Abstract
The use of programmed cell-death protein 1 (PD-1)/programmed cell-death ligand 1 (PD-L1) inhibitors is the standard therapy for the first-line or second-line treatment of patients with non-small-cell lung cancer (NSCLC). In contrast to current traditional treatments such as chemotherapy or radiotherapy, anti-PD-1 and anti-PD-L1 treatments can directly attenuate tumour-mediated exhaustion and effectively modulate the host anti-tumour immune response in vivo. In addition, compared with traditional therapy, PD-1/PD-L1 inhibitor monotherapy can significantly prolong survival without obvious side effects in the treatment of advanced NSCLC. Ideally, several biomarkers could be used to monitor the safety and effectiveness of anti-PD-1 and anti-PD-L1 treatments; however, the current lack of optimal prognostic markers remains a widespread limitation and challenge for further clinical applications, as does the possibility of immune-related adverse events and drug resistance. In this review, we aimed to summarise the latest progress in anti-PD-1/anti-PD-L1 treatment of advanced NSCLC, worldwide, including in China. An exploration of underlying biomarker identification and future challenges will be discussed in this article to facilitate translational studies in cancer immunotherapy.
Keywords: immunotherapy, non-small cell lung cancer (NSCLC), PD-1/PD-L1 inhibitors, predictive biomarkers, progress and challenges
Introduction
Lung cancer is the leading cause of cancer-related deaths and is associated with a poor prognosis.1,2 Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancers, 15–30% of which are squamous-cell carcinomas (SQCs) of the lung.3,4 The pathogenic genes of NSCLC have been discovered and widely investigated, and genotyping-based targeted therapy has been developed as one of the most successful therapeutic methods.5–8 At present, with a greater understanding of tumour immunity, targeted molecular therapy and immunotherapy have become important strategies for the conventional treatment of NSCLC.9–12 In recent years, the successful application of immune-checkpoint inhibitors (ICIs) of cytotoxic T-lymphocyte antigen-4 (CTLA-4), and anti-programmed cell-death protein 1 (PD-1) and programmed cell-death protein ligand 1 (PD-L1) in various advanced cancers has attracted widespread attention in the field of immuno-oncology.13–18 Two antibodies against PD-1, nivolumab and pembrolizumab, and two antibodies against PD-L1, atezolizumab and durvalumab, have been approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) for treating advanced NSCLC.19–24 The PD-1/PD-L1 inhibitors licensed for NSCLC treatment are listed in Table 1.
Table 1.
The PD-1/PD-L1 inhibitors licensed for NSCLC treatment.
| Checkpoint | Blocking agent | IgG isotype and characteristics | Clinical stage | Manufacturer |
|---|---|---|---|---|
| PD-1 | Pembrolizumab | Humanized IgG4 mAb | EMA, FDA approved for first-line and second-line NSCLC treatment | Merck |
| Nivolumab | Humanized IgG4 mAb | FDA approved for second-line NSCLC | Bristol-Myers Squibb | |
| Toripalimab | Humanized IgG4 mAb | Clinical trial ongoing | Shanghai Junshi | |
| Sintilimab | Fully human IgG4 mAb | Clinical trial ongoing | Innovent Biologics | |
| Camrelizumab | Humanized IgG4 mAb | Clinical trial ongoing | Hengrui Medicine | |
| Tislelizumab | Humanized IgG4 mAb | Clinical trial ongoing | BeiGene | |
| PDR001 | Humanized IgG4 mAb | Clinical trial ongoing | Novartis | |
| REGN2810 | Humanized IgG4 mAb | Clinical trial ongoing | Regeneron-Sanofi | |
| PD-L1 | Atezolizumab | High-affinity human IgG1 | FDA approved for second-line NSCLC | Roche |
| Durvalumab | Human IgG1 mAb | FDA approved for treatment of unresectable stage III NSCLC without relapse after chemoradiation | AstraZeneca | |
| BMS-936559 | Fully high-affinity human IgG4 | Clinical trial ongoing | Bristol-Myers Squibb |
EMA, European Medicines Agency; FDA, US Food and Drug Administration; IgG, immunoglobulin G; mAb, monoclonal antibody; NSCLC, non-small-cell lung cancer; PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1.
In this review, we aimed to outline the recent development of PD-1/PD-L1 inhibitors as first-, second-, and third-line treatments, and to discuss driver-gene mutation in NSCLC patients. Moreover, we summarised current and ongoing clinical trials of PD-1 and PD-L1 inhibitors in China. In the absence of optimal prognostic biomarkers, we elaborate on the presence of immune-related adverse events (irAEs), primary and acquired drug resistance, and the challenge of administering immunotherapy for the treatment of advanced NSCLC.
Regulatory mechanisms of PD-1/PD-L1 in NSCLC
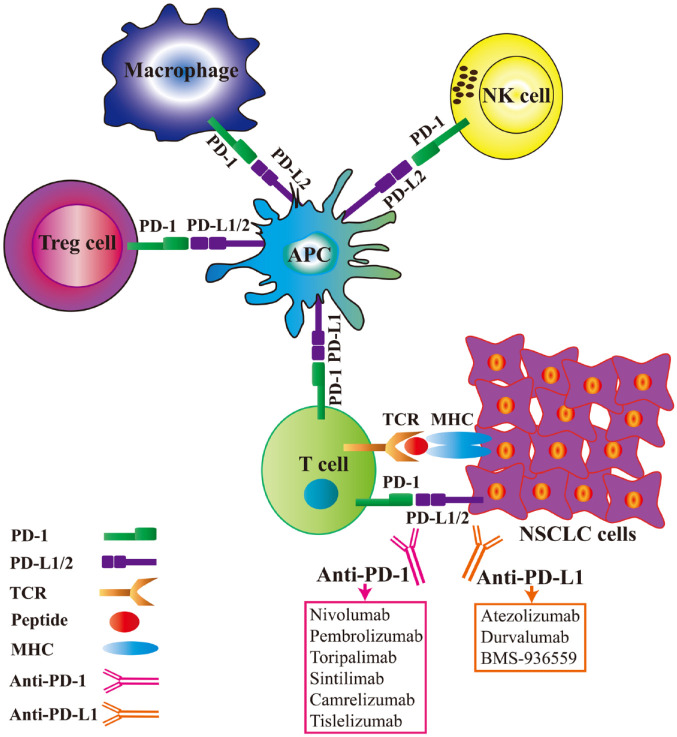
Modulation of immune checkpoints is an important mechanism for evading immune surveillance by inhibiting activated T cells.25 For example, PD-1+ immune cells can interact with PD-L1+ malignant tumour cells or other host cells. Mechanistically, immune escape might occur by interfering with related anti-tumour functions.26 In addition, PD-1 is expressed by CD4+ T cells, CD8+ T cells and some other immune cell types. PD-1 interaction with its ligand, PD-L1, can weaken the effector functions of T lymphocytes such as activation, proliferation and survival, promote antigen-specific T-cell apoptosis, and affect CD4+ and CD8+ T cells, natural killer (NK) cells, and CD11c+ M1 macrophage functions to promote tumour development.27–29 Antibodies that suppress the interaction between PD-1 and PD-L1 can largely improve and restore the functionality of exhausted T cells and enhance their anti-tumour immune response.30 PD-1 is highly expressed by regulatory T cells (Tregs) and has a significant inhibitory effect on tumour immunity.31 The immune-related regulatory mechanism of action of PD-1/PD-L1 inhibitors is shown in Figure 1. Modulation of PD-1/PD-L interactions effectively inhibits the anti-tumour response by weakening the inhibitory activity of Tregs.32 In addition to T-cell immunity, tumour-associated macrophages,33,34 as well as NK cells and dendritic cells in the tumour microenvironment (TME) can enhance anti-tumour effects.35–37
Figure 1.
Regulatory mechanism of programmed cell-death protein 1 (PD-1)/programmed cell-death ligand 1 (PD-L1) inhibitors.
The PD-1 receptor is expressed on activated T cells, macrophages, regulatory T cells (Tregs) and natural killer (NK) cells. Binding of PD-1 to its ligands, PD-L1 or PD-L2, results in suppression of proliferation and inhibition of the immune response of T cells. Anti-PD-1 antibodies or PD-L1 inhibitors reverse this process, resulting in enhanced anti-tumour immune responses.
APC, antigen-presenting cell; MHC, major histocompatibility complex; NSCLC, non-small-cell lung cancer; TCR, T cell receptor.
PD-1/PD-L1 inhibitor therapy in advanced NSCLC
Although there are many ways to treat NSCLC, such as radiotherapy, platinum-based chemotherapy, surgical resection and targeted molecular therapy, the long-term efficacy of these treatments is still not ideal, and the 5-year survival rate of patients with NSCLC is less than 18%.38 Thus, there is an urgent demand for novel clinical therapies that are associated with less toxicity and higher efficacy to further prolong the survival time of patients. At present, some anti-PD-1/PD-L1 antibodies have been approved by the FDA for clinical treatment of advanced NSCLC;39 these clinical studies will have a very important role in guiding long-term clinical practice.
PD-1/PD-L1 inhibitors as first-line treatment of advanced NSCLC
Multiple KEYNOTE trials (either alone or as part of a combined study) are being investigated using PD-1/PD-L1 inhibitors in various tumour types, with a focus on NSCLC. In the open-label phase Ib KEYNOTE-001 trial, patients with advanced NSCLC expressing PD-L1 in tumour tissues who were randomly assigned to the pembrolizumab group exhibited an objective response rate (ORR) of 27%, a median progression-free survival (mPFS) of 6.2 months [95% confidence interval (CI) 4.1–8.6], and a median overall survival (mOS) of 22.1 months (95% CI 17.1–27.2). The ORR, 1-year mPFS, and 1-year mOS were significantly higher in patients with a PD-L1 tumour proportion score (TPS) ⩾50% than in the overall population. The results showed that pembrolizumab was well tolerated; only 12 cases (11.9%) experienced grade 3 treatment-related adverse events (AEs), and there were no treatment-induced deaths.40 The 3 years of data from KEYNOTE-001 showed that 79.7% patients with a PD-L1 TPS ⩾50% reached the mOS of 34.9 months (95% CI 20.3–not reached).41 The KEYNOTE-189 study is a phase III clinical trial of non-squamous NSCLC patients without first-line sensitised epidermal growth-factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations. These patients received platinum-based drugs plus either pembrolizumab or placebo. The mPFS of the monoclonal antibody (mAb) group was significantly longer than the control group (8.8 versus 4.9 months, respectively; p < 0.001), and the incidences of grade 3 or higher AEs in the mAb group and the control group were 67.2% and 65.8%, respectively.42 The update data from the KEYNOTE-189 study (23.1 months follow up) showed mOS was 22.0 months (95% CI 19.5–25.2) in the pembrolizumab–combination group versus 10.7 months (95% CI 8.7–13.6) in the placebo–combination group [hazard ratio (HR) 0.56; 95% CI 0.45–0.70]; the mPFS were 9.0 months (95% CI 8.1–9.9) and 4.9 months (95% CI 4.7–5.5), respectively (HR 0.48; 95% CI 0.40–0.58).43
For patients with advanced squamous NSCLC, the KEYNOTE-407 trial indicated that concomitant administration of carboplatin plus paclitaxel and pembrolizumab provided longer mOS and mPFS than traditional chemotherapy alone.44 The efficacy outcome from the protocol-specified final analysis of KEYNOTE-407 showed that, after a median follow up of 14.3 months, pembrolizumab plus chemotherapy exhibited a clinically meaningful improvement over placebo plus chemotherapy for mOS (17.1 versus 11.6 months) and mPFS (8.0 versus 5.1 months).45
Another phase I/II trial, KEYNOTE-021, was designed to assess the efficacy and frequency of grade 3–4 AEs associated with combination treatment with platinum-based doublet chemotherapy with pembrolizumab. The results demonstrated that combination chemotherapy with pembrolizumab was associated with fewer grade 3–4 AEs compared with platinum-based chemotherapy or pembrolizumab monotherapy.46 A randomised, open-label, phase II, multi-cohort study, KEYNOTE-021, was performed with 123 patients with primary stage IIIb, or IV NSCLC without EGFR or ALK mutations. The mPFS of the pembrolizumab plus chemotherapy group was 13.0 months (95% CI 8.3–not reached), whereas the mPFS of the group receiving chemotherapy alone was only 8.9 months (95% CI 4.4–10.3).47 After a median time of 49.4 months from KEYNOTE-021, ORR (58% versus 33%) and mPFS (24.5 versus 9.9 months; HR 0.54; 95% CI 0.35–0.83) was improved with the pembrolizumab combination compared with chemotherapy, regardless of PD-L1 status; the median OSs were, respectively, 34.5 versus 21.1 months (HR 0.71; 95% CI 0.45–1.12).48
In the KEYNOTE-024 trial, compared with platinum-based chemotherapy alone, pembrolizumab significantly improved OS (30.0 versus 14.3 months), PFS (10.3 versus 6.0 months), and ORR (44.8% versus 27.8%) in the first-line therapy of advanced NSCLC cases with a PD-L1 TPS ⩾50%.49,50 Moreover, pembrolizumab was associated with longer mOS and mPFS in contrast to chemotherapy alone in patients with a high PD-L1 TPS ⩾50% in the KEYNOTE-042 trial.51 A real-world study in Japan, HOPE-001, analysed clinical data from patients receiving pembrolizumab as first-line treatment for NSCLC. The ORR, mPFS, and mOS were 51.2%, 8.3 months (95% CI 6.0–10.7), and 17.8 months (95% CI 17.8–not reached), respectively,52 similar to the results of the KEYNOTE-024 and KEYNOTE-042 trials.
