Abstract
Background:
Posterior cruciate ligament (PCL) reconstruction is commonly performed to restore joint stability and prevent posterior tibial translation at higher flexion angles. However, persistent knee laxity after reconstruction is often reported.
Purpose:
To biomechanically evaluate the effect of independent suture tape (ST) reinforcement on different PCL reconstruction techniques.
Study Design:
Controlled laboratory study.
Methods:
PCL reconstruction using porcine bones and quadrupled bovine tendons was performed using 2 techniques: (1) an all-inside method using suspensory adjustable loop devices (ALDs) in the tibia and femur and (2) a method using an interference screw on the tibial and an ALD on the femoral site. Both were tested with and without an additional ST for 4 groups (n = 8 per group). Each construct underwent biomechanical testing involving 3000 loading cycles in 3 stages. After position-controlled cycles simulating full range of motion, force-controlled loading from 10 to 250 N and then from 10 to 500 N were performed before pull-to-failure testing. Elongation, stiffness, and ultimate strength were evaluated.
Results:
The highest ultimate load (1505 ± 87 N), a small total elongation (2.60 ± 0.97 mm), and stiffness closest to the native human ligament (156.3 ± 16.1 compared with 198.9 ± 33.5 N/mm; P = .192) was seen in the all-inside technique using ST. Intragroup comparison revealed that reinforcement with ST produced a smaller total elongation for the screw fixation (Screw-ALD, 6.06 ± 3.60 vs Screw-ALD ST, 2.50 ± 1.28 mm; P = .018) and all-inside techniques (ALD-ALD, 4.77 ± 1.43 vs ALD-ALD ST, 2.60 ± 0.97 mm; P = .077), albeit the latter was not significantly different. Elongation for constructs without ST increased more rapidly at higher loads compared with elongation for ST constructs. The ultimate strength was significantly increased only for constructs using the all-inside technique using ST (ALD-ALD, 1167 ± 125 vs ALD-ALD ST, 1505 ± 87 N; P = .010).
Conclusion:
Adding an independent ST to PCL reconstruction led to improvement in the studied metrics by reducing the total elongation and increasing the ultimate strength, independent of the technique used.
Clinical Relevance:
PCL reconstruction using additional ST reinforcement was biomechanically favorable in this study. ST reinforcement in the clinical setting could decrease knee laxity after PCL reconstruction, providing better joint stability and improved functional outcomes.
Keywords: PCL reconstruction, suture tape reinforcement, biomechanical testing, cyclic loading
The posterior cruciate ligament (PCL), as the strongest ligament in the knee, plays an important role in maintaining normal knee kinematics. A rupture of the PCL is a rare injury and commonly arises in combination with additional ligamentous and/or knee injuries.44,45 A better understanding of the biomechanics of the ligament has recently led to several advances in the treatment of the PCL-deficient knee.18,29 However, the optimal treatment remains controversial. Given the self-healing capacity of the injured PCL,41 an acute isolated grade I or II PCL rupture can be treated nonoperatively.3,40,52 However, there is a risk that the ligament may heal in a lax position.4,40,41 Patients with failed conservative treatment, multiligament injuries, or severe symptoms usually require surgical treatment.10,32
Surgical techniques to reconstruct the PCL include the tibial inlay technique31 aiming to avoid the so-called killer turn, as well as single-bundle—restoring only the stronger anterolateral (AL) bundle—and double-bundle PCL reconstruction.9,47 Both autografts and allografts are regularly utilized in PCL reconstruction. Hamstring tendons are commonly used as autografts, followed by bone-patellar tendon-bone and quadriceps tendons.21 Reasons for the use of autografts include legal requirements, better availability, and reduced costs.22 In contrast, the use of allografts prevents additional donor-site morbidity and provides grafts with a sufficient length and diameter. However, the use of allografts involves a risk of a rejection reaction1 or disease transmission.13,16
Tibial and femoral graft fixation in PCL reconstruction surgery can be realized using interference screws to fixate the graft in full bone tunnels. Alternatively, all-inside PCL reconstruction using only bone sockets instead of full tunnels and suspensory fixation devices can be utilized.17 With this novel technique, bone material is conserved, and the risk of tunnel convergence related to the treatment of multiligament surgery can be reduced. Moreover, the utilization of adjustable loop devices (ALDs) for suspensory fixation allows for adjustable cortical fixation and graft preconditioning by applying tension to the graft. In comparison with the all-inside technique, PCL reconstruction using screw fixation shows a decreased ultimate strength, as the screw is more aligned with the load axis. However, the graft may show more elongation with all-inside PCL reconstruction because of a larger free graft portion.1,17,24,42,50
Even though clinical studies related to single-bundle PCL reconstruction have demonstrated satisfactory outcome data, pain and knee instability due to abnormal laxity after surgery are often still reported.12,20,23,27,51 Reasons for remaining knee laxity after surgery might be the healing of the ligament with a subluxated tibial plateau and therefore lax position.49 However, excessive tensioning and malpositioning of the graft can also lead to restrictions in knee movement.5,43,54 Consequences of PCL insufficiency may include cartilage degeneration and an increased risk of osteoarthritis.19,20,48
Previous biomechanical as well as clinical studies have revealed that an additional suture tape (ST) reinforcement in anterior cruciate ligament (ACL) reconstruction was biomechanically associated with a smaller elongation as well as higher ultimate strength and clinically led to improved patient-reported outcome measures and less pain.6,7,35 For PCL injuries treated using reconstruction and/or an additional ST, promising results in a clinical setting were also recently reported.39
Therefore, the aim of this biomechanical full-construct study was to compare different single-bundle PCL reconstruction techniques with and without an additional ST reinforcement to evaluate the influence of the reinforcement on studied metrics. We hypothesized that the ST would decrease elongation during cyclic loading while increasing the ultimate strength of the construct when compared with constructs without additional ST.
Methods
PCL Reconstruction With and Without Suture Tape Reinforcement
Full-construct PCL reconstruction using porcine bones and quadrupled bovine tendons was performed utilizing 2 different techniques: first, an all-inside technique using TightRope (Arthrex) as suspensory ALDs on both the femoral and tibial site (ALD-ALD group); and second, a screw fixation technique using a 11 x 28 mm biocomposite interference screw (Arthrex) on the tibial and an ALD on the femoral site (Screw-ALD group). Both configurations were biomechanically tested with an additional 2 mm–wide, high-strength braided polyblend ST (FiberTape; Arthrex) (ALD-ALD ST and Screw-ALD ST groups) and without (ALD-ALD and Screw-ALD groups) an independent ST reinforcement. The ST was threaded through the femoral ALD button allowing for independent fixation of the tape. A sample size of 8 was tested in each testing group. Subsequent power analysis revealed a mean power >0.8, leading us to conclude that our sample size was sufficient.
