Abstract
Objective
Following thyroid lobectomy, patients are at risk for hypothyroidism. This study sought to determine the incidence of postlobectomy thyroid hormone replacement as well as predictive risk factors to better counsel patients.
Study Design
Retrospective cohort study.
Setting
Patients aged 18 to 75 years treated in a single academic institution who underwent thyroid lobectomy from October 2006 to September 2017.
Methods
Patients were followed for an average of 73 months. Demographic data, body mass index, size of removed and remnant lobe, preoperative thyroid-stimulating hormone (TSH) level, final thyroid pathology, and presence of thyroiditis were collected and analyzed. Risk factors were evaluated with chi-square analyses, t tests, logistic regression, and Kaplan-Meier analysis.
Results
Of the 478 patients reviewed, 369 were included in the analysis, 30% of whom eventually required thyroid hormone replacement. More than 39% started therapy >12 months postoperatively, with 90% treated within 36 months. Patient age ≥50 years and preoperative TSH ≥2.5 mIU/L were associated with odds ratios of 2.034 and 3.827, respectively, for thyroid hormone replacement. Malignancy on final pathology demonstrated an odds ratio of 7.76 for hormone replacement. Sex, body mass index, volume of resected and remaining lobes, and weight of resected lobe were not significant predictors.
Conclusion
Nearly a third of patients may ultimately require thyroid hormone replacement. Age at the time of surgery, preoperative TSH, and final pathology are strong, clinically relevant predictors of the need for future thyroid hormone replacement. After lobectomy, patients should have long-term thyroid function follow-up to monitor for delayed hypothyroidism.
Keywords: thyroid lobectomy, postoperative hypothyroidism, thyroid hormone replacement
Thyroid lobectomy has generally been considered a safe and frequently preferred surgical option for the treatment of symptomatic unilateral goiter, toxic adenoma, cytologically indeterminate nodule, and low-risk differentiated thyroid cancer.1-3 As compared with total thyroidectomy, lobectomy has a significantly lower rate of postoperative complications, including hypocalcemia, bleeding, hematoma, tracheostomy, and vocal cord paralysis.4 Additionally, patients who undergo total thyroidectomy will require long-term routine office follow-up, regular blood tests, and lifelong thyroid hormone supplementation. Most patients who undergo lobectomy do not require thyroid hormone replacement. The reported incidence of postlobectomy hypothyroidism is quite variable, from 6.5% to 60%, with a meta-analysis reporting a pooled risk of 22%.1,2,5-8 This wide range is in part due to the varying follow-up durations, hypothyroidism definitions, timing of thyroid function assessments, and type of thyroid disease included in the studies.3
Determining the incidence of postoperative hypothyroidism following thyroid lobectomy has been complicated by its varying definitions.6,9 The National Health and Nutrition Examination Survey III suggested 0.4 to 4.0 mIU/L as the reference range for thyroid-stimulating hormone (TSH). However, others have suggested a lower limit of 2.5 mIU/L and upper limits of 3.59, 5.5, 7.0, 8.0, and 10.0 mIU/L.6,10 This makes interinstitutional studies and larger pooled data sets difficult to interpret and generalize.2,11 Furthermore, there is growing disagreement on the criteria to initiate thyroid hormone replacement.2,5,6,9 It is accepted that untreated hypothyroidism may cause symptoms such as dry skin, memory impairment, fatigue, cold intolerance, voice changes, and constipation.9 Yet, chronic replacement therapy with levothyroxine may increase the risk of atrial fibrillation or osteoporosis.11-13 This is particularly true in postmenopausal women, who have been shown to have a 3-fold higher risk of atrial fibrillation and aggravation of ischemic heart disease when on chronic thyroid hormone replacement.7,9 Additionally, the European Thyroid Association and the American Thyroid Association suggest that treating subclinical hypothyroidism when TSH is <10 mIU/L may be of minimal clinical benefit, as hormone replacement below these levels may not relieve the symptoms of concern.9,10
Within this context, the reported incidence of postlobectomy hypothyroidism from 6.5% to 60% must be viewed critically. Furthermore, the rate of thyroid replacement therapy is relevant, as one of the purported benefits of lobectomy over total thyroidectomy is avoiding the requirement of lifelong medication. Only a small subset of studies has selected the prescription of thyroid hormone replacement as the primary endpoint over a more stringent biochemical definition of hypothyroidism, with 2 such studies reporting replacement rates of 7.4% and 48%.6,8