In the CheckMate 012 trial, 52 patients received nivolumab until disease progression or toxicity was experienced. The ORR was 28% in patients regardless of the tumour expression level of PD-L1, and the mPFS and mOS were 3.6 months (95% CI 0.1–28.0) and 19.4 months (95% CI 0.2–35.8), respectively.53 In another CheckMate 012, open-label, phase I trial, 78 patients who received either nivolumab every 2 weeks plus ipilimumab every 12 weeks, or nivolumab every 2 weeks plus ipilimumab every 6 weeks, were assessed. The use of nivolumab plus ipilimumab as first-line treatment resulted in good tolerability and safety, and encouraging clinical efficacy, with a better ORR in each group.54 Based on CheckMate 012, in the CheckMate 227 trial, nivolumab plus ipilimumab as first-line therapy significantly prolonged the mPFS compared with chemotherapy alone in patients with advanced NSCLC.55,56 CheckMate 568, a phase II trial of nivolumab plus ipilimumab in NSCLC patients, defined a cut-off of the tumour mutational burden (TMB) of at least 10 mutations per base pair for selecting patients most likely to respond to treatment, regardless of the PD-L1 expression level.57 A TMB ⩾10 was associated with a high ORR and mPFS. However, in the CheckMate 026 study, compared with first-line chemotherapy alone in advanced NSCLC patients, patients that were treated with antibody therapy did not obtain significant survival benefits.58
Atezolizumab and durvalumab are engineered and humanised mAbs against PD-L1 immunoglobulin G1 (IgG1), which can combine with PD-L1 to block the PD-1/PD-L1-mediated signalling pathway. BIRCH was designed to measure the efficacy of atezolizumab in advanced NSCLC as first-, second- and third-line therapies. Of 139 patients with advanced NSCLC who underwent first-line treatment with atezolizumab, the ORR, mPFS and mOS were 22%, 5.4 months (95% CI 3.0–6.9) and 20.1 months (95% CI 20.1–not reached), respectively.59 IMpower150 was an open-label phase III study that aimed to assess the therapeutic efficacy of atezolizumab combined with bevacizumab and chemotherapy as first-line treatment in patients with advanced NSCLC. Patients received a combination therapy of atezolizumab + carboplatin + paclitaxel (ACP), bevacizumab + carboplatin + paclitaxel (BCP), or atezolizumab + BCP (ABCP) every 3 weeks for a total of four to six cycles. Following this, they then received atezolizumab, bevacizumab, or both, for maintenance treatment. Regardless of the expression levels and genetic status of PD-L1, the mPFS and mOS of patients with metastatic non-squamous NSCLC in the ABCP group were significantly improved when compared with the ACP or BCP group.60 In the IMpower132 trial, atezolizumab combined with carboplatin and the chemotherapeutic agent pemetrexed significantly decreased mortality and improved the mPFS and mOS compared with chemotherapy alone in patients with advanced non-squamous NSCLC.61 The IMpower130 trial reported an obvious improvement in mOS and mPFS, and no change in AEs were found in the group receiving atezolizumab plus chemotherapy compared with the group receiving chemotherapy alone as the first-line treatment of patients with metastasised non-squamous NSCLC without driver gene mutations.62 The IMpower131 study showed that an atezolizumab plus platinum-based chemotherapy significantly improved mPFS in patients with first-line squamous NSCLC; however, the mOS was similar between groups.63 Impower110 was performed to analyse the efficacy and safety of atezolizumab, as compared with platinum-based chemotherapy, as a first-line treatment for patients with metastatic NSCLC. The primary measurement was the OS among patients in the intention-to-treat population. This study found that the mOS was longer in the atezolizumab group than in the chemotherapy group (20.2 versus 13.1 months; HR for death 0.59; 95% CI 0.40–0.89; p = 0.01) as was mPFS (8.1 versus 5.0 months; HR for death 0.63; 95% CI 0.45–0.88) in the population with the highest expression of PD-L1. This study indicated that atezolizumab treatment results in significantly longer OS than platinum-based chemotherapy among patients with NSCLC with high PD-L1 expression, regardless of histologic type.64 Recently, an ongoing APPLE study (WJOG11218L) was performed in Japan to evaluate the additional effect of bevacizumab administered with platinum combination therapy and atezolizumab in patients with advanced non-squamous NSCLC.65
In a phase Ib trial, durvalumab and the anti-CTLA-4 antibody tremelimumab were administered to patients with advanced NSCLC. Regardless of the level of PD-L1 expression, the ORR was 23% in the group that received durvalumab combined with tremelimumab.66 The MYSTIC trial was an open-label, phase III trial of first-line treatment with durvalumab with or without tremelimumab versus platinum-based chemotherapy alone in patients with advanced NSCLC. Compared with chemotherapy alone, the durvalumab group exhibited a clinically significant improvement in mOS when tumour cell PD-L1 expression was ⩾25%.67 The SAKK study was performed to evaluate the safety of first-line durvalumab in patients with advanced NSCLC and a performance status of 2. The data demonstrate that using first-line durvalumab in patients with PS2 and metastatic NSCLC with PD-L1 expression ⩾25% resulted in an unexpectedly high number of fatal early events due to rapid tumour progression.68 All the clinical data described here are summarised in Table 2.
Table 2.
Efficacy of anti-PD-1/PD-L1 agents for advanced NSCLC in first line.
| Study | Histology | Phase | PD-L1 TPS (%) | Arms | Case number | ORR% (95% CI or p value) | mPFS (95% CI or p value, months) | mOS (95% CI or p value, months) | Grade 3–5 AE% | References |
|---|---|---|---|---|---|---|---|---|---|---|
| KEYNOTE-001 | Squamous or non- squamous | Ib | ⩾50 | Pembrolizumab | 27 | 51.9 (31.9–71.3) | 12.5 (6.2–NR) | NR (22.1–NR) | 11.9 | Hui et al.40 |
| 1–49 | 52 | 17.3 (8.2–30.3) | 4.2 (3.1–6.4) | 19.5 (10.7–22.2) | ||||||
| <1 | 12 | 8.3 (0.2–38.5) | 3.5 (2.1–19.0) | 14.7 (3.4–NR) | ||||||
| KEYNOTE-189 | Non- squamous | III | Any | Pembrolizumab plus chemotherapy | 410 | 47.6 (42.6–52.5) | 8.8 (7.6–9.2) | NR | 67.2 | Gandhi et al.42 |
| Chemotherapy | 206 | 18.9 (13.8–25.0) p < 0.001 | 4.9 (4.7–5.5) p < 0.001 | 11.3 (8.7–15.1) | 65.8 | |||||
| KEYNOTE-189 | Non- squamous | III | Any | Pembrolizumab plus chemotherapy | 410 | 48.0 (43.1–53.0) | 9.0 (8.1–9.9) | 22.0 (19.5–25.2) | 71.9 | Gadgeel et al.43 |
| Chemotherapy | 206 | 19.4 (14.2–22.0) | 4.9 (4.7–5.5) HR: 0.48; (0.40–0.58) | 10.7 (8.7–13.6) HR: 0.56; (0.45–0.70) | 66.8 | |||||
| KEYNOTE-407 | Squamous | III | Any | Pembrolizumab plus chemotherapy | 278 | 57.9 (51.9–63.8) | 6.4 (6.2–8.3) | 15.9 (13.2–NR) | 69.8 | Paz-Ares et al.44 |
| Chemotherapy | 281 | 38.4 (32.7–44.4) | 4.8 (4.3–5.7) p < 0.001 | 11.3 (9.5–14.8) p < 0.001 | 68.2 | |||||
| KEYNOTE-407 | Squamous | III | Any | Pembrolizumab plus chemotherapy | 278 | 62.6 (56.6–68.3) | 8.0 (6.3–8.4) | 17.1 (14.4–19.9) | 74.1 | Paz-Ares et al.45 |
| Chemotherapy | 281 | 38.4 (32.7–44.4) | 5.1 (4.3–6.0) HR: 0.57; (0.47–0.69) | 11.6 (10.1–13.7) HR: 0.71; (0.58–0.88) | 69.6 | |||||
| KEYNOTE-021 | Squamous or non- squamous | I/II | Any | Pembrolizumab plus carboplatin plus paclitaxel | 25 | 48 | 10.3 (6.1–14.6) | 21.4 (10.5–NR) | 40 | Gadgeel et al.46 |
| Carboplatin plus paclitaxel plus bevacizumab | 25 | 56 | 7.1 (4.2–14.3) | 16.7 (8.5–NR) | 42 | |||||
| Carboplatin plus pemetrexed | 24 | 75 | 10.2 (6.5–13.9) | 16.7 (13.9–29.2) | 46 | |||||
| KEYNOTE-021 | Non-squamous | II | Any | Pembrolizumab and chemotherapy | 60 | 55 (42.0–68.0) | 13.0 (8.3–NR) | NR | 39 | Langer et al.47 |
| Chemotherapy alone | 63 | 29 (18.0–41.0) p = 0.0016 | 8.9 (4.4–10.3) p = 0.010 | NR p = 0.39 | 26 | |||||
| KEYNOTE-021 | Non-squamous | II | Any | Pembrolizumab and chemotherapy | 60 | 58 | 24.5 (9.7–36.3) | 34.5 (24.0–NR) | 39 | Awad et al.48 |
| Chemotherapy alone | 63 | 33 | 9.0 (6.2–15.2) HR: 0.54; (0.35–0.83) | 21.1 (14.9–35.6) HR: 0.71; (0.45–1.12) | 31 | |||||
| KEYNOTE-024 | Squamous or non- squamous | III | ⩾50 | Pembrolizumab | 154 | 44.8 (36.8–53.0) | 10.3 (6.3–NR) | 30.0 (18.3–NR) | 31.2 | Reck et al.49,50 |
| Chemotherapy | 151 | 27.8 (20.8–35.7) | 6.0 (4.2–6.2) p < 0.001 | 14.2 (9.8–19.0) p = 0.002 | 53.3 | |||||
| KEYNOTE-042 | Squamous or non- squamous | III | ⩾50 | Pembrolizumab | 299 | 39 | 7.1 (5.9–9.0) | 20.0 (15.4–24.9) | 18 | Mok et al.51 |
| Chemotherapy | 300 | 32 | 6.4 (6.1–6.9) p = 0.0170 | 12.2 (10.4–14.2) p = 0.0003 | 41 | |||||