Specimen Preparation
Porcine knees and bovine hind limbs from freshly slaughtered animals (pigs and cattle at the age of 8 months and 2 years, respectively) were acquired from a local slaughterhouse. Animal material is more reproducible and is often used as a substitute in cruciate ligament testing.6,14 Furthermore, previous literature has also demonstrated that porcine tibias best resemble human bones that commonly sustain sports injuries.2,33
Porcine tibias and femurs were dissected by carefully removing the soft tissue from the bone. For femurs, the lateral condyle was sawed off, allowing an enhanced view on the reconstruction during biomechanical testing. Both the tibias and femurs were then embedded using a bicomponent polyurethane system (RenCast; Huntsman Advanced Materials).
Bovine digital extensor tendons were shown to have similar material properties when compared with human hamstring tendons.15 The Y-shaped bovine extensor digitorum longus tendons were harvested from hind limbs and then cut in half. Subsequently, the grafts were adjusted to a length of 360 mm and, if necessary, cautiously trimmed in fiber direction to a diameter of 10 mm when quadrupled.
Graft Preparation
All-Inside Constructs
For all-inside constructs (ALD-ALD and ALD-ALD ST groups), bovine tendons were placed on a graft preparation station. The tendon was passed through a TightRope RT (Arthrex) for femoral fixation and a no-button TightRope TN (Arthrex) for tibial fixation, leaving 1 longer and 1 shorter-end. The long tendon end was again passed through both devices until the tendon ends met to create a quadrupled graft construct. Graft ends were overlapped for approximately 5 mm and then U-stitched together using a No. 0 FiberWire (Arthrex), forming a continuous loop. Before the first linked stitch, the graft construct was exposed to an initial tension of 20 N. The graft construct was completed using 3 further linked stitches under a tension of 80 N. The preparation of the all-inside grafts without additional ST is illustrated in Figure 1A. The additional ST for the ALD-ALD ST group was threaded through the femoral button, allowing for independent fixation of the ST. The final quadrupled graft construct measured 90 mm in length and 10 mm in diameter, representing a standard allograft in PCL reconstruction.1,24 The final construct is illustrated in Figure 2.
Figure 1.
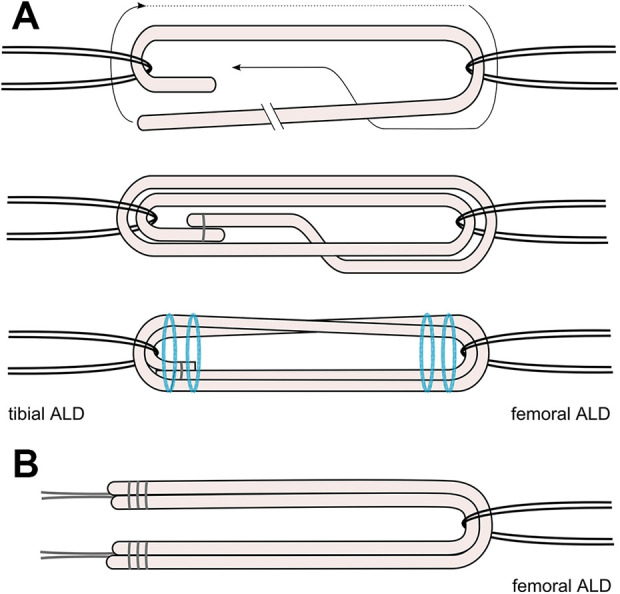
(A) Preparation and final construct of all-inside quadrupled grafts (ALD-ALD and ALD-ALD ST groups) without suture tape (ST). (B) Final construct of screw fixation constructs (Screw-ALD and Screw-ALD ST groups) without ST. ALD, adjustable loop device.
Figure 2.
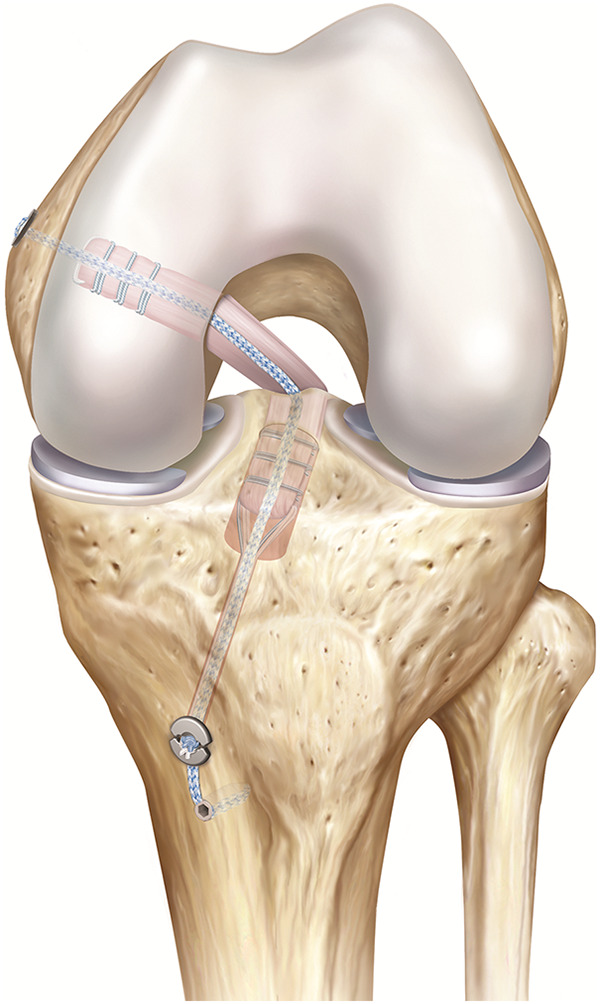
Illustration of final all-inside posterior cruciate ligament reconstruction with additional suture tape.
Screw Fixation Constructs
For the screw fixation constructs (Screw-ALD and Screw-ALD ST groups), 2 tendons measuring 210 mm in length and 10 mm in diameter when quadrupled were used for graft preparation. Subsequently, the tendons were threaded through a TightRope RT for femoral fixation. The 2 tendons were overlapped and whipstitched together at both ends. Last, the whipstitching sutures were knotted, allowing the strands to be equally tensioned. The final screw fixation construct without additional ST can be seen in Figure 1B. The additional ST for the Screw-ALD ST group was threaded through the femoral button, allowing for independent fixation of the ST. The final quadrupled graft construct measured around 105 mm in length and 10 mm in diameter.
