Abstract
Purpose
To determine if earlier initiation of renal replacement therapy (RRT) is associated with improved survival in patients with severe acute kidney injury.
Methods
We performed a retrospective case–control study of propensity-matched groups with multivariable logistic regression using Akaike Information Criteria to adjust for non-matched variables in a surgical ICU in a tertiary care hospital.
Results
We matched 169 of 205 (82%) patients with new initiation of RRT (EARLY group) to 169 similar patients who did not initiate RRT on that day (DEFERRED group). Eighteen (11%) of DEFERRED eventually received RRT before discharge. By univariate analysis, ICU mortality was higher in EARLY (n = 60 (36%) vs. n = 23 (14%), p < 0.001) as was hospital mortality (n = 73 (43%) vs. n = 44 (26%), p = 0.001). Of the 18 RRT patients in DEFERRED, 12 (67%) died in ICU and 13 (72%) in hospital. After propensity matching and logistic regression, we found that EARLY initiation of RRT was associated with a more than doubling of ICU mortality (aOR = 2.310, 95% confidence interval = 1.254–4.257, p = 0.007). However, after similar adjustment, there was no difference in hospital mortality (aOR = 1.283, 95% CI = 0.753–2.186, p = 0.360).
Conclusions
While ICU mortality was increased in the EARLY group, there was no difference in hospital mortality between EARLY and DEFERRED groups.
Keywords: Acute kidney injury, renal replacement therapy, propensity matching, creatinine
Introduction
Acute kidney injury (AKI) occurs commonly in hospitalized patients (∼20%) and more frequently (36%) in ICU patients.1,2 Treatment with renal replacement therapy (RRT) is indicated for severe acidosis, hyperkalemia, fluid overload, and uremia not responsive to medical therapy. The incidence of AKI patients receiving RRT has increased over the years, from 0.2% in 1995 to 0.6% in 2009 in elective surgical patients.3,4 However, it is 4–5% in ICU patients.5 Some of the increased incidence of RRT may be from changing indications, or indication creep, rather than increases in severe AKI. A recent study has shown that the indications for RRT have expanded with only 56% of RRT patients having “conventional” indications for RRT, and the other 44% receiving RRT for other reasons.6 This may be partially driven by retrospective studies and meta-analyses showing reduced mortality when RRT is initiated earlier.7 The optimal timing of RRT initiation in critically ill patients remains unknown. Earlier initiation may improve outcomes by removing toxins and fluids that may contribute to or cause worsened organ dysfunction. But, earlier initiation may expose patients to unneeded RRT, with its intrinsic hazards and morbidity.
Approximately half of the ICU patients receiving RRT will die before hospital discharge.8,9 However, patients who develop AKI and receive RRT typically are sicker than those who do not develop AKI or develop AKI but do not receive RRT. In cardiac surgery patients with postoperative AKI, patients who received RRT had almost twice the risk of death as patients with similar levels of AKI and no RRT. However, the patients who received RRT were sicker and the study, unfortunately, did not adjust for severity of illness.10
Retrospective studies have mostly found an association between early initiation of RRT and improved survival. Gettings et al.11 found that early initiation (BUN < 21.4 mmol/L [60 mg/dL]) patients had lower mortality than late (BUN > 21.4 mmol/L [60 mg/dL]) initiation patients. Similar benefits to early initiation were found in several other studies.12–14 More recently, Shiao et al. found a U-shaped association between timing (based on days after arrival in the ICU) of RRT and mortality.9 However, a limitation of these and other retrospective studies is that they only included patients who received dialysis. Excluding patients with a similar severity of kidney injury who did not receive RRT introduces a bias in these studies.
Recently, three prospective studies have been conducted to compare early versus later initiation of RRT and arrived at different conclusions.15–17 One trial found that early initiation of RRT was associated with better survival, while the other two studies found no difference in outcomes.15–17 While there were differences in patient populations including different inclusion and exclusion criteria, all three studies have been criticized for their entrance criteria not reflecting clinical practice. Specifically, relying on creatinine, oliguria, and NGAL thresholds as the major decider of RRT is dissimilar to the clinical “impression shaped by the patient’s global condition and trajectory” which is typically used in the ICU to decide on RRT.18
The purpose of this study is to evaluate initiation of RRT in a surgical ICU population using clinical impression compared to similar patients where the decision was made to defer RRT, while controlling for the patients’ global conditions and trajectories. Specifically, we hypothesized that the earlier initiation of RRT was associated with improved mortality.