| HOPE-001 | Squamous or non- squamous | Real-word study | ⩾50 | Pembrolizumab | 213 | 51.2 | 8.3 (6.0–10.7) | 17.8 (17.8–NR) | 18.3 | Tamiya et al.52 |
| CheckMate 012 | Squamous or non- squamous | I | Any | Nivolumab | 52 | 23 (12.0–52.0) | 3.6 (0.1–28.0) | 19.4 (0.2–35.8) | 19 | Gettinger et al.53 |
| CheckMate 012 | Squamous or non- squamous | I | Any | 2 weeks nivolumab plus 12 weeks ipilimumab | 38 | 47 (31–64) | NA | NR | 37 | Hellmann et al.54 |
| 2 weeks nivolumab plus 6 weeks ipilimumab | 40 | 38 (23–55) | NA | NR | 33 | |||||
| CheckMate 568 | Squamous or non- squamous | II | Any | Nivolumab (TMB ⩾10) | 48 | 44 | 7.1 (3.6–11.3) | NR | 29 | Ready et al.57 |
| Nivolumab (TMB <10) | 50 | 12 | 2.6 (1.4–5.4) | NR | ||||||
| CheckMate 026 | Squamous or non- squamous | III | ⩾5 | Nivolumab | 211 | 26 (20–33) | 4.2 (3.0–5.6) | 14.4 (11.7–17.4) | 18 | Carbone et al.58 |
| Chemotherapy | 212 | 33 (27–40) | 5.9 (5.4–6.9) p = 0.25 | 13.2 (10.7–17.1) HR: 1.01; (0.80–1.30) | 51 | |||||
| BIRCH | Squamous or non- squamous | NA | ⩾5 | Atezolizumab | 139 | 22 | 5.4 (3.0–6.9) | 20.1 (20.1–NR) | 40 | Peters et al.59 |
| IMpower150 | Non- squamous | III | Any | ACP | 402 | 49.2 | 7.0 | 18.6 | NA | Socinski et al.60 |
| BCP | 400 | 53.5 | 6.8 | 14.7 | 47.7 | |||||
| ABCP | 400 | 69.3 | 8.3 (p < 0.001) | 19.2 (p = 0.02) | 57.7 | |||||
| IMpower132 | Non- squamous | III | Any | APP | 292 | 49.6 | 7.6 (6.6–8.5) | 18.1 (13.0–NR) | 53.6 | Papadimitrakopoulou et al.61 |
| PP | 286 | 32.2 | 5.2 (4.3–5.6) p < 0.0001 | 13.6 (11.4–15.5) p = 0.0797 | 39.1 | |||||
| IMpower130 | Non- squamous | III | Any | Atezolizumab plus chemotherapy | 451 | 49.2 | 7.0 (6.2–7.3) | 18.6 (16.0–21.2) | 81.0 | Planchard et al.62 |
| Chemotherapy | 228 | 31.9 | 5.5 (4.4–5.9) p < 0.0001 | 13.9 (12.0–18.7) p = 0.033 | 71.0 | |||||
| IMpower131 | Squamous | III | Any | Atezolizumab + CnP | 343 | 49.7 | 6.3 | 14.2 | 69.2 | Jotte et al.63 |
| CnP | 340 | 41.0 | 5.6 (p = 0.0001) | 13.5 (p = 0.16) | 58.4 | |||||
| Impower110 | Squamous or non- squamous | III | ⩾50 | Atezolizumab | 107 | 38.3 | 8.1 | 20.2 (16.5–NR) | 33.9 | Herbst et al.64 |
| Chemotherapy | 98 | 28.6 | 5.0 HR: 0.63; (0.45–0.88) | 13.1 (7.4–16.5) p = 0.01) | 56.7 |
ABCP, atezolizumab plus BCP; AE, adverse event; ACP, atezolizumab plus carboplatin plus paclitaxel; APP, atezolizumab plus pemetrexed plus carboplatin or cisplatin; BCP, bevacizumab plus carboplatin plus paclitaxel; CI, confidence interval; CnP, carboplatin + nab-paclitaxel; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; NSCLC, non-small-cell lung cancer; NR, not reached; ORR, objective response rate; PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1; PP, pemetrexed plus carboplatin or cisplatin; TMB, tumour mutational burden; TPS, tumour proportion score.
PD-1/PD-L1 inhibitors as second-line treatment in advanced NSCLC
The human anti-PD-1 mAb nivolumab was the first to be approved as second-line therapy for stage IV NSCLC.69 CheckMate 017, a randomised, open-label, phase III study assessed the efficacy and safety of nivolumab for advanced squamous NSCLC; greater efficacy was obtained during or after first-line chemotherapy. Regardless of PD-L1 expression levels, the mOS, ORR, and mPFS were markedly better in the nivolumab group than in the docetaxel group.69,70 The CheckMate 057 trial evaluated the efficacy of nivolumab in patients with advanced non-squamous NSCLC who experienced obvious disease progression after first-line treatment. Compared with docetaxel, nivolumab treatment resulted in a remarkably longer mOS, with a more favourable safety profile.71,72 In both the CheckMate 017 and CheckMate 057 trials, the 2-year OS rates of patients with squamous NSCLC treated with nivolumab compared with docetaxel were about 23% and 8%, respectively. In those with non-squamous NSCLC, the 2-year OS rates of those treated with nivolumab were 29% and 16% in the CheckMate 017 and CheckMate 057 trials, respectively.73 The CheckMate 078 trial compared the safety and efficacy of nivolumab with docetaxel in patients with squamous or non-squamous NSCLC.74 The results were consistent with those of the CheckMate 017 and CheckMate 057 trials. Another Argentinian study was conducted to explore the efficacy and safety of nivolumab in previously treated patients with advanced NSCLC; the results showed that nivolumab was well tolerated, with promising prognostic outcomes in patients with NSCLC who had undergone previous treatment.75 A multicentre retrospective observational study in Japan was performed to evaluate the efficacy of nivolumab in second-line treatment in NSCLC patients. The mOS was 14.6 months (95% CI 12.3–15.9); 1-year survival rate was 54.3%; mPFS was 2.1 months (95% CI 1.9–2.4); and ORR was 20.5%. Subgroup analysis showed that PS, EGFR mutation status, smoking status, and PD-L1 were associated with the effectiveness of nivolumab.76
KEYNOTE-010, an open-label, randomised phase II/III trial, included pretreated NSCLC patients with an expression level of PD-L1 ⩾1% in tumour cells who had received treatment with pembrolizumab or docetaxel. This study showed that treatment with pembrolizumab significantly prolonged mOS in patients with previously treated, advanced NSCLC, especially in those with high PD-L1 expression levels.77 Patients in the KEYNOTE-010 trial were followed up to assess long-term outcomes. At a median follow-up time point of 42.6 months (range 35.2–53.2 months), those treated with pembrolizumab experienced a significantly improved mOS compared with those treated with docetaxel. The 3-year OS rates were 34.5% versus 12.7% (for those with PD-L1 TPS ⩾50%) and 22.9% versus 11.0% (for those with PD-L1 TPS ⩾1%), respectively.78 The KEYNOTE-025 trial assessed the safety and efficacy of pembrolizumab in patients with NSCLC who had been previously treated in Japan.79 The results showed that pembrolizumab was associated with a generally high ORR in those with a PD-L1 expression level ⩾50%. The phase I/II KEYNOTE-021 study evaluated combination therapy with pembrolizumab plus ipilimumab in patients with previously treated advanced NSCLC. This study showed that among patients who received pembrolizumab (2 mg/kg) plus ipilimumab (1 mg/kg), ORR was 30%, mPFS was 4.1 months (95% CI 1.4–5.8), and mOS was 10.9 months (95% CI 6.1–23.7) but was associated with meaningful toxicity.80
A randomised phase III clinical trial, OAK, also showed that regardless of the expression level of PD-L1, the survival rate following atezolizumab treatment was significantly improved compared with docetaxel. Based on the OAK findings, atezolizumab has been approved by the FDA and the EMA for further treatment of those previously treated for advanced NSCLC. For long-term survivors in the atezolizumab group, the ORR was 14% and the mOS was 13.8 months (95% CI 11.8–15.7).81,82 In the POPLAR study, an open-label, phase II trial, atezolizumab treatment significantly improved OS compared with docetaxel in patients with NSCLC who were previously treated.83 The BIRCH study also proved the efficacy of atezolizumab in patients with advanced NSCLC as second-line therapy. The ORR, mPFS, and mOS were 19%, 2.8 months (95% CI 1.5–3.9), and 15.5 months (95% CI 12.3–not reached), respectively.59 It is well known that approximately 10% of patients with NSCLC are complicated with comorbid interstitial pneumonia, with a poor prognosis. The Thoracic Oncology Research Group 1936/AMBITIOUS study is an ongoing, multicentre, single-arm, phase II trial to assess the safety and efficacy of atezolizumab for pretreated advanced/recurrent patients with NSCLC complicated with idiopathic chronic fibrotic interstitial pneumonia. We are looking forward to the results of this clinical study.84 Another multicentre, phase II, open-label, single-arm VinMetAtezo trial was designed to assess the safety and efficacy of metronomic oral vinorelbine in combination with atezolizumab in advanced NSCLC progressing after first-line platinum-based chemotherapy. The primary outcome was PFS at 4 months.85 In France, Marine et al. conducted a study to evaluate the cost effectiveness of atezolizumab versus docetaxel as a second-line treatment for stage IV NSCLC. Their results indicated that atezolizumab was more efficacious and more costly than docetaxel in treating advanced NSCLC.86 All studies that have assessed PD-1/PD-L1 inhibitors as second-line therapies are summarised in Table 3.
Table 3.
Efficacy of anti-PD-1/PD-L1 agents for advanced NSCLC in second line and third line.