Tunnel Preparation
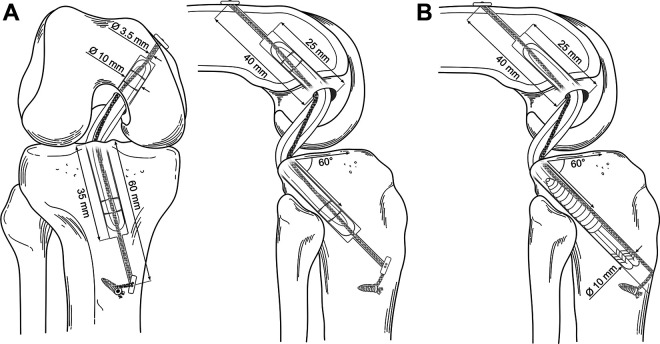
For the all-inside constructs (ALD-ALD and ALD-ALD ST groups), a tunnel measuring 60 mm in length and 3.5 mm in diameter was drilled from the lateral side of the tibia to the posterior attachment site of the PCL. The tunnel was placed at an angle of 60° relative to the tibial plateau. A tibial socket was retrodrilled using a flip cutter (Arthrex) and measured 10 mm in diameter and 35 mm in depth.
For the screw fixation constructs (Screw-ALD and Screw-ALD ST groups), the tibial bone tunnel measured 10 mm in diameter during the entire tunnel length.
The femoral tunnel was similar for all testing groups: the tunnel had a total length of 40 mm and a diameter of 3.5 mm. The retrodrilled femoral socket also measured 10 mm in diameter and 25 mm in depth. When the grafts were inserted into the bone tunnel, it was ensured that a space of 5 mm in the femoral and tibial socket was left, allowing the graft to be fully tensioned.
Measurements of the all-inside and screw fixation constructs can be seen in Figure 3.
Figure 3.
Illustration and measurements for all-inside constructs (A) and screw fixation construct (B) with suture tape reinforcement. Joint space was set at 40 mm.
Testing Protocol
The testing protocol included both a position- and load-controlled cyclic loading block, similar to that described in present biomechanical PCL and ACL studies.6,25,46 Position-controlled cyclic loading simulated early rehabilitation using passive range-of-motion (ROM) exercises41 and was based on the AL bundle length-knee flexion angle relationship. As this study focused on single-bundle reconstruction, which is designed to replace the stronger AL bundle, only the AL bundle was considered. According to Li et al,26 the AL bundle is at its maximum length at 90° of knee flexion and experiences a length decrease of –0.5 and 8.5 mm when the knee is flexed to 75° and 0°, respectively.
Position-controlled cyclic loading simulated full ROM from +0.5 mm (90° of knee flexion) to –8 mm (full extension). Load-controlled cyclic loading involved cyclic loading from 10 to 250 N and 500 N, respectively.
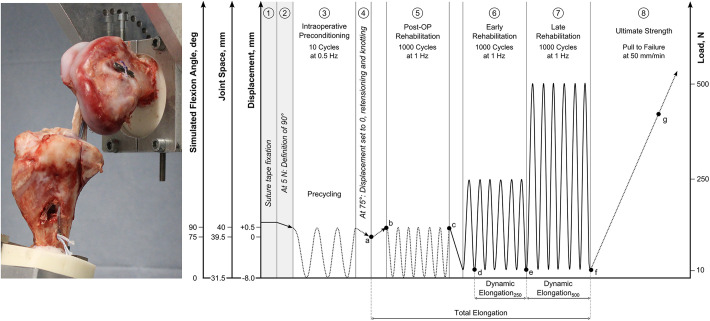
The full-construct testing setup as well as the biomechanical testing protocol including working steps 1 to 8 is illustrated in Figure 4.
Figure 4.
(Left) Full-construct test setup. (Right) Testing protocol including points of data evaluation: force loss during position-controlled cycling (Δde), dynamic elongation250 (Δde), dynamic elongation500 (Δdf), total elongation (Δbc), and stiffness during pull to failure (e). Dotted lines represent position-controlled loading; solid lines represent load-controlled loading. Post-OP, postoperative.
Biomechanical Testing
Custom-made testing fixtures for the femur and tibia allowing for an inclined bone fixation to position the PCL aligned with the load axis were fixated to the actuator and the base plate of a tensile testing machine (ElectroPuls 10000; Instron). The femur and tibia were clamped in an anatomic position at a knee flexion angle of 90°, as this presents the worst-case scenario for PCL testing.11,28
Before testing, each graft was placed into a graft tube and preconditioned for 5 minutes at 80 N.
Graft Insertion of All-Inside Constructs
For PCL reconstructions with ST reinforcement (ALD-ALD ST group), the initial intra-articular distance between both bone tunnel exits was set to 40 mm. The graft and the ST were inserted into the bone tunnels without tensioning the graft. In step 1, the ST was fixated in the tibia using a 4.75-mm knotless PEEK (polyether ether ketone) anchor (Arthrex). Subsequently, the machine was moved slightly until 5 N was reached (step 2). That position was then defined as a simulated knee flexion angle of 90°, ensuring that the ST was equally loaded at 90° for all constructs.
For PCL reconstructions without ST reinforcement (ALD-ALD group), steps 1 and 2 were not performed because no ST was involved. The initial distance was set to 40 mm, which directly corresponds to a simulated knee flexion angle of 90°.
The femoral ALD was shortened until the graft was pulled into the femoral socket for a distance of 20 mm, allowing enough space for retensioning. The graft in the ALD-ALD and ALD-ALD ST groups was then slightly tensioned on the tibial site until a load of 50 N was reached and then knotted. This load level for the initial tensioning was chosen because during pretests, it was observed that moderate manually tensioning resulted in approximately 50 N.
Graft Insertion of Screw Fixation Constructs
For PCL reconstructions with ST reinforcement (Screw-ALD ST group), the initial intra-articular distance was set to 40 mm. The graft and the ST were inserted into the bone tunnels without tensioning the graft. In step 1, the ST was fixated in the tibia using a knotless anchor. Subsequently, the machine was slightly moved until 5 N was reached (step 2). That position was then defined as a simulated knee flexion angle of 90°.
For PCL reconstructions without ST reinforcement (Screw-ALD group), steps 1 and 2 were not performed. The initial distance was set to 40 mm, which directly corresponds to a simulated knee flexion angle of 90°.
For the Screw-ALD and Screw-ALD ST groups, a weight of 50 N was suspended from the whipstitching sutures, and an interference screw was inserted for tibial fixation. To ensure equal starting conditions for all screw fixation constructs, the machine’s displacement was then changed until 50 N was reached.
Preconditioning and Cyclic Loading
Subsequent to graft insertion and tibial fixation of the constructs, testing was started with 10 precycles between +0.5 and −8.0 mm of displacement, respectively (simulating ROM from 90° to 0° of knee flexion) at a frequency of 0.5 Hz (step 3). In step 4, the machine moved to a displacement of 0 mm, corresponding to a simulated knee flexion angle of 75°, and the digital displacement was set to zero. At this position, the graft was retensioned on the femoral site to approximately 200 N, and knots were tied on the femoral ALD. Then, position-controlled cyclic loading started between +0.5 and –8.0 mm of displacement (simulating ROM from 90° to 0° of knee flexion) for 1000 cycles at 1 Hz (step 5). Upon completion, load-controlled cyclic loading from 10 to 250 N and 500 N, respectively, simulating a progressive rehabilitation protocol (steps 6 and 7), was performed before a pull to failure at 50 mm/min (step 8) at a knee flexion angle of 90°.