Multiple studies have proposed risk factors for the development postlobectomy hypothyroidism, including elevated preoperative TSH, the presence of antithyroid antibody and lymphocytic infiltration, higher resected lobe size, and lower remaining lobe volume.1,2,5,8,11,14 However, there is no set consensus within the literature on these risk factors, and additional research is needed to better identify their clinical predictive utility. Once identified and validated, such risk factors could be used to better council patients during the preoperative evaluation. The decision to proceed with a thyroid lobectomy versus a total thyroidectomy is often clear-cut when dictated by the underlying pathology; however, when the choice is less distinct, a thorough understanding of the risks and benefits of each option is essential for both the surgeon and the patient. This study aims to identify the incidence of thyroid hormone replacement following thyroid lobectomy and to identify predictive risk factors for needing hormone replacement.
Methods
This study employed a retrospective cohort study design. The electronic medical record was queried to identify patients who underwent thyroid lobectomy between October 2006 and September 2017 at our academic medical center, with CPT codes 60210, 60212, 60220, and 60225 (Current Procedural Terminology). Exclusion criteria included having undergone completion thyroidectomy within the study period, having preoperative thyroid hormone supplementation, preoperative TSH not available, and age ≥75 years. Chart review was performed on each identified patients to include all encounters and all prescriptions dispensed from the time of surgery to July 31, 2020. The endpoint for the follow-up period for each patients was defined as the most recent documented service or encounter within the electronic medical record. The study protocol was approved by the Naval Medical Center Portsmouth institutional review board.
Data abstracted included patient age, sex, body mass index, family history of thyroid disease, preoperative TSH, postoperative symptoms (<3 months), postoperative complications (<3 months), length of postoperative follow-up, volume of remaining thyroid lobe, volume of resected thyroid lobe, weight of resected thyroid lobe, presence of thyroiditis on pathology report, and final pathology of resected thyroid lobe. The normal range for TSH was defined as 0.464 to 4.679 mIU/L, in accordance with our institution’s laboratory standards. The volume of each lobe and isthmus was calculated by the formula for an ovoid (width × depth × length ×π/6) with the measurements determined on preoperative thyroid ultrasound.15
All statistical analyses were performed with R statistics software (R Foundation for Statistical Computing, v3.6.4). Demographics were presented with descriptive statistics and compared between groups of patients with and without documented thyroid hormone replacement. The prescription of hormone replacement over time was presented as Kaplan-Meier survival curves. Risk factors for hormone replacement were compared between groups prescribed replacement and not. Independent-sample t tests, chi-square analyses, and Fisher exact tests were performed depending on variable characteristics. Odds ratios (ORs) were calculated with univariate logistic regression, with hormone replacement as the dependent variable. Furthermore, multivariate logistic regression analysis was used to asses the risk factors of malignant pathology. Kaplan-Meier curves were generated for time to hormone replacement in the groups with benign and malignant pathology, with curves compared per the nonparametric log-rank test. Additional subgroup analysis was performed on those requiring hormone replacement <3 months versus ≥3 months. Simple 2-sample t tests were used for continuous data and Fisher exact test for discrete data.
Results
A total of 478 patients underwent a lobectomy during the study period, and 369 were ultimately included in the analysis ( Figure 1 ). Demographics and perioperative data are presented in Table 1 . The average in-person postoperative follow-up was 2.6 months (range, 1 day–41.3 months). However, the average follow-up time via electronic chart review was 73.8 months (range, 13 days–160 months). This resulted in 30% of patients being followed >96 months after surgery and 16% for >120 months. The overall incidence of thyroid hormone replacement was 30.1%, with 29% of these patients starting medication within postoperative 6 months and 39% at >1 year. Additionally, 90% of patients who started hormone therapy did so within 36 months following surgery ( Figure 2 ).