| Study | Histologies | Phase | PD-L1 TPS (%) | Arms | Case number | ORR (95% CI or p value) | mPFS (95% CI or p value, months) | mOS (95% CI or p value, months) | Grade 3–4 AE% | Refences |
|---|---|---|---|---|---|---|---|---|---|---|
| Checkmate 017 (second) | Squamous | III | Any | Nivolumab | 135 | 20 (14–28) | 3.5 (2.1–4.0) | 9.2 (7.3–13.3) | 7 | Brahmer et al.69; Reck et al.70 |
| Docetaxel | 137 | 9 (5–15) p = 0.008 | 2.8 (2.1–3.5) p < 0.01 | 6.0 (5.1–7.3) p < 0.001 | 55 | |||||
| Checkmate 057 (second) | Non-squamous | III | Any | Nivolumab | 292 | 19 (15–24) | 2.3 (2.2–3.3) | 12.2 (9.7–15.0) | 10 | Borghaei et al.71; Reck et al.72 |
| Docetaxel | 290 | 12 (7–17) p = 0.02 | 4.2 (3.5–4.9) p = 0.39 | 9.4 (8.1–10.7) p = 0.002 | 54 | |||||
| Checkmate 078 (second) | Squamous or non- squamous | III | Any | Nivolumab | 338 | 16.6 (12.2–21.0) | 2.8 (2.4–3.4) | 12.0 (10.4–14.0) | 10 | Wu et al.74 |
| Docetaxel | 166 | 4.2 (1.7–8.5) p < 0.0001 | 2.8 (1.6–2.9) p = 0.0147 | 9.6 (7.6–11.2) p = 0.0006 | 48 | |||||
| Keynote-010 (second) | Squamous or non- squamous | II/III | ⩾1% | Pembrolizumab 2 mg/kg | 345 | 18 | 3.9 (3.1–4.1) p = 0.07 | 10.4 (9.4–11.9) p = 0.0008 | 13 | Herbst et al.77,78 |
| Pembrolizumab 10 mg/kg | 346 | 18 | 4.0 (2.7–4.3) p = 0.004 | 12.7 (10.0–17.3) p < 0.0001 | 16 | |||||
| Docetaxel | 343 | 9 (p = 0.0005) | 4.0 (3.1–4.2) | 8.5 (7.5–9.8) | 35 | |||||
| ⩾50% | Pembrolizumab 2 mg/kg | 139 | 30 | 5.0 (4.0–6.5) p = 0.0002 | 14.9 (10.4–NR) p = 0.0002 | NA | ||||
| Pembrolizumab 10 mg/kg | 151 | 29 | 5.2 (4.1–8.1) p < 0.0001 | 17.3 (11.8–NR) p < 0.0001 | NA | |||||
| Docetaxel | 152 | 8 (p < 0.0001) | 4.1 (3.6–4.3) | 8.2 (6.4–10.7) | NA | |||||
| Keynote-025 (second) | Squamous or non- squamous | Ib | ⩾1% | Pembrolizumab | 38 | 22 (10–38) | 3.9 (2.0–6.2) | 19.2 (8.0–26.7) | 29 | Nishio et al.79 |
| ⩾50% | 12 | 27 (6–61) | 4.1 (1.6–19.1) | 17.9 (5.9–NR) | NA | |||||
| Keynote-021 (⩾2) | NSCLC | I/II | Any | Pembrolizumab plus ipilpmumab | 44 | 30 | 4.1 (1.4–5.8) | 10.9 (6.1–23.7) | 29 | Gubens et al.80 |
| OAK (second) | Squamous or non- squamous | III | Any | Atezolizumab | 425 | 14 | 2.8 (2.6–3.0) | 13.8 (11.8–15.7) | 15 | Rittmeyer et al.81; von Pawel et al.82 |
| Docetaxel | 425 | 13 | 4.0 (3.3–4.2) p = 0.049 | 9.6 (8.6–11.2) p = 0.0003 | 43 | |||||
| POPLAR (second) | Squamous or non- squamous | II | Any | Atezolizumab | 144 | 17 (11.0–23.8) | 2.7 | 12.6 (9.7–16.4) | 11 | Fehrenbacher et al.83 |
| Docetaxel | 143 | 15 (9.3–21.4) | 3.0 HR: 0.94; (0.72–1.23) | 9.7 (8.6–12.0) p = 0.04 | 39 | |||||
| BIRCH (second) | Squamous or non- squamous | NA | ⩾5% | Atezolizumab | 268 | 19 | 2.8 (1.5–3.9) | 15.5 (12.3–NR) | NA | Peters et al.59 |
| BIRCH (third) | Squamous or non- squamous | NA | ⩾5% | Atezolizumab | 252 | 18 | 2.8 (1.5–3.9) | 13.2 (10.3–17.5) | NA | Peters et al.59 |
| ATLANTIC (third) | Squamous or non- squamous | II | ⩾25% | EGFR and ALK positivity (durvalumab) | 74 | 12.2 (5.7–21.8) | 1.9 (1.803.6) | 13.3 (8.1–NR) | 6 | Garassino et al.87 |
| EGFR and ALK negativity (durvalumab) | 146 | 16.4 (10.8–23.5) | 3.3 (1.9–3.7) | 10.9 (8.6–13.6) | 9 | |||||
| ⩾90% PD-L1 expression (durvalumab) | 68 | 30.9 (20.2–43.3) | 2.4 (1.8–5.5) | NR (9.5–NR) | 18 | |||||
| ARCTIC (⩾3) | Squamous or non- squamous | III | ⩾25 | durvalumab | 62 | 35.5 | 3.8 (1.9–5.6) | 11.7 (8.2–17.4) | 9.7 | Planchard et al.88 |
| Standard of care | 64 | 12.5 | 2.2 (1.9–3.7) HR: 0.71 (0.49–1.04) | 6.8 (4.9–10.2) HR: 0.63 (0.42–0.93) | 44.4 | |||||
| <25 | Durvalumab + tremelimumab | 174 | 14.9 | 3.5 (2.3–4.6) | 11.5 (8.7–14.1) | 22.0 | ||||
| Standard of care | 118 | 6.8 | 3.5 (1.9–3.9) p = 0.056 | 8.7 (6.5–11.7) p = 0.109 | 36.4 |
AE, adverse event; ALK, anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth-factor receptor; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; NSCLC, non-small-cell lung cancer; NR, not reached; ORR, objective response rate; PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1; TMB, tumour mutational burden; TPS, tumour proportion score.
PD-1/PD-L1 inhibitors as third-line or greater treatment in advanced NSCLC
The BIRCH study examined the clinical efficacy of atezolizumab as third-line or greater therapy in advanced NSCLC. The mOS, mPFS, and ORR were 13.2 months (95% CI 10.3–17.5), 2.8 months (95% CI 1.5–3.9), and 18%, respectively as third-line treatment, suggesting that atezolizumab has good clinical efficacy as third-line therapy for advanced NSCLC.59
The ATLANTIC trial was an open-label, phase II, multicentre clinical trial conducted to assess the efficacy of durvalumab as third-line or greater therapy for advanced NSCLC. The ORR was 12.2% in EGFR+ and ALK+ patients who had expression levels of PD-L1 ⩾25% and received durvalumab, which was lower than that of patients who were EGFR− and ALK− with the same expression level of PD-L1 ⩾25% (16.4%) or those with expression of PD-L1 ⩾90% (30.9%). The mPFS rates in the three groups were 1.9 months, 3.3 months, and 2.4 months, respectively.87
ARCTIC was a phase III, randomised, open-label study that evaluated the efficacy of durvalumab with or without tremelimumab versus the standard of care for advanced NSCLC as a greater than third-line therapy. For patients with expression levels of PD-L1 ⩾25%, durvalumab resulted in significant improvements in mOS and mPFS compared with the standard of care. For patients with expression of PD-L1 < 25%, durvalumab combined with tremelimumab resulted in obvious improvements in mOS compared with the standard of care.88 A detailed summary of the clinical trials of third-line or greater treatment of advanced NSCLC is shown in Table 3.
PD-1/PD-L1 inhibitors in cases of advanced NSCLC with driver-gene mutations
Inhibition of the PD-1/PD-L1 axis has resulted in favourable responses in NSCLC patients with EGFR mutations; however, the efficacy was significantly lower in these patients compared with those without EGFR mutations.89,90 Although high expression levels of PD-L1 in patients with advanced NSCLC may be associated with EGFR mutations,91 studies have shown that EGFR-tyrosine kinase inhibitors (TKIs) downregulate the messenger ribonucleic acid (mRNA) expression of PD-L1, suggesting that EGFR-TKIs may activate T-cell immunocompetence in the TME of NSCLC patients with EGFR-mutations.92–94 A phase II clinical trial showed that pembrolizumab was less effective in advanced NSCLC patients who were naïve to TKIs and were PD-L1+, with EGFR mutations, even those with a PD-L1 expression level ⩾50%; this indicated that anti-PD-1/PD-L1 treatment was not the ideal first choice for patients with EGFR mutations.95
The phase I/II KEYNOTE-021 trial analysed the feasibility of administering erlotinib or gefitinib combined with pembrolizumab as first-line treatment in patients with stage IIIb/IV EGFR-mutant NSCLC. Pembrolizumab plus gefitinib combination therapy had to be continued because of severe AEs, and another study showed that pembrolizumab plus erlotinib administration did not improve the ORR when compared with pembrolizumab plus gefitinib.96 The CheckMate 370 trial evaluated the safety of first-line treatment with nivolumab combined with crizotinib in patients with ALK+ NSCLC. Again, the combination treatment had to be discontinued, and enrolment was closed because of observed grade ⩾3 hepatic toxicities.97
A subgroup analysis of advanced NSCLC patients with EGFR mutations and ALK rearrangements was performed to assess the use of anti-PD-1/PD-L1 therapy as second-line treatment in the CheckMate 057, KEYNOTE-010, POLAR and OAK studies. Their results revealed that EGFR mutations and ALK rearrangements in patients with advanced NSCLC were associated with beneficial outcomes and prolonged mOS from anti-PD-1/PD-L1 treatment compared with chemotherapy when administered as second-line treatment.71,72,77,78,81–83 In a study assessing the effects of nivolumab, the ORR was significantly lower in patients with EGFR-mutant non-squamous NSCLC than in patients without the mutation (8.8% versus 19.6%, p = 0.007).98 It is well known that the acquired EGFR-T790M mutation is a major reason for patients developing resistance to EGFR-TKIs. A clinical study showed that of 25 NSCLC patients who received nivolumab following previous TKI administration, 8 patients exhibited the T790M mutation, rendering them resistant to EGFR-TKI treatment, whereas 17 patients did not. The mPFS times were 1.3 months (95% CI 0.1–1.8) and 2.1 months (95% CI 1.3–3.4) for the T790M-positive and T790M-negative patients, respectively (p = 0.099), and the mOS was not achieved at the follow up at 7.3 months. Overall, this study indicated that patients resistant to EGFR-TKI with negative expression of T790M probably benefited from subsequent treatment with nivolumab.99 Hence, some studies have explored combination therapy in advanced NSCLC patients who are resistant to TKIs.100,101 Gettinger et al. performed a study to assess the efficacy of nivolumab combined with erlotinib in advanced NSCLC patients with EGFR mutations who were treated with an EFGR-TKI but no chemotherapy.102 Another multicentre, open-label, phase Ib dose-escalation clinical trial was performed with patients previously treated with an ALK-TKI or chemotherapy who had advanced, ALK-rearranged NSCLC.103 The patients received either nivolumab plus ceritinib (450 mg daily) or nivolumab plus ceritinib (300 mg daily). The results showed that the ORR and mPFS were improved in the nivolumab plus ceritinib (450 mg) group; however, this combination of drugs was also associated with increased toxicity. Based on safety considerations, an alternative regimen is being investigated in which ceritinib is administered as a monotherapy for two cycles before the initiation of combination treatment with nivolumab.
One of the studies intended to identify a biomarker for patients most likely to positively respond to immunotherapy. Tumour deoxyribonucleic acid (DNA) was derived from advanced NSCLC patients receiving nivolumab or pembrolizumab therapy as second- or third-line treatment. The most common mutated genes observed in these patients were tumour protein p53 (TP53; 49%), Kirsten rat sarcoma 2 viral oncogene homolog (KRAS; 43%), and v-erb-b2 erythroblastic leukaemia viral oncogene homolog 2 (ERBB2; 13%). This study confirmed that patients with KRAS mutations experienced a longer mPFS and mOS than patients without KRAS mutations. Furthermore, patients with ERBB-family mutations failed to respond to treatment with PD-1 antibodies.104 The efficacy data of anti-PD-1/PD-L1 for advanced NSCLC with driver gene mutations is shown in Table 4.
Table 4.
