covid-19-pandemic-specific-micronutrient-composition-inhibits-coronavirus-cell-entry-receptor-ace2-expression/
- 17. Goc A, Sumera W, Ivanov V, Niedzwiecki A, Rath M. Micronutrient combination inhibits two key steps of coronavirus (SARS-CoV-2) infection: viral binding to ACE2 receptor and its cellular expression. J Cell Med & Nat. Health. Published online 14 August 2020. https://www.jcmnh.org/micronutrient-combination-inhibits-two-key-steps-of-coronavirus-sars-cov-2-infection-viral-binding-to-ace2-receptor-and-its-cellular-expression/
- 18. Sumera W, Goc A, Niedzwiecki A, Rath M. The micronutrient combination with immune-enhancing pleiotropic effects. J Cell Med & Nat. Health. Published online 28 August 2020. https://www.jcmnh.org/the-micronutrient-combination-with-immune-enhancing-effects/
- 19. Goc A, Niedzwiecki A, Rath M. Synergistic Anti-Borreliae efficacy of a composition of naturally-occurring compounds: an in vitro study. J Nutri Bio. 2019;5:350-363. [Google Scholar]
- 20. Goc A, Gehring G, Baltin H, Niedzwiecki A, Rath M. Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: in vivo and human observational study. Ther Adv Chronic Dis. 2020;11:1-13. doi: 10.1177/2040622320922005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jariwalla RJ, Gangapurkar B, Pandit A, Kalinovsky T, Niedzwiecki A, Rath M. Micronutrient cooperation in suppression of HIV production in chronically and latently infected cells. Mol Med Rep. 2010;3:377-385. doi: 10.3892/mmr_00000268 [DOI] [PubMed] [Google Scholar]
- 22. Barbour EK, Rayya EG, Shaib H, et al. Alleviation of histopathologic effects of Avian Influenza virus by a specific nutrient synergy. Int J Appl Res Vet Med. 2007;5:9-16. [Google Scholar]
- 23. Harakeh S, Jariwalla RJ, Pauling L. Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc Natl Acad Sci USA. 1990;87:7245-7249. doi: 10.1073/pnas.87.18.7245 [DOI] [PMC free article] [PubMed] [Google Scholar]