Figure 1.
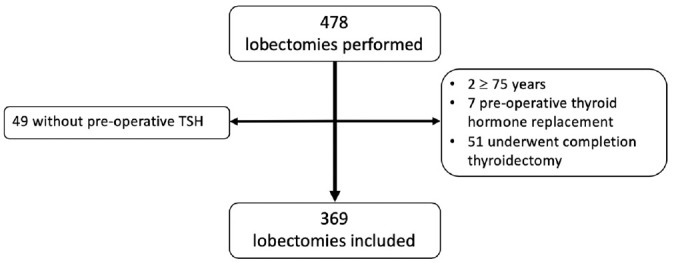
Patient inclusion flow diagram. TSH, thyroid-stimulating hormone.
Table 1.
Demographics and Clinical Characteristics of Patients Who Required Thyroid Hormone Replacement Following Thyroid Lobectomy.
| Patients, No. (%)a | ||||
|---|---|---|---|---|
| Total (N = 369) | No hormone replacement (n = 258) | Hormone replacement (n = 111) | P value | |
| Age, y | ||||
| Mean ± SD | 43.8 ± 12.3 | 42.6 ± 12.3 | 46.6 ± 12.4 | .005 |
| 18-29 | 65 (17.6) | 50 (19) | 15 (14) | |
| 30-49 | 174 (47) | 130 (50) | 44 (40) | |
| ≥50 | 130 (35) | 78 (46) | 52 (47) | .002 |
| Sex: female | 278 (75) | 191 (74) | 87 (78.4) | .364 |
| Body mass index, mg/kg2, mean ± SD | 28.8 ± 6.3 | 28.6 ± 6.0 | 29.4 ± 7.0 | .288 |
| Family history of thyroid disease | 75 (24.5) | 45 (21.5) | 30 (30.9) | .087 |
| Preoperative TSH, mIU/L | ||||
| Mean ± SD | 1.34 ± 1.08 | 1.65 ± 1.13 | 1.21 ± 1.06 | <.001 |
| <0.4 | 36 (11) | |||
| 0.4 to <2.5 | 262 (79) | |||
| 2.5 to <4.0 | 27 (8) | |||
| ≥4.0 | 7 (2) | |||
| ≥2.5 | 34 (10) | 14 (6) | 20 (20) | <.001 |
| Postoperative (<3 mo) | ||||
| Symptoms | 50 (13.7) | 31 (12) | 19 (17) | .202 |
| Complications | 21 (4) | 22 (9) | 9 (8.1) | .886 |
| Temporary TVC weakness | 5 (1.4) | |||
| Permanent TVC weakness | 0 | |||
| Seroma | 3 (0.83) | |||
| Hematoma | 2 (0.55) | |||
| Hypocalcemia | 1 (0.28) | |||
| Lobe volume, cm3, mean ± SD | ||||
| Remaining | 6.5 ± 5.8 | 6.5 ± 5.3 | 6.4 ± 7.0 | .902 |
| Resected | 22.2 ± 22.3 | 22.7 ± 22.6 | 21.2 ± 21.8 | .563 |
| Resected lobe weight, g, mean ± SD | 20.7 ± 18.7 | 20.7 ± 18.6 | 20.6 ± 19.1 | .949 |
| Final pathology | ||||
| Benign | 329 (89) | 240 (92.9) | 89 (79.6) | <.001 |
| Follicular adenoma | 188 (57.1) | |||
| Multinodular goiter | 129 (39.2) | |||
| Otherb | 4 (1.2) | |||
| Malignant | 40 (11.0) | 18 (7.1) | 22 (20.4) | .0002 |
| Papillary thyroid carcinoma | 35 (87.5) | |||
| Follicular thyroid carcinoma | 4 (10) | |||
| Squamous cell carcinoma | 1 (2.5) | |||
| Thyroiditis on pathology report: positive | 68 (19.0) | 43 (17.1) | 25 (23.6) | .184 |
| Follow-up, mo, mean ± SD (range) | ||||
| In person | 2.17 ± 4.0 (0.03-31.2) | 1.69 ± 2.6 | 3.38 ± 6.2 | .01 |
| Electronic | 74.8 ± 41.7 (0.43-160) | 72.9 ± 42.7 | 79.7 ± 39.3 | .138 |
Abbreviations: THS, thyroid-stimulating hormone; TVC, true vocal cord.