Efficacy of anti-PD-1/PD-L1 agents for advanced NSCLC with driver gene mutations.
| Study | Therapy line | Phase | Driver gene mutant | PD-L1 TPS (%) | Arms | Case number | ORR (95% CI or p value) | mPFS (95% CI or p value, months) | mOS (95% CI or p value, months) | Grade 3–4 AE% | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02879994 | First line | II | EGFR | ⩾1% | Pembrolizumab | 11 | 9 | 3.9 | NR | NA | Lisberg et al.95 |
| KEYNOTE-021 (NCT02039674) | First line | I/II | EGFR | Any | Pembrolizumab plus gefitinib | 7 | 14.3 | 1.4 (0.2–13.0) | 13.0 (0.2–NR) | 71.5 | Yang et al.96 |
| Pembrolizumab plus erlotinib | 12 | 41.7 | 19.5 (3.0–19.5) | NR | 33.3 | ||||||
| Checkmate 370 | First line | I/II | ALK | Any | Nivolumab Plus Crizotinib | 13 | 38 | NA | NA | 38 | Spigel et al.97 |
| Garassino et al.98 | ⩾2 | NA | WT- EGFR | Any | Nivolumab | 1293 | 19.6 (17.4–21.7) | 3.0 (2.8–31) | 11.0 (10.0–12.0) | 6 | Garassino et al.98 |
| EGFR-mutant | 102 | 8.8 (3.3–14.3) p = 0.007 | 3.0 (2.7–3.3) p = 0.004 | 8.3 (2.2–14.4) p = 0.046 | 7 | ||||||
| Haratani et al.99 | Second line | NA | T790M-negative | Any | Nivolumab | 17 | 24 | 2.1 (1.3–3.4) | NR | NA | Haratani et al.99 |
| T790M-positive | 8 | 13 p = 1.000 | 1.3 (0.1–1.8) p = 0.099 | NR | NA | ||||||
| Gettinger et al.102 | Second line | I | EGFR | Any | Nivolumab Plus erlotinib | 21 | 15 | 5.1 (2.3–12.2) | 18.7 (7.3–NR) | 24 | Gettinger et al.102 |
| Felip et al.103 | Second line | Ib | ALK | Any | Ceritinib 450 mg plus nivolumab | 8 | 50 (16–84) | 6.4 (0.8–13.7) | NA | 93 | Felip et al.103 |
| Ceritinib 300 mg plus nivolumab | 12 | 25 (6–57) | 3.7 (1.8–NR) | NA | 82 |
ABCP, atezolizumab plus bevacizumab plus carboplatin plus paclitaxel; AE, adverse event; ALK, anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth-factor receptor; mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; NSCLC, non-small-cell lung cancer; NR, not reached; ORR, objective response rate; PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1; TPS, tumour proportion score; WT, wild type.
Application of PD-1/PD-L1 inhibitors in advanced NSCLC cases in China
Toripalimab (also known as JS001 or TAB001) is a recombinant human anti-PD-1 mAb developed by Shanghai Junshi Biotechnology Co., Ltd. that selectively inhibits PD-1 and PD-L1/PD-L2 interactions to facilitate T-cell activation.105 Yang et al. conducted a phase I dose escalation and expansion clinical study to evaluate the safety and tolerability of toripalimab in patients with advanced or recurrent malignancies in China.106 Thirty-three patients participated in this study, seven of which had NSCLC. The results showed that toripalimab was well tolerated at a dose of 10 mg/kg every 2 weeks, and no dose-limiting toxicity was apparent; in addition, long-term anti-tumour activity was achieved in patients with alveolar soft-part sarcomas.106
Sintilimab is a human IgG4 mAb coupled to PD-1; it was jointly developed by Innovent Biologics and Eli Lilly and Company. Sintilimab was approved for treating patients with classical Hodgkin’s lymphoma who had relapsed or were refractory after at least two rounds of systemic chemotherapy in China.107 A phase Ib study was performed to assess the safety and efficacy of sintilimab as a neoadjuvant therapy for NSCLC; the results showed that sintilimab was well tolerated in NSCLC patients, with an encouraging pathologic response rate.108
Camrelizumab, a PD-1 inhibitor, developed by Jiangsu Hengrui Pharmaceutical Co., Ltd., has recently been approved for the treatment of relapsed or refractory classical Hodgkin’s lymphoma in China.109 A study evaluated the efficacy and safety of camrelizumab combined with microwave ablation (MWA) in patients with metastatic NSCLC. The results indicated that the ORR was 33.3%, with two patients achieving a complete response (CR) and five patients achieving a partial response (PR). The mPFS was 5.1 months; OS was not reached. The most common AE related to camrelizumab administration was reactive capillary hyperplasia of the skin110 that could be alleviated by substitution with apatinib.111
The anti-PD-1 antibody, tislelizumab, has demonstrated clinical tolerability and safety in treating solid tumours, including NSCLC in Chinese patients.112 Toripalimab, sintilimab, camrelizumab and tislelizumab are all currently undergoing clinical trials for the treatment of various solid tumours, including advanced NSCLC; these are summarised in Table 5.
Table 5.
Application of PD-1/PD-L1 inhibitors in the advanced NSCLC in China.
| Drug | ClinicalTrials.gov identifier | Phase | Condition | Therapy line | Design | Patients (n) | Recruiting status | Locations |
|---|---|---|---|---|---|---|---|---|
| Toripalimab | NCT03513666 | II | Advanced NSCLC with EGFR-mutation positive and T790M negative | Second line | Toripalimab combined with pemetrexed plus carboplatin | 40 | Not yet | Jiangsu Province Hospital |
| Toripalimab | NCT04316351 | II | IIIb/IV NSCLC with T790M-positive mutations failed to osimertinib therapy | Second line | Toripalimab (JS001) combined with pemetrexed and anlotinib | 60 | Not yet | Guangzhou Institute of Respiratory Disease |
| Toripalimab | NCT03856411 | III | Advanced NSCLC | First line | Toripalimab injection or placebo combined with standard chemotherapy | 450 | Recruiting | Cancer Hospital Chinese Academy of Medical Sciences |
| Toripalimab | NCT03924050 | III | Advanced NSCLC with TKI-resistant EGFR mutated | Second line | Toripalimab or placebo combined with chemotherapyin | 350 | Recruiting | Shanghai Pulmonary Hospital |
| Sintilimab | NCT03798743 | II | Advanced NSCLC | Second line | Sintilimab combined with docetaxel | 30 | Recruiting | Hunan Province Tumor Hospital |
| Sintilimab | NCT03765775 | II | Advanced NSCLC with first-generation EGFR-TKI resistance along with T790M negative | Second line | Anlotinib combined with sintilimab | 20 | Recruiting | First Hospital of Shijiazhuang |
| Sintilimab | NCT04252365 | II | Advanced NSCLC | First line | Sintilimab and pembrolizumab | 20 | Not yet | Guangdong Association of Clinical Trials |
| Sintilimab | NCT03812549 | I | Advanced NSCLC | First line | Sintilimab combination with radiation | 29 | Recruiting | West China Hospital, Sichuan University |
| Sintilimab | NCT04124731 | II | Advanced NSCLC | First line | Sintilimab combined with anlotinib | 98 | Recruiting | Tianjin Medical University Cancer Institute and Hospital |
| Sintilimab | NCT03802240 | III | EGFR-mutant advanced non-squamous NSCLC | Second line | Sintilimab ± IBI305 combined with pemetrexed and cisplatin | 600 | Recruiting | Shanghai Chest Hospital |
| Sintilimab | NCT04213170 | II | Advanced NSCLC with brain matastases | NA | Sintilimab combined with bevacizumab | 60 | Recruiting | Sun Yat-sen University |
| Sintilimab | NCT03830411 | II | Nonsquamous NSCLC with wild-type EGFR | Second line | Sintilimab compared with docetaxel or pemetrexed | 76 | Recruiting | China Three Gorges University |
| Camrelizumab | NCT04211090 | II | Nonsquamous NSCLC with brain matastases and without driver gene mutations | First line | Camrelizumab combined with pemetrexed/carboplatin | 64 | Recruiting | Sun Yat-sen University |
| Camrelizumab | NCT04203485 | III | PD-L1-positive advanced NSCLC | First line | Camrelizumab combined with apatinib mesylate or camrelizumab alone | 762 | Recruiting | Jiangsu HengRui Medicine Co., Ltd |
| Camrelizumab | NCT04303130 | II | Advanced squamous NSCLC | First line | Camrelizumab combined with endostar | 52 | Recruiting | Beijing Cancer Hospital |
| Camrelizumab | NCT04167774 | II | Advanced NSCLC | Second line | Camrelizumab combined with paclitaxel | 62 | Recruiting | Sun Yat-sen University |
| Tislelizumab | NCT03663205 | III | Advanced non-squamous NSCLC | First line | Tislelizumab with chemotherapy versus chemotherapy | 334 | Not yet | Anhui Provincial Hospital |
| Tislelizumab | NCT03594747 | III | Advanced squamous NSCLC | First line | Tislelizumab with chemotherapy versus chemotherapy | 360 | Not yet | Anhui Provincial Hospital |
EGFR, epidermal growth-factor receptor; NSCLC, non-small-cell lung cancer; PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1; TKI, tyrosine kinase inhibitor.
Predictive biomarkers in anti-PD-1/PD-L1 immunotherapy
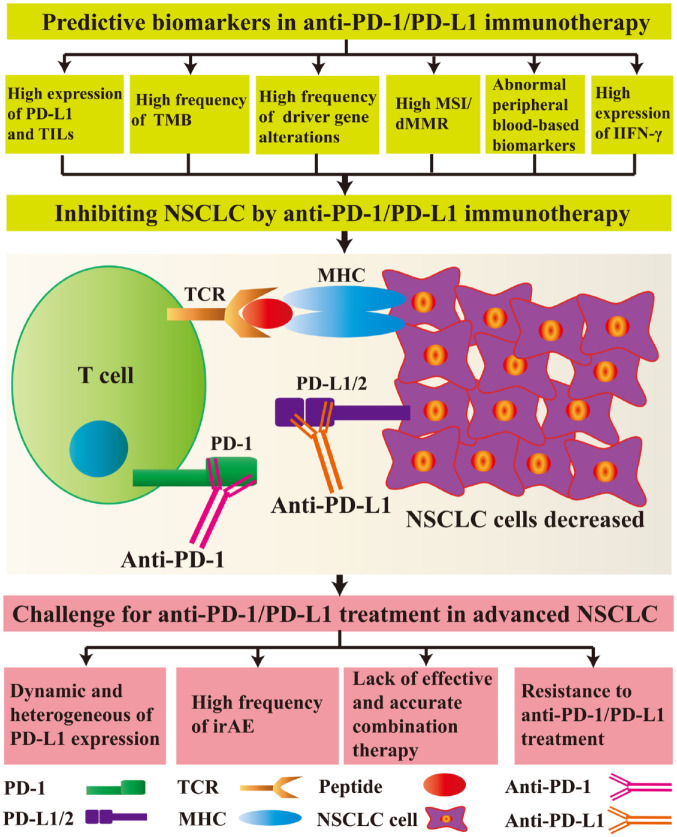
In order to predict the response to immunotherapy, the biomarkers initially used by the researchers were the expression of PD-1 receptor and PD-L1, and the prediction method was assessed only by immunohistochemistry.113 More recently, with the development with newer technology, there are many available biomarkers that could be used to further predict the efficacy and prognosis of immunotherapy.113–117 These include PD-L1 expression, the number of tumour-infiltrating lymphocytes (TILs), the TMB, mutations in certain driver genes, microsatellite instability (MSI)/defective mismatch repair (dMMR), peripheral blood-based biomarkers and interferon gamma (IFN-γ) levels (Figure 2).
Figure 2.
Predictive biomarkers and challenges facing anti-programmed cell-death protein 1 (PD-1)/programmed cell-death ligand 1 (PD-L1) treatment in advanced non-small-cell lung cancer (NSCLC).
For inhibiting NSCLC cells, the predictive biomarkers include high expression levels of PD-L1, high numbers of tumour infiltrating lymphocytes (TILs), high tumour mutational burden (TMB), a high frequency of driver-gene alterations, high microsatellite instability (MSI)/defective mismatch repair (dMMR), abnormal peripheral blood-based biomarkers, and high expression levels of interferon gamma (IFN-γ). After administration of anti-PD-1 or anti-PD-L1 antibodies, the interaction between PD-1 and PD-L1/L2 is disrupted, inhibiting cell signalling pathways. T cells and other immune-related cells exert anti-tumour effects; however, there are still many unresolved problems in the clinical use of anti-PD-1/PD-L1 therapies, such as dynamic changes and heterogeneous differences in PD-L1 expression, a high frequency of immune-related adverse events (irAEs), the lack of effective and accurate combination therapies, and resistance to anti-PD-1/PD-L1 treatment.
MHC, major histocompatibility complex; TCR, T cell receptor.