Methods
This study was approved by the Institutional Review Board which waived informed consent of analysis of these de-identified data sets. This retrospective case–control study examined patients admitted to the surgical intensive care unit (SICU) at the University of Michigan Hospital, a large mid-western quaternary-care hospital, between 1 July 2011 and 7 June 2014. Patients were excluded if they spent <24 h in the SICU, received RRT prior to SICU admission, or if they received ECMO at any time during the SICU stay. The SICU database with patient demographics, hospital outcome and day of RRT, if any, was merged with the electronic health record (Centricity, GE Healthcare, Chicago, IL, and Epic, Epic Systems Corp, Verona, WI) for laboratory values, fluids, and medicines.
Continuous RRT (CRRT) was performed using dedicated machines (Prismaflex®, Baxter International Inc., Deerfield, IL) in continuous veno-venous hemodiafiltration mode with post-filter replacement. Blood flow rates were 150–200 mL/min. Effluent dose was typically prescribed with a target of 25–30 mL/kg/h in order to achieve a minimum delivered dose of 20–25 mL/kg/h per KDIGO guidelines.19 CRRT was conducted using regional citrate anticoagulation in all patients.
Statistical methods
Patients who received RRT during their SICU stay (EARLY) were compared to propensity score-matched SICU patients who had not received RRT (DEFERRED) by the day of the match. The propensity score used clinically relevant factors associated with the decision for RRT or with mortality in critically ill patients. For full details, see the online supplement.20–26 Data are presented as frequency (percentage), mean ± standard deviation, and median [interquartile range].
Power analysis
To have 80% power to find a difference in mortality from an estimated 40% in the EARLY initiation group to 62% in the control group with alpha = 0.05, required 160 patients in each group.
Results
During the study years, 205 SICU patients received new onset CRRT. We were able to use propensity scores to match 169 (82%) patients on their day of CRRT initiation (EARLY group) to 169 for whom the decision to implement CRRT was deferred (DEFERRED group). We were unable to match 36 (18%) new onset CRRT patients. Overall, the match was good with all 14 variables (prior day’s and current day’s BUN, creatinine, potassium, bicarbonate, vasopressor use, urine output and fluid balance) in the match having standardized differences <10% (Figure 1). The EARLY patients had higher APACHE III scores and lower hemoglobin and platelet counts, while DEFERRED patients were older (Table 1). By KDIGO criteria of oliguria and creatinine increases, the groups had similar numbers of stage 2 [44 (26%) vs. 50 (30%), p = .544] and stage 3 patients [69 (41%) vs. 76 (45%], p = .510; DEFEERED vs. EARLY, respectively).
Figure 1.
(a) Box and whisker plots showing the continuous variables from the prior day used in calculating the propensity scores in the renal replacement therapy (RRT) and the no RRT group. All standardized differences were <10%. (b) Box and whisker plots showing the continuous variables from the current day used in calculating the propensity scores in the renal replacement therapy (RRT) and the no RRT group. All standardized differences were <10%.
Table 1.
Characteristics of patients.
|
DEFERRED RRT
|
EARLY RRT | |||||||
|
Factor
|
N
|
%
|
|
N
|
%
|
p
|
stan diff
|
|
| Diuretic prior daya | 35 | 21 | 36 | 21 | 0.999 | 1.5 | ||