Values are presented as No. (%) unless noted otherwise.
Two cases of colloid cysts and 2 cases of parathyroid cysts.
Figure 2.
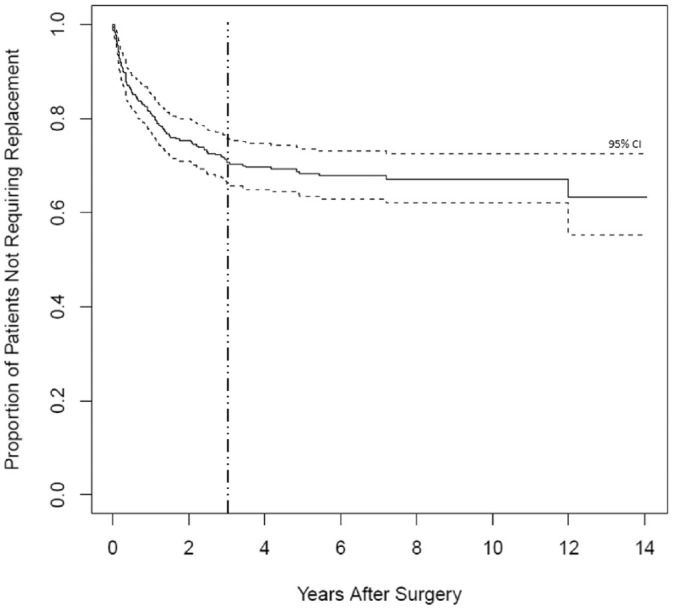
Kaplan-Meier curve for prescription of postoperative thyroid hormone replacement as a function of time. Dashed line indicates point at which 90% of patients who were prescribed postoperative thyroid hormone replacement had started therapy.
Need for thyroid hormone replacement was associated with older age, higher preoperative TSH, and presence of malignant thyroid pathology. Age and preoperative TSH were categorized into groups (≥50 vs <50 years, ≥2.5 vs <2.5 mIU/L) to align with accepted clinical cutoffs. Patients ≥50 years old had an increased risk of requiring hormone replacement as compared with those <50 years old (OR, 2.034; 95% CI, 1.287-3.215). Patients with a preoperative TSH ≥2.5 mIU/L had an increased risk of needing hormone replacement as compared with <2.5 mIU/L (OR, 3.827; 95% CI, 1.846-7.935). The remaining variables did not demonstrate a significant association with the eventual need for hormone replacement ( Figure 3 ).
Figure 3.
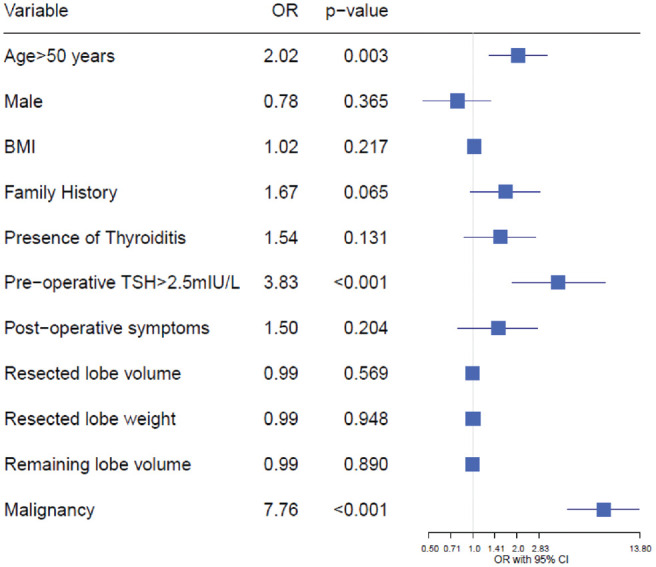
Odds ratios (ORs) generated by logistic regression for the prescription of postlobectomy thyroid hormone replacement dependent on patient and surgical characteristics. BMI, body mass index; TSH, thyroid-stimulating hormone.