Level of PD-L1 expression and the number of TILs
Currently, the level of PD-L1 is commonly used as a tumour biomarker to evaluate the efficacy of anti-PD-1/PD-L1 therapy in patients with NSCLC.34,118 The KEYNOTE-024 and KEYNOTE-042 trials49–51 suggested that pembrolizumab could significantly improve the survival rate in patients with high expression levels of PD-L1. The KEYNOTE-001 study also indicated that patients with a PD-L1 TPS ⩾50% would likely experience better outcomes, including improved ORR, mPFS, and mOS.40 Therefore, pembrolizumab has been approved by the FDA for advanced NSCLC patients without driver gene mutations as a first-line treatment. In a single-arm study assessing early ICIs in selected patients with high expression levels of PD-L1, Ito et al. found that ORR and mPFS could be used as an endpoint to predict the survival of patients with high PD-L1 expression levels.119 Teramoto et al. analysed PD-L1 immunohistochemistry in tumour patients at stage I–IIIa NSCLC; they found that high PD-L1 expression is strongly related to early-stage NSCLC and, therefore, is a representative biomarker for predicting a good prognosis.120 Recently, Ricci et al. investigated 150 advanced NSCLC patients, they suggested PD-L1 expression with a cut-off of 50% can be effectively evaluated by the cytological smears.121 Another study showed that PD-L1 expression, CD8+ T cell infiltration, the TMB, and the human leukocyte antigen (HLA) class I were outstanding predictive biomarkers of the anticipated response to anti-PD-1 immunotherapy.122 The mPFS was significantly improved in patients with both a high level of PD-L1 expression and TMB or in those without a loss of HLA class I molecules.
The TME plays a vital role in the growth and progression of cancer cells and distant metastasis, and affects the efficacy of immunotherapy in clinical oncology.123 TILs mainly include NK cells and cytotoxic T cells, both of which are routinely assessed in histopathology reports as predictive biomarkers.124,125 In hyperprogressive disease patients, a high number of CD2− CD4 T lymphocytes during the first and second line of immunotherapy was associated with a lower mOS.126 A retrospective study by Chen et al., assessed tumour PD-L1 expression and T-cell infiltration in NSCLC patients with rare EGFR mutations and found that the frequency of a high PD-L1 expression level and CD8+ TIL infiltration was 36.7%, with a worse prognosis.127
TMB
The TMB is representative of the number of tumour mutations; the higher the frequency of mutations in somatic exonic regions, the greater the number of corresponding tumour-related antigens that are expressed.128,129 For patients with advanced NSCLC, a high TMB detected by next-generation sequencing seems to predict the lasting clinical effects of PD-1 or PD-L1 blockades.130 This results in the subsequent increase in activation of lymphocytes to maintain an immunological anti-tumour response. A previous study evaluated the predictive role of the TMB in 76 NSCLC patients treated with anti-PD-1/PD-L1 therapy. Patients with a high TMB exhibited significantly longer mPFS and mOS.131 In a Chinese study, the TMB was analysed in NSCLC patients receiving anti-PD-1/PD-L1 treatment; the mPFS was significantly longer at 10.6 months in the high TMB group compared with just 3.9 months in the low TMB group (p = 0.0007), and the mOS was 21.0 months in the high TMB group and just 11.6 months in the low TMB group (p = 0.0126).132 The circulating tumour DNA (ctDNA)-related TMB was also assessed in NSCLC patients who received anti-PD-1 or anti-PD-L1 therapy;133 however, higher ctDNA-related TMBs were obviously related to shorter mOS and mPFS, which indicated that a higher ctDNA TMB reflected worse clinical outcomes.134 Another group detected TMB in plasma through blood-based cell-free DNA (cfDNA), and they were able to successfully identify NSCLC patients whose PFS was significantly improved after anti-PD-L1 therapy.135 Chen et al. collected tumour tissue samples from 183 patients with NSCLC, and then assessed PD-L1 expression levels by immunohistochemical analysis, as well as TMB status to assess correlations between PD-L1 expression, TMB, and clinical pathological characteristics.136 They found that in addition to quantifying existing PD-L1 expression levels, monitoring the TMB could be a valuable non-invasive biomarker for predicting the success of chemotherapy and more targeted treatments. This difference may be associated with the large amount of tumour heterogeneity in NSCLCs. A meta-analysis also found that the combination of CD8+ T-cell TILs, PD-L1 expression and TMB was strongly related to a reliable prognosis for advanced NSCLC.137 On the other hand, the precise cut-off values and methods of optimally monitoring TMBs need to be further explored in future clinical studies.
Driver-gene alterations
Next-generation sequencing technology has been able to identify thousands of mutations in large cohorts for various cancers.138 Ono et al. investigated 242 patients with NSCLC to assess driver mutations using whole exome sequencing; they found that the existence of driver-gene alterations might be an independent factor that affects prognosis in NSCLC.139 KRAS is one of the most common driver oncogenes associated with NSCLC.140 A study investigated NSCLC-specific driver-gene alterations in the hope of identifying potential immunotherapy biomarkers. The most common driver genes were TP53 (49%), KRAS (43%) and ERBB2 (13%). Patients with KRAS mutations responded better to PD-1 inhibitors and exhibited improved mPFS and mOS than those without such mutations.104 However, patients with ERBB-family mutations failed to respond to anti-PD-1 therapy. There was a case report of a 64-year-old non-smoking man with squamous NSCLC with co-mutations of TP53 and KRAS who received pembrolizumab combined with gemcitabine treatment; this patient achieved PR, which had persisted for over 7 months at the time of publication.141 However, another study retrospectively identified patients with NSCLC who were harbouring KRAS mutations who were treated with immunotherapy, and there was no significant difference between patients with or without KRAS mutations in terms of mPFS.142 Another study showed that the reason patients with KRAS mutations responded better to PD-1/PD-L1 inhibitors was related to tumour immunogenicity. NSCLC patients with KRAS mutations show good clinical outcomes following treatment with PD-1/PD-L1 inhibitors.143
MSI/dMMR
In addition to PD-1/PD-L1 expression analysis, the presence of MSI is also a key reaction marker.144 Studies have shown that genetic mutations in the dMMR pathway can lead to MSI, and high MSI is closely related to the increased immunogenicity and immune response produced by tumours. MSI is an excellent predictive biomarker. Therefore, the FDA has approved pembrolizumab to treat unresectable solid tumours with high MSI or dMMR.145 The DNA MMR pathway includes the mutL homolog 1 (MLH1), MSH (mutS homolog)-2, postmeiotic segregation increased 2 (PMS2) and MSH-6 proteins. When this pathway is defective (dMMR), it can be used as a means to select patients for immunotherapy. In addition, MSI frequency can also be used to predict NSCLC.117 Similarly, NSCLC patients with DNA polymerase epsilon (POLE) and DNA polymerase delta 1 (POLD1) gene mutations also have an objective clinical response to pembrolizumab treatment and a corresponding degree of association.146,147 Therefore, clinicians can further determine whether a patient can use the epidemiology of the patient’s MSI/dMMR to predict NSCLC prognosis.148
Peripheral blood-based biomarkers
Because biopsies of lung cancer tissue samples are difficult to obtain, some alternative methods include minimally invasive biopsy of peripheral blood for practical reasons.149 Peripheral blood contains DNA, RNA, and proteins released from tumour tissues reflecting the dynamic changes in the TME. The level of ctDNA in the peripheral blood in the early stage of treatment provides valuable clues to the therapeutic effect. In various cancer types, including NSCLC, the detection of highly mutated ctDNA is positively correlated with a better clinical response to immune-checkpoint blockade.150 Zhang et al. analysed individual peripheral T-cell clones of 110 peripheral T cells of NSCLC patients before and after the initiation of PD-1 blockade.151 They found that 25 genes were significantly changed during tumour progression. These analyses help to understand the dynamic changes of T-cell clones in the peripheral blood during PD-1 blockade of NSCLC. Tanizaki et al. investigated 134 patients with advanced or recurrent NSCLC treated with nivolumab to evaluate the association between survival and peripheral blood parameters; they found that a low absolute neutrophil count, high absolute lymphocyte count, and high absolute eosinophil count were closely related to a better clinical outcome in patients with NSCLC treated with nivolumab.152 In addition, Jiang et al. investigated 76 NSCLC patients to evaluate the relationship between survival and peripheral blood markers with ICI monotherapy; they found that a high platelet-to-lymphocyte ratio and low albumin in peripheral blood might be important indicators in advanced NSCLC patients treated with nivolumab and durvalumab monotherapy.153
IFN-γ
IFN-γ is a vital immune-regulatory molecule in the TME released by tumour-reactive T cells.154 Recent studies have suggested that IFN-γ is an important driver of PD-L1 expression in tumours, and it can significantly improve the response to anti-PD-1 therapy.155 The increased IFN-γ levels at baseline and at 3 months after anti-PD-1 therapy were significantly correlated with improved mOS.156 Recent studies have shown that in 17 NSCLC patients treated with nivolumab, mPFS was obviously prolonged in those with high expression levels of IFN-γ, in contrast to those with low expression of IFN-γ (5.1 versus 2 months, respectively; p = 0.0124). This indicated that IFN-γ is a vital biomarker for NSCLC patients treated with anti-PD-1 therapy.157
Other potential biomarkers
Other potential biomarkers being investigated in anti-PD-1/PD-L1 immunotherapy on NSCLC include the pretreatment haemoglobin level,158 serum vascular endothelial growth factor (VEGF) concentration,159 serum lactate dehydrogenase level,160 gut microbiome composition116 and neo-antigen load.161
Challenges for anti-PD-1/PD-L1 treatment in advanced NSCLC
Currently, there are still many unresolved problems associated with the clinical use of anti-PD-1/PD-L1 therapies (Figure 2). First, PD-L1 expression is a predictive biomarker of outcomes of anti-PD-1/PD-L1 treatment, as it is correlated with the efficacy of PD-1/PD-L1 inhibitor therapy in NSCLC; however, this association can be quite variable. For example, the improvement in mOS by nivolumab and durvalumab treatment for NSCLC was independent of PD-L1 expression levels.71,162 In immunohistochemical analysis, the expression levels of PD-L1 are dynamic and heterogeneous, and this is further complicated by issues related to antibody specificity and detection techniques.163 In the future, more valuable biomarkers may be identified by neoantigen prediction techniques, microbiota signatures, or high microsatellite instability (MSI), all of which should be further explored in clinical medicine.
The second challenge is the risk of irAEs associated with the use of anti-PD-1/PD-L1 monotherapy for advanced NSCLC. Although the safety profile of individual antibodies is favourable compared with standard chemotherapy; the frequency of irAEs in combination therapies was much higher (Tables 2–4). For example, 53.6% of patients who received atezolizumab plus chemotherapy developed irAEs, whereas only 29.1% of patients in the group receiving chemotherapy alone did.61 Therefore, better strategies to reduce the risks of serious irAEs need to be explored in future studies.
The third challenge of immunotherapy is deciding how to effectively and accurately select the best combination therapy for patients with advanced NSCLC. Anti-PD-1/PD-L1 treatment combined with chemotherapy is proven to have great potential for improving ORRs.164,165 Apart from chemotherapy, treatments with VEGF, CTLA-4, chimeric antigen receptor T cell, CRISPR-Cas9 gene editing or lymphocyte-activation-protein-3 therapies could provide additional choices for combination strategies in future clinical studies.17,166–169 Therefore, it is important to establish a standard to guide and determine the most appropriate combination therapy for treating advanced NSCLC.
The fourth challenge is that some patients who are resistant to anti-PD-1/PD-L1 therapy experience a poor prognosis after treatment. The underlying resistance mechanism is a very complicated process, and little is known about the potential tumour-related antigens associated with TMB resistance to anti-PD-1/PD-L1 therapy or the genetic determinants of a good prognosis.