Data were recorded at an acquisition rate of 500 Hz, and the mode of failure was noted. All specimens were constantly kept moist using a physiologic saline solution during preparation and testing.
Outcome Data
Outcome measures included percentage force loss during position-controlled cycling (Δde in Figure 4), dynamic and total elongation, and stiffness and ultimate load during pull to failure. Points of data evaluation are also given in Figure 4. The elongation experienced during load-controlled cyclic loading was the dynamic elongation (Δbc and Δbd, respectively, in Figure 4), and the total elongation was the elongation occurring from the retensioning of the graft until the last load-controlled cycle was completed (Δad in Figure 4). Stiffness was measured within a linear portion of the pull-to-failure curve (e in Figure 4). Ultimate failure load was the absolute maximum during the pull-to-failure testing.
Statistical Analysis
Statistical analysis was performed using JMP 14 (SAS Institute, Inc), and statistical methods to evaluate differences among the 4 different groups included a 1-way analysis of variance before Tukey post hoc test for pairwise analysis. Differences in tension before and after screw insertion for the Screw-ALD and Screw-ALD ST groups were investigated using a paired t test and a Brown-Forsythe test for the equality of group variances. The significance level was set at P < .05 and the desired power at 0.8.
Properties of the Native Human PCL
To compare the properties of the PCL reconstruction to those of the native human ligament, a pilot test was conducted using human cadaveric knees (Science Care, Inc). For this purpose, 10 fresh-frozen cadaveric knees (7 men, 3 women) originating from donors with a mean age of 55 ± 12 years were biomechanically tested.
Specimen Preparation
All soft tissue, with the exception of the PCL, was removed. Subsequently, the lateral femoral condyle was carefully sawed off using an orthopedic saw to enable a sufficient view of the ligament. Both the tibia and the femur were embedded in a bicomponent embedding material (RenCast; Huntsman Advances Materials).
Biomechanical Testing
A tensile testing machine (ElectroPuls E10000; Instron) with a 10-kN load cell installed to the crosshead was used for biomechanical testing. Custom-made testing fixtures were secured to the actuator and to an X-Y table mounted to the base plate, respectively. The embedded bones were fixated within cup holders at a knee flexion angle of 90°. This flexion angle constitutes the most critical angle for the PCL, as it is maximally loaded in that position,11,28 and was therefore chosen for biomechanical testing.
A preload of 30 N was applied, and subsequently, the machine’s displacement was set to zero. Afterward, 10 precycles between 0 and 2 mm at 0.5 Hz, reducing settling effects, were performed before a pull to failure at 200 mm/min.53 Data were recorded using Wavematrix software (Instron) at an acquisition rate of 1000 Hz. Load-displacement curves were evaluated to determine the PCL function zone of the native ligament as well as the stiffness during pull-to-failure and ligament measurements. All specimens were constantly kept moist using a physiologic saline solution during preparation and testing.
Results
PCL Reconstruction With and Without Suture Tape Reinforcement
Biomechanical testing results of PCL reconstruction with and without ST reinforcement are listed in Table 1, and P values for pairwise comparison after Tukey post hoc tests are shown in Table 2.
TABLE 1.
Biomechanical Testing Results of PCL Reconstruction With and Without ST Reinforcementa
| ALD-ALD (n = 8) | ALD-ALD ST (n = 8) | Screw-ALD (n = 8) | Screw-ALD ST (n = 8) | |
|---|---|---|---|---|
| Cyclic loading | ||||
| Force loss, % | 17.8 ± 5.3 | 10.1 ± 3.2 | 16.4 ± 3.5 | 13.1 ± 2.9 |
| Dynamic elongation250 N, mm | 2.28 ± 0.25 | 1.81 ± 0.27 | 1.67 ± 0.18 | 1.62 ± 0.22 |
| Dynamic elongation500 N, mm | 4.46 ± 1.16 | 2.78 ± 0.66 | 6.21 ± 3.54 | 3.06 ± 1.23 |
| Total elongation, mm | 4.77 ± 1.43 | 2.60 ± 0.97 | 6.06 ± 3.60 | 2.50 ± 1.28 |
| Pull to failure | ||||
| Stiffness, N/mm | 136.1 ± 7.2 | 156.3 ± 16.1 | 142.8 ± 15.3 | 151.0 ± 9.8 |
| Ultimate failure load, N | 1167 ± 125 | 1505 ± 87 | 1014 ± 194 | 1184 ± 276 |
| Method of failure (%) | ALD failure tibial (62.5), ALD + bone failure tibial (12.5), ALD failure femoral (12.5), ALD failure tibial + femoral (12.5) | ALD failure tibial (50), ALD failure femoral (25), bone failure femoral (12.5), bone failure tibial + femoral (12.5) | Graft slippage (62.5), ALD failure femoral (37.5) | Graft + ST slippage (75), Graft + ST slippage + bone failure femoral (12.5), Graft + ST slippage + ALD failure femoral (12.5) |
aValues are presented as mean ± SD unless otherwise noted. ALD, adjustable loop device; PCL, posterior cruciate ligament; ST, suture tape.
TABLE 2.
P Values for Tukey Post Hoc Analysisa
| ALD-ALD | ALD-ALD ST | Screw-ALD | Screw-ALD ST | |
|---|---|---|---|---|
| Position and load-controlled block | ||||
| Force loss | ||||
| ALD-ALD | — | .004 | .901 | .119 |
| ALD-ALD ST | .004 | — | .022 | .476 |
| Screw-ALD | .901 | .022 | — | .380 |
| Screw-ALD ST | .119 | .476 | .380 | — |
| Dynamic elongation250 N | ||||
| ALD-ALD | — | .004 | <.001 | <.001 |
| ALD-ALD ST | .004 | — | .443 | .320 |
| Screw-ALD | <.001 | .443 | — | .667 |
| Screw-ALD ST | <.001 | .320 | .667 | — |
| Dynamic elongation500 N | ||||
| ALD-ALD | — | .127 | .816 | .151 |
| ALD-ALD ST | .127 | — | .012 | >.999 |
| Screw-ALD | .816 | .012 | — | .015 |
| Screw-ALD ST | .151 | >.999 | .015 | — |
| Total elongation | ||||
| ALD-ALD | — | .077 | .982 | .051 |
| ALD-ALD ST | .077 | — | .029 | .999 |
| Screw-ALD | .982 | .029 | — | .018 |
| Screw-ALD ST | .051 | .999 | .018 | — |
| Pull to failure | ||||
| Stiffness | ||||
| ALD-ALD | — | .250 | >.999 | .988 |
| ALD-ALD ST | .250 | — | >.999 | >.999 |
| Screw-ALD | >.999 | >.999 | — | >.999 |
| Screw-ALD ST | .988 | >.999 | >.999 | — |
| Native PCL | <.001 | .192 | <.001 | .030 |
| Ultimate failure load | ||||
| ALD-ALD | — | .010 | .250 | .868 |
| ALD-ALD ST | .010 | — | <.001 | .012 |
| Screw-ALD | .250 | <.001 | — | .267 |
| Screw-ALD ST | .868 | .012 | .267 | — |
aP values in bold (P < .05) indicate statistical significance. Dashes indicate that no statistical test was performed within the same testing group. ALD, adjustable loop device; PCL, posterior cruciate ligament; ST, suture tape.