For malignant pathology, there was a 7.76-times increase in odds (95% CI, 4.41-13.63) of requiring postoperative medication relative to whether the pathology was benign. Upon adjusting for age at the time of surgery and preoperative TSH level, the adjusted OR remained significant at 4.45 (95% CI, 2.16-9.48). A log-rank test for equivalence was performed for the malignant versus benign survival curves and found to have a chi-square statistic of 21.3 (P < .001), rejecting the null hypothesis of equivalence ( Figure 4 ).
Figure 4.
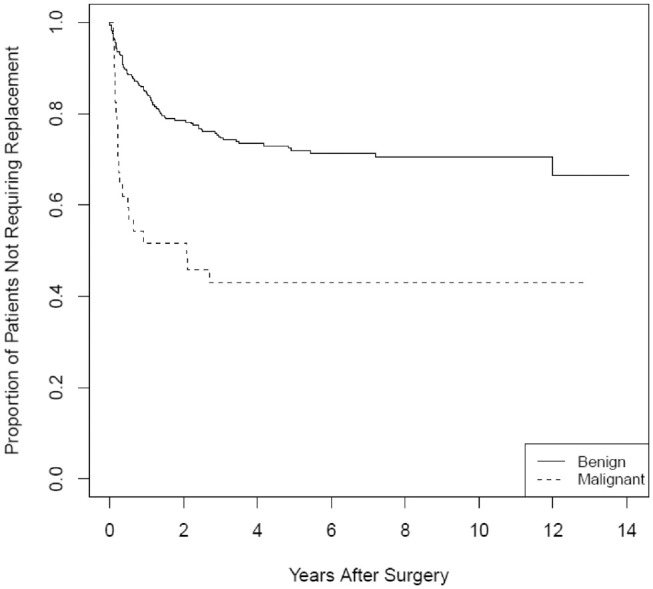
Kaplan-Meier curves for prescription of postoperative thyroid hormone replacement as a function of time for the malignant and benign pathology subgroups (log-rank test, P < .001).
Additional subgroup analysis was performed comparing those requiring early versus late replacement (<3 vs ≥3 months postoperatively). The early replacement group showed a significantly smaller remaining thyroid lobe volume (mean [SD], 4.8 [2.6] cm3) when compared with late replacement group (7.2 [8.25] cm3, P = .038). Early replacement was also associated with a final malignant pathology of 40.6%, as opposed to 12.2% for late replacement (P = .002; Table 2 ).
Table 2.
Demographics and Clinical Characteristics of Patients Who Required Early vs Late Thyroid Hormone Replacement Following Thyroid Lobectomy.a
| Within 3 mo (n = 32) | After 3 mo (n = 77) | P value | |
|---|---|---|---|
| Age, y | 44.5 ± 11.2 | 47.1 ± 12.9 | .281 |
| Sex: female | 27 (84.4) | 58 (75.3) | .299 |
| Body mass index, mg/kg2 | 28.6 ± 5.5 | 29.7 ± 7.6 | .417 |
| Family history of thyroid disease | 7 (21.9) | 23 (29.8) | .323 |
| Preoperative TSH, mIU/L | 1.56 ± 1.01 | 1.66 ± 1.07 | .656 |
| ≥2.5 | 6 (20.7) | 13 (18.6) | .786 |
| Lobe volume, cm3 | |||
| Remaining | 4.8 ± 2.6 | 7.2 ± 8.25 | .038b |
| Resected | 18.5 ± 14.0 | 22.5 ± 24.6 | .292 |
| Resected lobe weight, g | 17.5 ± 14.9 | 21.9 ± 20.8 | .249 |
| Final pathology: malignant | 13 (40.6) | 9 (12.2) | .002b |
| Thyroiditis on pathology report: positive | 8 (25.8) | 16 (21.6) | .641 |
Abbreviation: THS, thyroid-stimulating hormone.