| Diuretic todaya | 20 | 12 | 19 | 11 | 0.999 | 1.9 | ||
| Vasopressor prior daya | 47 | 28 | 50 | 30 | 0.810 | 3.9 | ||
| Vasopressor todaya | 111 | 66 | 118 | 70 | 0.485 | 8.9 | ||
| Female | 55 | 33 | 73 | 43 | 0.057 | 22.1 | ||
| Diagnosis | <0.001 | |||||||
| ARDS | 11 | 7 | 29 | 17 | ||||
| Transplants | 15 | 9 | 18 | 11 | ||||
| Sepsis | 38 | 22 | 31 | 18 | ||||
| Other | 105 | 62 | 91 | 54 | ||||
|
Factor
|
N
|
median
|
IQ
|
N
|
median
|
IQ
|
p
|
stan diff
|
| Age (y) | 153 | 61 | 52, 70 | 153 | 57 | 46, 64 | 0.004 | 29.6 |
| Prior day’s values | ||||||||
| Urine (mL)a | 141 | 775 | 290, 1801 | 141 | 560 | 215, 1865 | 0.551 | 9.0 |
| Fluid balance (mL) | 136 | 287 | −941, 2188 | 139 | 969 | −308, 2993 | 0.085 | 17.8 |
| Creatinine (µmol/L)a,b | 169 | 221 | 110, 327 | 169 | 274 | 164, 385 | 0.005 | 9.3 |
| BUN (mmol/L)a,b | 169 | 18 | 12, 24 | 169 | 18 | 12, 25 | 0.599 | 9.0 |
| Potassium (mmol/L)a | 169 | 4.3 | 4.0, 4.9 | 169 | 4.4 | 4.0, 5.0 | 0.390 | 3.1 |
| Bicarbonate (mmol/L)a | 169 | 22 | 19, 25 | 169 | 22 | 19, 25 | 0.875 | 0.8 |
| Current day’s values | ||||||||
| Creatinine (µmol/L)a,b | 169 | 230 | 124, 352 | 169 | 256 | 175, 354 | 0.066 | 3.2 |
| BUN (mmol/L) a,b | 169 | 18 | 13, 28 | 169 | 16 | 11, 24 | 0.351 | 6.0 |
| Potassium (mmol/L)a | 169 | 4.4 | 4.0, 4.9 | 169 | 4.5 | 4.0, 5.0 | 0.717 | 0.5 |
| Bicarbonate (mmol/L)a | 169 | 22 | 18, 25 | 169 | 22 | 19, 25 | 0.407 | 5.1 |
| Fluid input (mL) | 169 | 1912 | 1260, 3509 | 169 | 2656 | 1747, 4457 | <0.001 | 18.4 |
| Fluid output (mL) | 169 | 1135 | 550, 2248 | 169 | 1814 | 710, 3162 | 0.004 | 23.7 |
| Fluid balance (mL) | 169 | −48 | −1940, 1325 | 169 | −378 | −2143, 1165 | 0.385 | 1.1 |
| Urine today (mL)a | 169 | 950 | 352, 1951 | 169 | 1208 | 250, 2319 | 0.159 | 9.3 |
| APACHE III Score | 169 | 75 | 61, 88 | 169 | 90 | 75, 110 | <0.001 | 68.0 |
| Heart rate (/min) | 169 | 91 | 79, 103 | 169 | 94 | 78, 107 | 0.378 | 11.6 |
| Respirations (/min) | 166 | 20 | 16, 24 | 161 | 22 | 16, 26 | 0.081 | 13.3 |
| Temperature (℃) | 166 | 36.9 | 36.6, 37.4 | 163 | 36.8 | 36.5, 37.2 | 0.006 | 30.2 |
|
Factor
|
N
|
median
|
IQ
|
N
|
median
|
IQ
|
p
|
stan diff
|
| Systolic blood pressure (kPa) | 169 | 15.2 | 13.3, 16.9 | 169 | 14.8 | 13.3, 16.9 | 0.628 | 1.1 |
| Diastolic blood pressure (kPa) | 169 | 7.2 | 6.5, 8.4 | 168 | 7.3 | 6.4, 8.9 | 0.855 | 2.2 |
| Sodium (mmol/L) | 169 | 139 | 136, 142 | 169 | 140 | 137, 144 | 0.086 | 9.0 |
| Phosphorous (mmol/L) | 163 | 1.61 | 1.16, 2.36 | 164 | 1.78 | 1.49, 2.39 | 0.029 | 13.5 |
| Magnesium (mmol/L) | 164 | 0.95 | 0.86, 1.07 | 164 | 0.99 | 0.91, 1.11 | 0.003 | 25.7 |
| Glucose high (mmol/L) | 166 | 7.71 | 6.55, 9.38 | 165 | 8.71 | 7.38, 10.6 | <0.001 | 41.5 |
| Glucose low (mmol/L) | 166 | 5.94 | 5.16, 6.83 | 165 | 5.94 | 5.11, 6.94 | 0.914 | 1.9 |
| Albumin (g/L) | 95 | 27 | 24, 31 | 125 | 28 | 24, 32 | 0.782 | 3.0 |
| Hemoglobin (g/L) | 164 | 81 | 74, 93 | 163 | 78 | 70, 86 | 0.011 | 25.2 |
| Platelets (109/L) | 164 | 141 | 85, 211 | 163 | 97 | 48, 177 | 0.001 | 19.6 |
| Bilirubin (µmol/L) | 94 | 23.9 | 10.3, 78.8 | 125 | 35.9 | 15.4, 101 | 0.049 | 26.4 |
Note: All current day values are based on values up to 12 noon of that day. If more than one lab value was determined, the highest creatinine, BUN, potassium, phosphorous, magnesium, bilirubin values are displayed; while the lowest bicarbonate, sodium, hemoglobin, and platelets values are displayed. aVariables in the propensity match.