Position and Load-Controlled Block
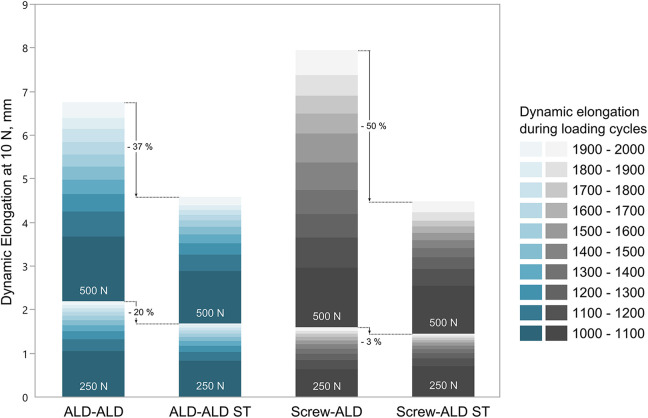
The least force loss during the position-controlled block was revealed for the ALD-ALD ST group (10.1% ± 3.2%), which was significantly less than for the ALD-ALD group (17.8% ± 5.3%) (P = .004). With an additional ST, total elongation was decreased by around 45%, from 4.77 ± 1.43 for the ALD-ALD group to 2.60 ± 0.97 mm for the ALD-ALD ST group, albeit this difference was not statistically significant (P = .077). For the Screw-ALD group, a statistically significant decrease in total elongation (P = .018) of approximately 58% from 6.06 ± 3.60 mm to 2.50 ± 1.28 mm with an additional ST was observed (Figure 5).
Figure 5.
Dynamic elongation measured during the 250- and 500-N block for every 100 cycles. ALD, adjustable loop device; ST, suture tape.
The greatest dynamic elongation during the 250-N and 500-N load blocks was revealed for the ALD-ALD (2.28 ± 0.25 mm) and the Screw-ALD (6.21 ± 3.54 mm) groups, respectively. Regarding intragroup comparison of the ALD-ALD and ALD-ALD ST constructs, the addition of the ST led to a decrease in dynamic elongation of 20% during the 250-N load block, which was statistically significant (P = .004). During the 500-N load block, a decrease of 37% was observed, albeit this decrease was not statistically significant. The Screw-ALD ST group showed 3% (P = .667) and 50% (P = .015) less dynamic elongation during the 250- and 500-N load blocks, respectively, compared with the Screw-ALD group.
Considering the intragroup comparison during the 500-N load block, the dynamic elongation was greater for groups without additional ST compared with groups with ST (P = .127 for ALD-ALD vs ALD-ALD ST; P = .015 for Screw-ALD vs Screw-ALD ST). Moreover, it was observed that the amount of elongation occurring within the cycling was constantly decreasing with continuous loading cycles during 250 N (Figure 6). When considering the amount of elongation occurring during the 500-N block, the elongation in the groups with additional ST (ALD-ALD ST and Screw-ALD ST) steadily decreased with proceeding cycles, whereas the elongation in the groups without ST (ALD-ALD and Screw-ALD) steadily increased.
Figure 6.
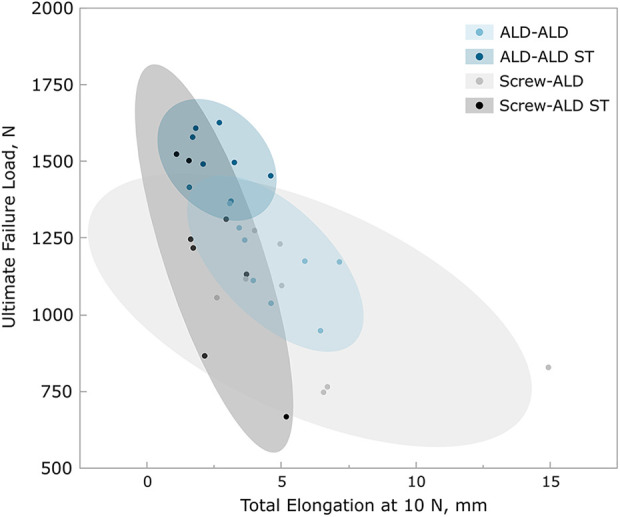
Ultimate failure load versus total elongation including 95% CI (ellipse). ALD, adjustable loop device; ST, suture tape.
Pull-to-Failure Testing
Stiffness values of all groups tested as well as the stiffness range of the native human PCL determined in pretests are illustrated in Figure 7. The ALD-ALD ST group had mean stiffness closest to that of the native human ligament (156.3 ± 16.1 vs 198.9 ± 33.5 N/mm; P = .192). Stiffness in the groups without additional ST (ALD-ALD and Screw-ALD groups) was significantly lower than that of the native ligament (P < .001).
Figure 7.
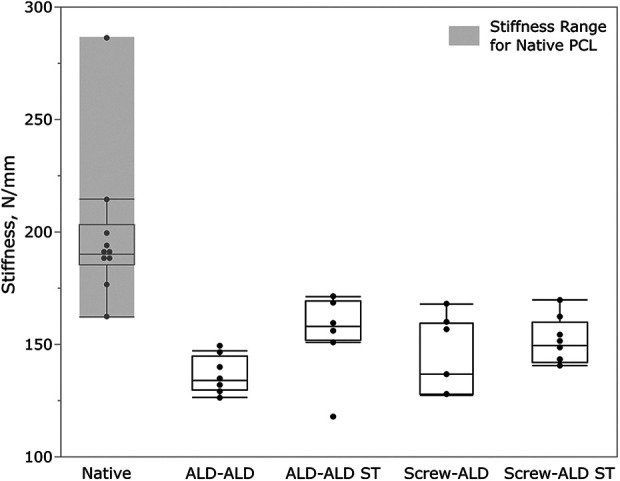
Stiffness during pull to failure of all groups tested compared with stiffness of the native human PCL. Box plot demonstrating individual data points (dots) along with mean value (horizontal bar) within the interquartile range. ALD, adjustable loop device; PCL, posterior cruciate ligament; ST, suture tape.