Conclusion
To date, four anti-PD-1/PD-L1 drugs, including pembrolizumab, nivolumab, atezolizumab and durvalumab, have been registered and approved for the neoadjuvant, first-line and second-line treatment of NSCLC. With the approval of ICIs, immune-checkpoint therapy based on modulating PD-1/PD-L1 signalling provides more choices for treating advanced NSCLC patients. However, the ORR after ICI treatment is only 14–20% in NSCLC patients who are not identified beforehand as being likely to experience a good prognosis. Therefore, identification of predictive biomarkers is crucial for selecting patients with NSCLC who would most benefit from anti-PD-1/PD-L1 therapy. Although the clinical application of anti-PD-1/PD-L1 therapy is still at an early stage, there are an increasing number of ongoing clinical trials to assess the efficacy of anti-PD-1/PD-L1 treatment. EGFR mutation rates and microbiome differences may affect the efficacy of anti-PD-1/PD-L1 treatment. ALK rearrangements in patients with advanced NSCLC are associated with beneficial outcomes and prolonged mOS from anti-PD-1/PD-L1 treatment compared with chemotherapy, when administered as second-line treatment. Patients with KRAS mutations experienced a longer mPFS and mOS than patients without a KRAS mutation using anti-PD-1/PD-L1 treatment, thus immunotherapy may be a promising strategy with KRAS mutation of NSCLC owing to regulating immune response. Therefore, with the continuous development of clinical trials, more retrospective and prospective clinical studies need to comprehensively evaluate more underlying biomarkers to improve treatment efficacy and enable more NSCLC patients to benefit from ICIs for anti-PD-1/PD-L1 therapy.
Acknowledgments
The authors thank Prof. Nong Yang from the Lung Cancer and Gastroenterology Department, Hunan Cancer Hospital, Affiliated Tumour Hospital of Xiangya Medical School of Central South University for technical help this manuscript. In addition, we would like to thank SAGE Author Services (https://languageservices.sagepub.com/en/services/editing.html) for English language editing.
Footnotes
Authors’ contributions: JZ and JQ wrote the manuscript; QM, LC, LL, TC and PW collected the references and modified the manuscript; LC and JZ designed the manuscript and approved the final manuscript for publication.
Availability of data and materials: Please contact the corresponding author for data requests.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the National Natural Science Foundation of China (81802278, 81900563, 81670017, 81960232, and 82001282) and the Natural Science Foundation of Hunan Province (2019JJ50361 and 2020JJ4418).
ORCID iDs: Jingjing Qu  https://orcid.org/0000-0002-8079-4722
https://orcid.org/0000-0002-8079-4722
Lijun Chen  https://orcid.org/0000-0001-9374-6466
https://orcid.org/0000-0001-9374-6466
Contributor Information
Jingjing Qu, Department of Respiratory Disease, Thoracic Disease Centre, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, PR China.
Quanhui Mei, Intensive Care Unit, The First People’s Hospital of Changde City, Changde, Hunan, PR China.
Li Liu, Lung Cancer and Gastroenterology Department, Hunan Cancer Hospital, Affiliated Tumour Hospital of Xiangya Medical School of Central South University, Changsha, Hunan, PR China.
Tianli Cheng, Thoracic Medicine Department 1, Hunan Cancer Hospital, Affiliated Tumour Hospital of Xiangya Medical School of Central South University, Changsha, Hunan, PR China.
Peng Wang, Ningxia Key Laboratory of Cerebrocranial Diseases, School of Basic Medical Science, Ningxia Medical University, Yinchuan, Ningxia, PR China.
Lijun Chen, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Centre for Infectious Diseases, Collaborative Innovation Centre for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou, Zhejiang 310003, PR China.
Jianying Zhou, Department of Respiratory Disease, Thoracic Disease Centre, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou, Zhejiang 310003, PR China.
References
- 1. Duruisseaux M, Esteller M. Lung cancer epigenetics: from knowledge to applications. Semin Cancer Biol 2018; 51: 116–128. [DOI] [PubMed] [Google Scholar]
- 2. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e1S–e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 4. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553: 446–454. [DOI] [PubMed] [Google Scholar]
- 5. Ohe Y, Imamura F, Nogami N, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol 2019; 49: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters S, Camidge DR, Shaw AT, et al. ; ALEX trial investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–838. [DOI] [PubMed] [Google Scholar]
- 7. Yang CY, Yang JC, Yang PC. Precision management of advanced non-small cell lung cancer. Annu Rev Med 2020; 71: 117–136. [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal C, Rolfo CD, Oxnard GR, et al. Strategies for the successful implementation of plasma-based NSCLC genotyping in clinical practice. Nat Rev Clin Oncol 2021; 18: 56–62. [DOI] [PubMed] [Google Scholar]
- 9. Doroshow DB, Herbst RS. Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol 2018; 4: 569–570. [DOI] [PubMed] [Google Scholar]
- 10. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 2020; 17: 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brozos-Vazquez EM, Diaz-Pena R, Garcia-Gonzalez J, et al. Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother. Epub ahead of print 28 October 2020. DOI: 10.1007/s00262-020-02752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Califano R, Kerr K, Morgan RD, et al. Immune checkpoint blockade: a new era for non-small cell lung cancer. Curr Oncol Rep 2016; 18: 59. [DOI] [PubMed] [Google Scholar]
- 13. Baraibar I, Melero I, Ponz-Sarvise M, et al. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf 2019; 42: 281–294. [DOI] [PubMed] [Google Scholar]
- 14. Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol 2015; 33: 23–35. [DOI] [PubMed] [Google Scholar]
- 15. Taghizadeh H, Marhold M, Tomasich E, et al. Immune checkpoint inhibitors in mCRPC - rationales, challenges and perspectives. Oncoimmunology 2019; 8: e1644109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh RJ, Soo RA. Resistance to immune checkpoint inhibitors in non-small cell lung cancer: biomarkers and therapeutic strategies. Ther Adv Med Oncol 2020; 12: 1758835920937902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chae YK, Arya A, Iams W, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer 2018; 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards SC, Hoevenaar WHM, Coffelt SB. Emerging immunotherapies for metastasis. Br J Cancer 2021; 124: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dang TO, Ogunniyi A, Barbee MS, et al. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev Anticancer Ther 2016; 16: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 21. Lim SH, Sun JM, Lee SH, et al. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Opin Biol Ther 2016; 16: 397–406. [DOI] [PubMed] [Google Scholar]
- 22. Sul J, Blumenthal GM, Jiang X, et al. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist 2016; 21: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017; 3: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munoz-Unceta N, Burgueno I, Jimenez E, et al. Durvalumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2018; 10: 1758835918804151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang K, Li J, Sun Z, et al. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Ther Adv Med Oncol 2020; 12: 175883592097535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noguchi T, Ward JP, Gubin MM, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 2017; 5: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Machicote A, Belen S, Baz P, et al. Human CD8+ HLA-DR+ regulatory T cells, similarly to classical CD4+Foxp3+ cells, suppress immune responses via PD-1/PD-L1 axis. Front Immunol 2018; 9: 2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yahata T, Mizoguchi M, Kimura A, et al. Programmed cell death ligand 1 disruption by clustered regularly interspaced short palindromic repeats/Cas9-genome editing promotes antitumor immunity and suppresses ovarian cancer progression. Cancer Sci 2019; 110: 1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bally AP, Austin JW, Boss JM. Genetic and epigenetic regulation of PD-1 expression. J Immunol 2016; 196: 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019; 30: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 31. Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 2020; 21: 1346–1358. [DOI] [PubMed] [Google Scholar]
- 32. Pio R, Ajona D, Ortiz-Espinosa S, et al. Complementing the cancer-immunity cycle. Front Immunol 2019; 10: 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res 2020; 26: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng Y, Fang YC, Li J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol Lett 2019; 18: 5399–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trefny MP, Kaiser M, Stanczak MA, et al. PD-1+ natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol Immunother 2020; 69: 1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayoux M, Roller A, Pulko V, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med 2020; 12: eaav7431. [DOI] [PubMed] [Google Scholar]
- 38. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016; 5: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marur S, Singh H, Mishra-Kalyani P, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol 2018; 45: 220–225. [DOI] [PubMed] [Google Scholar]
- 40. Hui R, Garon EB, Goldman JW, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol 2017; 28: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Resp Med 2019; 7: 347–357. [DOI] [PubMed] [Google Scholar]
- 42. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 43. Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2020; 38: 1505–1517. [DOI] [PubMed] [Google Scholar]
- 44. Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 45. Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 2020; 15: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 46. Gadgeel SM, Stevenson JP, Langer CJ, et al. Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non-small-cell lung cancer: phase 1 cohorts from the KEYNOTE-021 study. Lung Cancer 2018; 125: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 Investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Awad MM, Gadgeel SM, Borghaei H, et al. Long-term overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol 2021; 16: 162–168. [DOI] [PubMed] [Google Scholar]
- 49. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–546. [DOI] [PubMed] [Google Scholar]
- 50. Reck M, Rodriguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 51. Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 52. Tamiya M, Tamiya A, Hosoya K, et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001). Invest New Drugs 2019; 37: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 53. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer 2019; 116: 137–147. [DOI] [PubMed] [Google Scholar]
- 57. Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol 2019; 37: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 2017; 35: 2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 61. Papadimitrakopoulou V, Cobo M, Bordoni R, et al. OA05.07 IMpower132: PFS and safety results with 1l atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol 2018; 13: S332–S333. [Google Scholar]
- 62. Planchard D, Yokoi T, McCleod MJ, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer 2016; 17: 232–236.e231. [DOI] [PubMed] [Google Scholar]
- 63. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol 2020; 15: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 64. Herbst RS, Giaccone G, De Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 2020; 383: 1328–1339. [DOI] [PubMed] [Google Scholar]
- 65. Shiraishi Y, Kishimoto J, Tanaka K, et al. Treatment rationale and design for APPLE (WJOG11218L): a multicenter, open-label, randomized phase 3 study of atezolizumab and platinum/pemetrexed with or without bevacizumab for patients with advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2020; 21: 472–476. [DOI] [PubMed] [Google Scholar]
- 66. Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016; 17: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang X, Wang C, Wang J, et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater 2018; 30: e1707112. [DOI] [PubMed] [Google Scholar]
- 68. Mark M, Froesch P, Eboulet EI, et al. ; Swiss Group for Clinical Cancer. SAKK 19/17: safety analysis of first-line durvalumab in patients with PD-L1 positive, advanced nonsmall cell lung cancer and a performance status of 2. Cancer Immunol Immunother. Epub ahead of print 1 November 2020. DOI: 10.1007/s00262-020-02757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 study. J Thorac Oncol 2018; 13: 194–204. [DOI] [PubMed] [Google Scholar]
- 71. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reck M, Brahmer J, Bennett B, et al. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer 2018; 102: 23–30. [DOI] [PubMed] [Google Scholar]
- 73. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14: 867–875. [DOI] [PubMed] [Google Scholar]
- 75. Martin C, Lupinacci L, Perazzo F, et al. Efficacy and safety of nivolumab in previously treated patients with non-small-cell lung cancer: real world experience in Argentina. Clin Lung Cancer 2020; 21: e380–e387. [DOI] [PubMed] [Google Scholar]
- 76. Morita R, Okishio K, Shimizu J, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: a multicenter retrospective observational study in Japan. Lung Cancer 2020; 140: 8–18. [DOI] [PubMed] [Google Scholar]
- 77. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 78. Herbst RS, Garon EB, Kim DW, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1positive, advanced nonsmall-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol 2020; 38: 1580–1590. [DOI] [PubMed] [Google Scholar]
- 79. Nishio M, Takahashi T, Yoshioka H, et al. KEYNOTE-025: phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1-positive advanced non-small-cell lung cancer. Cancer Sci 2019; 110: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gubens MA, Sequist LV, Stevenson JP, et al. Pembrolizumab in combination with ipilimumab as second-line or later therapy for advanced non-small-cell lung cancer: KEYNOTE-021 cohorts D and H. Lung Cancer 2019; 130: 59–66. [DOI] [PubMed] [Google Scholar]