Values are presented as mean ± SD or No. (%).
P < .05.
Discussion
This study showed a hormone replacement rate of 30.1% following lobectomy for all indications. Two similar studies reported replacement rates of 7.4% and 48%.6,8 When compared with this study, those studies had smaller cohorts, shorter follow-up duration, and more restricted pathologic inclusion criteria. Farkas et al studied patients undergoing lobectomy for solitary nodules, while Su et al included only benign pathology.8,16
One potential reason for the reported higher rate of replacement therapy within this study is the longer follow-up time. Most studies evaluating postoperative hypothyroidism followed patients for 24 or 48 months and reported that 90% of patients were diagnosed in the first 12 months.2,3,5,14 Within our institution, access to medical care is universally covered, and there is no additional patient cost for follow-up visits or medications. Additionally, patient records within our nationwide health care network are reviewable from any location, and patient electronic health information is available even after patients have moved away from the surgical practice or even the geographic area, as long as they retain benefits within the health system. This strength allowed patient follow-up data to be captured for a mean 73.8 months postoperatively.
Our results show that while 29% of those receiving hormone replacement started therapy within the first 3 months postoperatively, an additional 39% began therapy after 12 months. Routine follow-up past 3 to 6 months is not standard after a thyroid lobectomy. It therefore follows that the diagnosis of hypothyroidism for 53% to 71% of patients and their subsequent need for hormone replacement therapy could be delayed or missed. To identify 90% of those requiring replacement, thyroid function surveillance would need to be performed until at least 36 months postoperatively.
In keeping with previous studies, our results showed that age and preoperative TSH have a significant correlation with the need for thyroid hormone replacement. Age >50 years more than doubles the risk for thyroid hormone replacement, while a preoperative TSH >2.5 mIU/L portends a nearly a 4-times higher risk. This study also revealed a significant correlation between the final pathology within the resected thyroid lobe and the need for eventual hormone replacement, with an increased odds of requiring hormone replacement at nearly 4.5 times that of the group with benign pathology, even when adjusting for age and preoperative TSH.
One explanation for this unique finding is that earlier studies primarily focused on lobectomies performed for benign disease. In part this was due to the previously customary practice of prescribing postoperative thyroid hormone replacement to suppress the development of additional disease in the thyroid remnant, which would therefore preclude these patients from evaluation within earlier incidence studies.7,12,13 This practice is less common, and each prescription of replacement hormone within this cohort was verified to be prescribed only for hypothyroidism rather than for tumor suppression. The underlying biochemical explanation of the impact of malignancy within the resected lobe on the remaining lobe’s endocrine function is not yet clear. This question warrants future study, but even without a known mechanism, our findings provide a new and powerful predictive risk factor to assist in counseling patients on the eventual need for thyroid hormone replacement following thyroid lobectomy.
Limitations of our study include its retrospective single-institution nature. Additionally, as with most retrospective studies, we encountered inconsistent preoperative collection of thyroid hormone levels, postoperative TSH, and pre- and postoperative thyroid antibody levels. Furthermore, while the design of the electronic medical record allowed for uncommonly long follow-up of medication prescriptions, it was not clear if and when patients stopped taking these medications. This prevents distinguishing between temporary and permanent postoperative hypothyroidism. However, this weakness would not likely have an impact on the observations of higher rates of delayed hypothyroidism (≥12 months) or the association with malignancy. Based on the promising prognostic findings from this study, a prospective trial is underway to better delineate these factors and their impact on hormone replacement.