The ALD-ALD ST group also demonstrated the highest ultimate failure load (1505 ± 87 N), which was statistically significantly different from that of all other groups (P = .010 vs ALD-ALD; P < .001 vs Screw-ALD; and P = .012 vs Screw-ALD ST). Moreover, the ALD-ALD ST group revealed the least variation regarding ultimate failure load (Figure 5).
Whereas the ALD-ALD and ALD-ALD ST groups failed due to suture rupture of the ALD (tibial and/or femoral) during pull-to-failure testing, failure in the Screw-ALD and Screw-ALD ST groups was mainly due to graft slippage.
Properties of the Native Human PCL
The measurements and biomechanical testing results of the native human PCL are listed in Table 3. Assuming that the PCL has an elliptical shape, the cross-sectional area was calculated for the tested human ligaments and was found to be a mean of 88.5 ± 27.8 mm2. The final bovine graft utilized in this study had a diameter of 10 mm corresponding to a cross-sectional area of 78.5 mm2.
TABLE 3.
Measurements and Biomechanical Testing Results of Native Human PCLa
| Diameter, mm | ||||
|---|---|---|---|---|
| Stiffness, N/mm | Mediolateral | Anteroposterior | CSA, mm2 | |
| Native human PCL | 198.9 ± 33.5 | 12.4 ± 2.0 | 8.6 ± 2.0 | 88.5 ± 27.8 |
aData are reported as mean ± SD. CSA, cross-sectional area; PCL, posterior cruciate ligament.
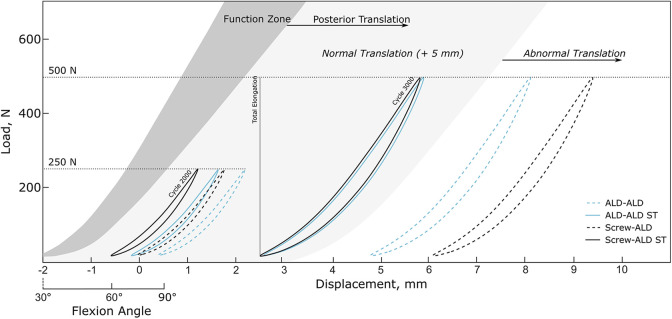
The load-displacement curves from the pull-to-failure tests of the native PCLs were used to create a native PCL reference model. Previous literature has reported a correlation of knee flexion angle and AL bundle length and tension, respectively.6,11,26 With regard to these data, a PCL Function Zone representing the in vivo behavior of the native ligament could have been established to help quantify and qualify the stabilization potential of the PCL reconstruction techniques tested. Given that a posterior translation after PCL reconstruction surgery of 5 mm or more is considered to be abnormal,37 a range of normal posterior translation was included (Figure 8).
Figure 8.
Function zone of the native human posterior cruciate ligament (dark gray) with ranges of normal posterior translation and abnormal posterior translation. Hysteresis curves at the end of the 250-N (cycle 2000) and of the 500-N load block (cycle 3000) of testing groups are additionally plotted. ALD, adjustable loop device; ST, suture tape.
Discussion
This full-construct biomechanical study included a testing methodology with intraoperative preconditioning simulating a loading scenario acting on the PCL during the rehabilitation phase. In general, it was revealed that adding an independent ST to a PCL reconstruction results in improved biomechanical metrics by decreasing the dynamic as well as total elongation while increasing the ultimate failure load, independent of the utilized fixation technique. The ALD-ALD ST group reported the highest ultimate failure load, and stiffness values closest to the native PCL. Additionally, this group presented the smallest total elongations.
During preconditioning including precycles and retensioning to 200 N, a high initial force level could have been created due to the use of an ALD. It was shown that this high initial force level might prevent knee laxity subsequent to surgical reconstruction30,36; however, extensive graft tensioning can cause abnormal articulation, causing cartilage or graft damage.5,54 The ALD-ALD group was the least able to maintain the initial force, resulting in the highest percentage of force loss after position-controlled cyclic loading was completed. By adding the ST, the percentage of force loss could have been significantly decreased, allowing for an increased final force level.
It is essential to avoid graft stretching and construct lengthening to protect the healing PCL before graft incorporation.30 Abnormal posterior tibial translation can otherwise result in an unstable knee joint. Considering the overall differences, the dynamic elongation measured during the 250 N was significantly the highest for the ALD-ALD group without additional ST reinforcement. Given the different graft fixation method, the grafts utilizing the all-inside technique have a longer free graft portion that might reveal a greater dynamic elongation. Because the dynamic elongation was significantly reduced by adding the ST, it can be assumed that the additional reinforcement already strengthens the construct even at these low loads. In contrast, the additional ST did not affect the dynamic elongation for screw fixation groups at 250 N. However, with increasing loads and proceeding cyclic loading, intragroup differences become more apparent. Given that the dynamic elongation during 500-N cyclic loading was the highest among the Screw-ALD group, additional graft slippage due to the fixation technique might also cause additional elongation. Dynamic elongation could have been decreased by 37% and 50% for all-inside and screw fixation groups, respectively. Therefore, it can be concluded that the ST appears to be like a “safety belt”, which becomes more dominant when the graft is exposed to higher loads where it demonstrates more plastic deformation. This principle was also stated in previous biomechanical ACL studies.6
When qualifying the PCL reconstruction results with the native PCL function zone, final cycle hysteresis curves of testing groups without ST were within the range of abnormal posterior translation. Total elongation exceeded the critical threshold of clinical failure of 5 mm37 for 4 (50%) and 3 samples (37.5%) in the Screw-ALD and ALD-ALD groups, respectively, indicating that PCL reconstruction without additional ST reinforcement is not able to sufficiently withstand plastic deformation and/or graft slippage at high loads. Moreover, it was observed that the increase of dynamic elongation remained constant with proceeding loading cycle during the 500-N load block whereas the dynamic elongation steadily decreased (steady state) with proceeding cycles for groups with additional ST reinforcement. Given that our testing protocol reflects only a small part of the rehabilitation phase after surgical PCL reconstruction, it can be assumed that the elongation would further increase with additional cyclic loading.
PCL reconstruction aims to restore knee function and provide joint stability, reducing knee laxity. Construct stiffness plays an essential role, as an overconstrained knee can lead to limitations regarding ROM and may cause graft or fixation failure.8,34 In contrast, a construct stiffness that is too low may result in an unstable knee joint. Stiffness measured during pull to failure was lower than stiffness of the native human PCL for all groups tested. However, the ALD-ALD ST construct revealed the greatest stiffness among all other groups that seem to best replicate the native properties of the ligament. Also, the ultimate failure load was the greatest for this group. In general, tibial suspensory fixation is superior regarding ultimate strength because the interference screw fixation is almost aligned with the force axis, causing the graft to slip out of the tibial bone tunnel. A high ultimate strength might be beneficial when it comes to peak loads during rehabilitation, which may cause construct deformation/slippage or even a rerupture.37 In general, constructs with additional ST were able to withstand higher loads until failure when compared with constructs without ST.