- 81. Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK study group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Von Pawel J, Bordoni R, Satouchi M, et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer 2019; 107: 124–132. [DOI] [PubMed] [Google Scholar]
- 83. Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR study group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 84. Ikeda S, Kato T, Kenmotsu H, et al. A phase II study of atezolizumab for pretreated advanced/recurrent non-small cell lung cancer with idiopathic interstitial pneumonias: rationale and design for the TORG1936/AMBITIOUS study. Ther Adv Med Oncol 2020; 12: 1758835920922022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vergnenegre A, Monnet I, Bizieux A, et al. Open-label phase II trial to evaluate safety and efficacy of second-line metronomic oral vinorelbine-atezolizumab combination for stage-IV non-small-cell lung cancer - VinMetAtezo trial, (GFPC‡ 04-2017). Future Oncol 2020; 16: 5–10. [DOI] [PubMed] [Google Scholar]
- 86. Marine S, Stephane R, Nicolas P, et al. Cost-effectiveness of atezolizumab versus docetaxel and nivolumab in the treatment of non-small cell lung cancer as a second line in France. J Med Econ 2020; 23: 464–473. [DOI] [PubMed] [Google Scholar]
- 87. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Planchard D, Reinmuth N, Orlov S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol 2020; 31: 609–618. [DOI] [PubMed] [Google Scholar]
- 89. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev 2018; 62: 39–49. [DOI] [PubMed] [Google Scholar]
- 91. D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015; 112: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol 2016; 49: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 93. Abdelhamed S, Ogura K, Yokoyama S, et al. AKT-STAT3 pathway as a downstream target of EGFR signaling to regulate PD-L1 expression on NSCLC cells. J Cancer 2016; 7: 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jiang XM, Xu YL, Huang MY, et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol Sin 2017; 38: 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol 2018; 13: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang JC, Gadgeel SM, Sequist LV, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol 2019; 14: 553–559. [DOI] [PubMed] [Google Scholar]
- 97. Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol 2018; 13: 682–688. [DOI] [PubMed] [Google Scholar]
- 98. Garassino MC, Gelibter AJ, Grossi F, et al. Italian nivolumab expanded access program in nonsquamous non-small cell lung cancer patients: results in never-smokers and EGFR-mutant patients. J Thorac Oncol 2018; 13: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 99. Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 2017; 28: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 100. Maione P, Sacco PC, Sgambato A, et al. Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application. Ther Adv Med Oncol 2015; 7: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018; 17: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol 2018; 13: 1363–1372. [DOI] [PubMed] [Google Scholar]
- 103. Felip E, De Braud FG, Maur M, et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: results of an open-label, multicenter, phase 1B study. J Thorac Oncol 2020; 15: 392–403. [DOI] [PubMed] [Google Scholar]
- 104. Cinausero M, Laprovitera N, De Maglio G, et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol 2019; 11: 1758835919885540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fu J, Wang F, Dong LH, et al. Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001, a programmed cell death protein-1 (PD-1) monoclonal antibody. Acta Pharmacol Sin 2017; 38: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang J, Dong L, Yang S, et al. Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer 2020; 130: 182–192. [DOI] [PubMed] [Google Scholar]
- 107. Hoy SM. Sintilimab: first global approval. Drugs 2019; 79: 341–346. [DOI] [PubMed] [Google Scholar]
- 108. Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol 2020; 15: 816–826. [DOI] [PubMed] [Google Scholar]
- 109. Markham A, Keam S. Camrelizumab: first global approval. Drugs 2019; 79: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 110. Wei Z, Yang X, Ye X, et al. Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer. J Cancer Res Ther 2019; 15: 1629–1634. [DOI] [PubMed] [Google Scholar]
- 111. Li W, Wei Z, Yang X, et al. Salvage therapy of reactive capillary hemangiomas: apatinib alleviates the unique adverse events induced by camrelizumab in non-small cell lung cancer. J Cancer Res Ther 2019; 15: 1624–1628. [DOI] [PubMed] [Google Scholar]
- 112. Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8: e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Darabi S, Braxton DR, Eisenberg BL, et al. Predictive biomarkers for immunotherapy response beyond PD-1/PD-L1. Oncology (Williston Park) 2020; 34: 321–327. [DOI] [PubMed] [Google Scholar]
- 114. Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol 2019; 20: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 115. Guven DC, Sahin TK, Dizdar O, et al. Predictive biomarkers for immunotherapy efficacy in non-small-cell lung cancer: current status and future perspectives. Biomark Med 2020; 14: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 116. Tray N, Weber JS, Adams S. Predictive biomarkers for checkpoint immunotherapy: current status and challenges for clinical application. Cancer Immunol Res 2018; 6: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 117. Ren D, Hua Y, Yu B, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer 2020; 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang B, Liu Y, Zhou S, et al. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: a meta-analysis. Int Immunopharmacol 2020; 80: 106214. [DOI] [PubMed] [Google Scholar]
- 119. Ito K, Miura S, Sakaguchi T, et al. The impact of high PD-L1 expression on the surrogate endpoints and clinical outcomes of anti-PD-1/PD-L1 antibodies in non-small cell lung cancer. Lung Cancer 2019; 128: 113–119. [DOI] [PubMed] [Google Scholar]
- 120. Teramoto K, Igarashi T, Kataoka Y, et al. Biphasic prognostic significance of PD-L1 expression status in patients with early- and locally advanced-stage non-small cell lung cancer. Cancer Immunol Immunother. Epub ahead of print 28 October 2020. DOI: 10.1007/s00262-020-02755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ricci C, Capizzi E, Giunchi F, et al. Reliability of programmed death ligand 1 (PD-L1) tumor proportion score (TPS) on cytological smears in advanced non-small cell lung cancer: a prospective validation study. Ther Adv Med Oncol 2020; 12: 1758835920954802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hurkmans DP, Kuipers ME, Smit J, et al. Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother 2020; 69: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chen F, Zhuang X, Lin L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med 2015; 13: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wein L, Savas P, Luen SJ, et al. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol 2017; 7: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-immunoscore in resected nonsmall-cell lung cancer. Ann Oncol 2016; 27: 225–232. [DOI] [PubMed] [Google Scholar]
- 126. Arasanz H, Zuazo M, Bocanegra A, et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers (Basel) 2020; 12: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen K, Cheng G, Zhang F, et al. PD-L1 expression and T cells infiltration in patients with uncommon EGFR-mutant non-small cell lung cancer and the response to immunotherapy. Lung Cancer 2020; 142: 98–105. [DOI] [PubMed] [Google Scholar]
- 128. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019; 30: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fumet JD, Truntzer C, Yarchoan M, et al. Tumour mutational burden as a biomarker for immunotherapy: current data and emerging concepts. Eur J Cancer 2020; 131: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 2018; 36: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Alborelli I, Leonards K, Rothschild SI, et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J Pathol 2020; 250: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huang D, Zhang F, Tao H, et al. Tumor mutation burden as a potential biomarker for PD-1/PD-L1 inhibition in advanced non-small cell lung cancer. Target Oncol 2020; 15: 93–100. [DOI] [PubMed] [Google Scholar]
- 133. Fang W, Ma Y, Yin JC, et al. Combinatorial assessment of ctDNA release and mutational burden predicts anti-PD(L)1 therapy outcome in nonsmall-cell lung cancer. Clin Transl Med 2020; 10: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chae YK, Davis AA, Agte S, et al. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist 2019; 24: 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 136. Chen Y, Liu Q, Chen Z, et al. PD-L1 expression and tumor mutational burden status for prediction of response to chemotherapy and targeted therapy in non-small cell lung cancer. J Exp Clin Cancer Res 2019; 38: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yu Y, Zeng D, Ou Q, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open 2019; 2: e196879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pon JR, Marra MA. Driver and passenger mutations in cancer. Annu Rev Pathol 2015; 10: 25–50. [DOI] [PubMed] [Google Scholar]
- 139. Ono A, Isaka M, Serizawa M, et al. Genetic alterations of driver genes as independent prognostic factors for disease-free survival in patients with resected non-small cell lung cancer. Lung Cancer 2019; 128: 152–157. [DOI] [PubMed] [Google Scholar]
- 140. Roman M, Baraibar I, Lopez I, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer 2018; 17: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fang C, Zhang C, Zhao WQ, et al. Co-mutations of TP53 and KRAS serve as potential biomarkers for immune checkpoint blockade in squamous-cell non-small cell lung cancer: a case report. BMC Med Genomics 2019; 12: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gianoncelli L, Spitaleri G, Passaro A, et al. Efficacy of anti-PD1/PD-L1 therapy (IO) in KRAS mutant non-small cell lung cancer patients: a retrospective analysis. Anticancer Res 2020; 40: 427–433. [DOI] [PubMed] [Google Scholar]
- 143. Liu C, Zheng S, Jin R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett 2020; 470: 95–105. [DOI] [PubMed] [Google Scholar]
- 144. Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016; 22: 813–820. [DOI] [PubMed] [Google Scholar]
- 145. Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 2019; 25: 3753–3758. [DOI] [PubMed] [Google Scholar]
- 146. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yao J, Gong Y, Zhao W, et al. Comprehensive analysis of POLE and POLD1 gene variations identifies cancer patients potentially benefit from immunotherapy in Chinese population. Sci Rep 2019; 9: 15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhao P, Li L, Jiang X, et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nassar FJ, Chamandi G, Tfaily MA, et al. Peripheral blood-based biopsy for breast cancer risk prediction and early detection. Front Med (Lausanne) 2020; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Shen H, Yang ES, Conry M, et al. Predictive biomarkers for immune checkpoint blockade and opportunities for combination therapies. Genes Dis 2019; 6: 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang F, Bai H, Gao R, et al. Dynamics of peripheral T cell clones during PD-1 blockade in non-small cell lung cancer. Cancer Immunol Immunother 2020; 69: 2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 2018; 13: 97–105. [DOI] [PubMed] [Google Scholar]
- 153. Jiang M, Peng W, Pu X, et al. Peripheral blood biomarkers associated with outcome in non-small cell lung cancer patients treated with nivolumab and durvalumab monotherapy. Front Oncol 2020; 10: 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Thibaut R, Bost P, Milo I, et al. Bystander IFN-gamma activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer 2020; 1: 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017; 127: 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Boutsikou E, Domvri K, Hardavella G, et al. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol 2018; 10: 1758835918768238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Karachaliou N, Gonzalez-Cao M, Crespo G, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol 2018; 10: 1758834017749748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang Z, Zhang F, Yuan F, et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol 2020; 12: 175883592097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Shibaki R, Murakami S, Shinno Y, et al. Predictive value of serum VEGF levels for elderly patients or for patients with poor performance status receiving anti-PD-1 antibody therapy for advanced non-small-cell lung cancer. Cancer Immunol Immunother 2020; 69: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang M, Yang J, Hua W, et al. Monitoring checkpoint inhibitors: predictive biomarkers in immunotherapy. Front Med 2019; 13: 32–44. [DOI] [PubMed] [Google Scholar]
- 161. Rosenthal R, Cadieux EL, Salgado R, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019; 567: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 163. Hofman P. PD-L1 immunohistochemistry for non-small cell lung carcinoma: which strategy should be adopted? Expert Rev Mol Diagn 2017; 17: 1097–1108. [DOI] [PubMed] [Google Scholar]
- 164. Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res 2018; 2018: 6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pirker R. Immunotherapy combinations in advanced nonsmall cell lung cancer. Curr Opin Oncol 2021; 33: 73–79. [DOI] [PubMed] [Google Scholar]
- 166. Meder L, Schuldt P, Thelen M, et al. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res 2018; 78: 4270–4281. [DOI] [PubMed] [Google Scholar]
- 167. Qu J, Mei Q, Chen L, et al. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother Epub ahead of print 6 October 2020. DOI: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lu Y, Xue J, Deng T, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med 2020; 26: 732–740. [DOI] [PubMed] [Google Scholar]
- 169. He Y, Yu H, Rozeboom L, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol 2017; 12: 814–823. [DOI] [PubMed] [Google Scholar]