Conclusion
To date, this study examined postlobectomy hormone replacement in the largest cohort over the longest duration. Findings demonstrated a 30% incidence of thyroid hormone replacement and identified age, preoperative TSH, and an underlying malignant pathology as powerful and clinically relevant risk factors for starting hormone replacement therapy. Delayed hypothyroidism following surgery was also identified, with 39% of patients starting therapy >1 year postoperatively. We therefore recommend long-term surveillance of thyroid function for patients following lobectomy.
Author Contributions
Charles Meyer, concept, research, writing, revision, submission; Danielle Anderson, concept, research, writing, revision; Zhiqiao Dong, research, writing, revision; Jeanelle Braxton Riddick, concept, research, revision; Marilisa Elrod, concept, research, writing, revision; Marco Ayala, concept, research, writing, revision.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
This article was presented at the AAO-HNSF 2020 Virtual Annual Meeting & OTO Experience; September 13–October 25, 2020.
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US government. We are military service members. This work was prepared as part of our official duties. Title 17 USC 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 USC 101 defines US government work as work prepared by a military service member or employee of the US government as part of that person’s official duties.
References
- 1. Lang BH, Wong CKH, Wong KP, Chu KK, Shek TWH. Effect of thyroid remnant volume on the risk of hypothyroidism after hemithyroidectomy: a prospective study. Ann Surg Oncol. 2017;24(6):1525-1532. [DOI] [PubMed] [Google Scholar]
- 2. Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP. Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec. 2013;75(1):6-17. [DOI] [PubMed] [Google Scholar]
- 3. Park S, Jeon MJ, Song E, et al. Clinical features of early and late postoperative hypothyroidism after lobectomy. J Clin Endocrinol Metab. 2017;102(4):1317-1324. [DOI] [PubMed] [Google Scholar]
- 4. Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21(12):3844-3852. [DOI] [PubMed] [Google Scholar]
- 5. Chong SS, Hoh SY, Huang SM. Post-hemithyroidectomy hypothyroidism in non autoimmune thyroiditis patients: incidence, risk factors and duration of follow up. Asian J Surg. 2019;42(11):957-962. [DOI] [PubMed] [Google Scholar]
- 6. Lee DY, Seok J, Jeong WJ, Ahn SH. Prediction of thyroid hormone supplementation after thyroid lobectomy. J Surg Res. 2015;193(1):273-278. [DOI] [PubMed] [Google Scholar]
- 7. McHenry CR, Slusarczyk SJ. Hypothyroidisim following hemithyroidectomy: incidence, risk factors, and management. Surgery. 2000;128(6):994-998. [DOI] [PubMed] [Google Scholar]
- 8. Su SY, Grodski S, Serpell JW. Hypothyroidism following hemithyroidectomy: a retrospective review. Ann Surg. 2009;250(6):991-994. [DOI] [PubMed] [Google Scholar]
- 9. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235. [DOI] [PubMed] [Google Scholar]
- 11. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243-2255. [DOI] [PubMed] [Google Scholar]
- 12. Miller FR, Paulson D, Prihoda TJ, Otto RA. Risk factors for the development of hypothyroidism after hemithyroidectomy. Arch Otolaryngol Head Neck Surg. 2006;132:36-38. [DOI] [PubMed] [Google Scholar]
- 13. Stoll SJ, Pitt SC, Liu J, Schaefer S, Sippel RS, Chen H. Thyroid hormone replacement after thyroid lobectomy. Surgery. 2009;146(4):554-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu KK, Lang BH. Clinicopathologic predictors for early and late biochemical hypothyroidism after hemithyroidectomy. Am J Surg. 2012;203(4):461-466. [DOI] [PubMed] [Google Scholar]
- 15. Malago R, D’Onofrio M, Ferdeghini M, et al. Thyroid volumetric quantification: comparative evaluation between conventional and volumetric ultrasoundography. J Ultrasound Med. 2008;27:1727-1733. [DOI] [PubMed] [Google Scholar]
- 16. Farkas E, King T, Bolton J, Fuhrman G. Comparison of total thyroidectomy and lobectomy in the treatment of dominant thyroid nodules. Am Surg. 2002;68:678-683. [PubMed] [Google Scholar]