Limitations
This biomechanical study had some inherent limitations. Animal material was used to create a full construct biomechanical test setup. Bovine tendons have been shown to have similar mechanical properties compared with human tendons,15 and porcine tibia is commonly used as a substitute for human bone, although some authors have discouraged the use of porcine tibias due to noteworthy differences in mechanical properties.38 However, using animal instead of human material allows for better comparability and reproducibility because of more consistent mechanical properties.
Moreover, this study was an in vitro time-zero biomechanical study not accounting for in vivo factors, such as biological healing, which also may affect construct lengthening. Finally, in vivo factors, such as biological healing, could not be addressed with this type of study; hence, further short- and long-term clinical studies are needed to confirm our biomechanical testing results.
Conclusion
The results of our biomechanical in vitro study indicated that adding an independent ST to a PCL reconstruction, reinforcing the graft construct, resulted in both a decrease of elongation and an increase of the ultimate strength. These improvements in studied metrics were independent of the utilized technique and applied to all-inside technique as well as to tibial screw fixation technique. Therefore, we assume that a ST reinforcement in the clinical setting could decrease knee laxity after PCL reconstruction, providing better joint stability and improved functional outcome. However, clinical data are needed to confirm our assumptions.
Footnotes
Final revision submitted July 7, 2020; accepted August 24, 2020
One or more of the authors has declared the following potential conflict of interest or source of funding: Testing was performed at the Department of Orthopedic Research, Arthrex GmbH, Munich, Germany. C.A.W. and M.P. are employees of Arthrex. B.A.L. has received consulting fees and nonconsulting fees from Arthrex and Smith & Nephew, faculty/speaker fees from Linvatec, and royalties from Arthrex. M.J.S. has received consulting fees, nonconsulting fees, and royalties from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Adler GG. All-inside posterior cruciate ligament reconstruction with a GraftLink. Arthrosc Tech. 2013;2(2):e111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. [DOI] [PubMed] [Google Scholar]
- 3. Agolley D, Gabr A, Benjamin-Laing H, Haddad FS. Successful return to sports in athletes following non-operative management of acute isolated posterior cruciate ligament injuries: medium-term follow-up. Bone Joint J. 2017;99(6):774–778. [DOI] [PubMed] [Google Scholar]
- 4. Ahn S, Lee YS, Song YD, Chang CB, Kang SB, Choi YS. Does surgical reconstruction produce better stability than conservative treatment in the isolated PCL injuries? Arch Orthop Trauma Surg. 2016;136(6):811–819. [DOI] [PubMed] [Google Scholar]
- 5. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6(suppl 1):S2–S12. [DOI] [PubMed] [Google Scholar]
- 6. Bachmaier S, Smith PA, Bley J, Wijdicks CA. Independent suture tape reinforcement of small and standard diameter grafts for anterior cruciate ligament reconstruction: a biomechanical full construct model. Arthroscopy. 2018;34(2):490–499. [DOI] [PubMed] [Google Scholar]
- 7. Bodendorfer BM, Michaelson EM, Shu HT, et al. Suture augmented versus standard anterior cruciate ligament reconstruction: a matched comparative analysis. Arthroscopy. 2019;35(7):2114–2122. [DOI] [PubMed] [Google Scholar]
- 8. Bylski-Austrow DI, Grood ES, Hefzy MS, Holden JP, Butler DL. Anterior cruciate ligament replacements: a mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8(4):522–531. [DOI] [PubMed] [Google Scholar]
- 9. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clancy WG, Jr, Sutherland TB. Combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13(3):629–647. [PubMed] [Google Scholar]
- 11. DeFrate LE, Gill TJ, Li G. In vivo function of the posterior cruciate ligament during weightbearing knee flexion. Am J Sports Med. 2004;32(8):1923–1928. [DOI] [PubMed] [Google Scholar]
- 12. Devitt BM, Dissanayake R, Clair J, et al. Isolated posterior cruciate reconstruction results in improved functional outcome but low rates of return to preinjury level of sport: a systematic review and meta-analysis. Orthop J Sports Med. 2018;6(10):2325967118804478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz-de-Rada P, Barriga A, Barroso JL, Garcia-Barrecheguren E, Alfonso M, Valenti JR. Positive culture in allograft ACL-reconstruction: what to do? Knee Surg Sports Traumatol Arthrosc. 2003;11(4):219–222. [DOI] [PubMed] [Google Scholar]
- 14. Domnick C, Kosters C, Franke F, et al. Biomechanical properties of different fixation techniques for posterior cruciate ligament avulsion fractures. Arthroscopy. 2016;32(6):1065–1071. [DOI] [PubMed] [Google Scholar]
- 15. Donahue TL, Gregersen C, Hull ML, Howell SM. Comparison of viscoelastic, structural, and material properties of double-looped anterior cruciate ligament grafts made from bovine digital extensor and human hamstring tendons. J Biomech Eng. 2001;123(2):162–169. [DOI] [PubMed] [Google Scholar]
- 16. Fowler JR, Truant AL, Sewards JM. The incidence of and clinical approach to positive allograft cultures in anterior cruciate ligament reconstruction. Clin J Sport Med. 2011;21(5):402–404. [DOI] [PubMed] [Google Scholar]
- 17. Freychet B, Desai VS, Sanders TL, et al. All-inside posterior cruciate ligament reconstruction: surgical technique and outcome. Clin Sports Med. 2019;38(2):285–295. [DOI] [PubMed] [Google Scholar]
- 18. Geissler WB, Whipple TL. Intraarticular abnormalities in association with posterior cruciate ligament injuries. Am J Sports Med. 1993;21(6):846–849. [DOI] [PubMed] [Google Scholar]
- 19. Gill TJ, DeFrate LE, Wang C, et al. The biomechanical effect of posterior cruciate ligament reconstruction on knee joint function: kinematic response to simulated muscle loads. Am J Sports Med. 2003;31(4):530–536. [DOI] [PubMed] [Google Scholar]
- 20. Hermans S, Corten K, Bellemans J. Long-term results of isolated anterolateral bundle reconstructions of the posterior cruciate ligament: a 6- to 12-year follow-up study. Am J Sports Med. 2009;37(8):1499–1507. [DOI] [PubMed] [Google Scholar]
- 21. Hudgens JL, Gillette BP, Krych AJ, Stuart MJ, May JH, Levy BA. Allograft versus autograft in posterior cruciate ligament reconstruction: an evidence-based systematic review. J Knee Surg. 2013;26(2):109–115. [DOI] [PubMed] [Google Scholar]
- 22. Jost PW, Dy CJ, Robertson CM, Kelly AM. Allograft use in anterior cruciate ligament reconstruction. HSS J. 2011;7(3):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y-M, Lee CA, Matava MJ. Clinical results of arthroscopic single-bundle transtibial posterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2010;39(2):425–434. [DOI] [PubMed] [Google Scholar]
- 24. King AH, Prince MR, Reardon PJ, Levy BA, Stuart MJ. All-inside posterior cruciate ligament reconstruction. Oper Tech Sports Med. 2015;23(4):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kitamura N, Yasuda K, Yamanaka M, Tohyama H. Biomechanical comparisons of three posterior cruciate ligament reconstruction procedures with load-controlled and displacement-controlled cyclic tests. Am J Sports Med. 2003;31(6):907–914. [DOI] [PubMed] [Google Scholar]
- 26. Li G, DeFrate LE, Sun H, Gill TJ. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am J Sports Med. 2004;32(6):1415–1420. [DOI] [PubMed] [Google Scholar]
- 27. Lien OA, Aas EJ, Johansen S, Ludvigsen TC, Figved W, Engebretsen L. Clinical outcome after reconstruction for isolated posterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1568–1572. [DOI] [PubMed] [Google Scholar]
- 28. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006;22(10):1100–1106. [DOI] [PubMed] [Google Scholar]
- 29. Markolf KL, Slauterbeck JL, Armstrong KL, Shapiro MM, Finerman GA. Effects of combined knee loadings on posterior cruciate ligament force generation. J Orthop Res. 1996;14(4):633–638. [DOI] [PubMed] [Google Scholar]
- 30. Markolf KL, Slauterbeck JR, Armstrong KL, Shapiro MS, Finerman GA. A biomechanical study of replacement of the posterior cruciate ligament with a graft, part 1: isometry, pre-tension of the graft, and anterior-posterior laxity. J Bone Joint Surg Am. 1997;79(3):375–380. [DOI] [PubMed] [Google Scholar]
- 31. McAllister DR, Hussain SM. Tibial inlay posterior cruciate ligament reconstruction: surgical technique and results. Sports Med Arthrosc Rev. 2010;18(4):249–253. [DOI] [PubMed] [Google Scholar]
- 32. Montgomery SR, Johnson JS, McAllister DR, Petrigliano FA. Surgical management of PCL injuries: indications, techniques, and outcomes. Curr Rev Musculoskelet Med. 2013;6(2):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67–71. [DOI] [PubMed] [Google Scholar]
- 34. Nicholas SJ, D’Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP. A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(8):1881–1886. [DOI] [PubMed] [Google Scholar]
- 35. Noonan BC, Bachmaier S, Wijdicks CA, Bedi A. Independent suture tape reinforcement of tripled smaller-diameter and quadrupled grafts for anterior cruciate ligament reconstruction with tibial screw fixation: a biomechanical full construct model. Arthroscopy. 2020;36(2):481–489. [DOI] [PubMed] [Google Scholar]
- 36. Noonan BC, Dines JS, Allen AA, Altchek DW, Bedi A. Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of retensioning and knot tying. Arthroscopy. 2016;32(10):2050–2059. [DOI] [PubMed] [Google Scholar]
- 37. Noyes FR, Barber-Westin SD. Posterior cruciate ligament revision reconstruction, part 1: causes of surgical failure in 52 consecutive operations. Am J Sports Med. 2005;33(5):646–654. [DOI] [PubMed] [Google Scholar]
- 38. Nurmi JT, Sievänen H, Kannus P, Järvinen M, Järvinen TLN. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765–771. [DOI] [PubMed] [Google Scholar]
- 39. Otto A, Helal A, Imhoff FB, et al. Promising clinical and magnetic resonance imaging results after internal bracing of acute posterior cruciate ligament lesions in multiple injured knees. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2543–2550. [DOI] [PubMed] [Google Scholar]
- 40. Pache S, Aman ZS, Kennedy M, et al. Posterior cruciate ligament: current concepts review. Arch Bone Jt Surg. 2018;6(1):8–18. [PMC free article] [PubMed] [Google Scholar]
- 41. Pierce CM, O’Brien L, Griffin LW, LaPrade RF. Posterior cruciate ligament tears: functional and postoperative rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1071–1084. [DOI] [PubMed] [Google Scholar]
- 42. Prince MR, Stuart MJ, King AH, Sousa PL, Levy BA. All-inside posterior cruciate ligament reconstruction: GraftLink technique. Arthrosc Tech. 2015;4(5):e619–e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenthal MD, Rainey CE, Tognoni A, Worms R. Evaluation and management of posterior cruciate ligament injuries. Phys Ther Sport. 2012;13(4):196–208. [DOI] [PubMed] [Google Scholar]
- 44. Sanders TL, Pareek A, Barrett IJ, et al. Incidence and long-term follow-up of isolated posterior cruciate ligament tears. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3017–3023. [DOI] [PubMed] [Google Scholar]
- 45. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186–191. [DOI] [PubMed] [Google Scholar]
- 46. Smith PA, Piepenbrink M, Smith SK, Bachmaier S, Bedi A, Wijdicks CA. Adjustable- versus fixed-loop devices for femoral fixation in ACL reconstruction: an in vitro full-construct biomechanical study of surgical technique-based tibial fixation and graft preparation. Orthop J Sports Med. 2018;6(4):2325967118768743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stannard JP, McKean RM. Anatomic PCL reconstruction: the double bundle inlay technique. Oper Tech Sports Med. 2009;17(3):148–155. [Google Scholar]
- 48. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Arthroscopic evaluation of articular cartilage lesions in posterior-cruciate-ligament-deficient knees. Arthroscopy. 2003;19(3):262–268. [DOI] [PubMed] [Google Scholar]
- 49. Vaquero-Picado A, Rodríguez-Merchán EC. Isolated posterior cruciate ligament tears: an update of management. EFORT Open Rev. 2017;2(4):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vasdev A, Rajgopal A, Gupta H, Dahiya V, Tyagi VC. Arthroscopic all-inside posterior cruciate ligament reconstruction: overcoming the “killer turn.” Arthrosc Tech. 2016;5(3):e501–e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang C-J, Chen H-S, Huang T-W. Outcome of arthroscopic single bundle reconstruction for complete posterior cruciate ligament tear. Injury. 2003;34(10):747–751. [DOI] [PubMed] [Google Scholar]
- 52. Wang D, Graziano J, Williams RJ, III, Jones KJ. Nonoperative treatment of PCL injuries: goals of rehabilitation and the natural history of conservative care. Curr Rev Musculoskelet Med. 2018;11(2):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. [DOI] [PubMed] [Google Scholar]
- 54. Yoshiya S, Andrish JT, Manley MT, Bauer TW. Graft tension in anterior cruciate ligament reconstruction: an in vivo study in dogs. Am J Sports Med. 1987;15(5):464–470. [DOI] [PubMed] [Google Scholar]