To facilitate comparisons with other studies that used mean and standard deviation to describe creatinine and BUN, our mean and standard deviation for creatinine for the prior day were 277 ± 272 µmol/L in the DEFERRED group vs. 298 ± 182 in the EARLY group, p = 0.393; for BUN for the prior day, it was 19 ± 10 mmol/L vs. 20 ± 11, p = 0.411; for current day’s creatinine, it was 283 ± 236 vs. 290 ± 176, p = 0.765; and for current day’s BUN, 19 ± 10 vs. 20 ± 11, p = 0.411, respectively.
IQ: interquartile range; SD: standard deviation; stan diff: standardized difference.
In the DEFERRED group, five patients (3%) with less than stage 2 progressed to stage 2 and two other patients (1%) to stage 3. Five patients (3%) initially with stage 2 progressed to stage 3. Eighteen (11%) patients in the DEFERRED group eventually received CRRT before discharge, a median of 2 [1, 5] days later. ICU mortality was higher in the EARLY group [n = 60 (36%) vs. n = 23 (14%), p < 0.001] as was hospital mortality [n = 73 (43%) vs. n = 44 (26%), p = 0.001]. Of the 18 patients in the DEFERRED group who eventually received RRT, 12 (67%) died in ICU and 13 (72%) in hospital. DEFERRED patients who eventually received RRT had worse ICU [n = 12 (67%) vs. n = 11 (7%), p < 0.001] and worse hospital [n = 13 (72%) vs. 31 (21%), p < 0.001] mortality than DEFERRED patients who never received RRT (Figure 2). Mortality in the DEFERRED patients who eventually received RRT was also higher than the mortality rate in the EARLY group (67% vs. 36%, p = 0.019 in the ICU and 72% vs. 43%, p = .025 in the hospital). However, patients in the DEFERRED group who received RRT worsened over the subsequent two days compared to DEFERRED patients who did not receive RRT. Specifically, the APACHE III score increased to 106 ± 21 in the DEFERRED group who eventually received RRT but decreased to 68 ± 25, p < 0.001 in the DEFERRED who never received RRT and vasopressor use remained high, 71%, but fell to 33%, p = 0.006 in the DEFERRED patients who never received RRT.
Figure 2.
Flowchart of unadjusted hospital mortality. EARLY RRT group had higher mortality than the DEFERRED group (p = 0.001), but lower mortality than the DEFERRED group who received RRT (p = 0.024). DEFERRED patients who eventually received RRT had higher mortality than DEFERRED patients who did not (p < 0.001).
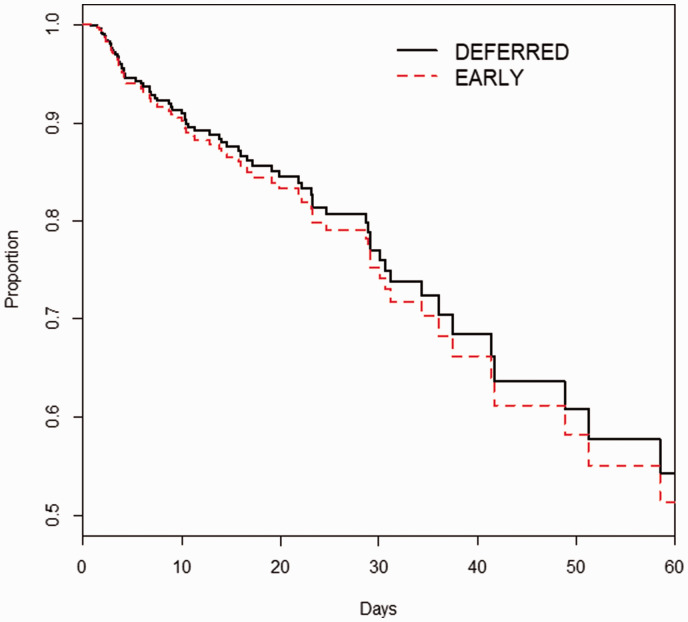
When we used logistic regression with Akaike Information Criteria to adjust for the factors that were not included in the calculation of the propensity score (Table 1), we found that EARLY initiation of RRT was associated with a more than doubling of ICU mortality (adjusted odds ratio (aOR) = 2.310, 95% confidence interval (CI) = 1.254–4.257, p = .007). However, after similar adjustment, there was no difference in hospital mortality (aOR = 1.283, 95% CI = 0.753–2.186, p = 0.360) (Table 2). As a confirmatory analysis using LASSO logistic regression, we similarly found that EARLY was associated with ICU death (aOR = 2.253), but not with hospital death (aOR = 1.285) (Table 3). This lack of survival difference was confirmed by Cox analysis (Figure 3).
Table 2.
Multivariable logistic regression of factors associated with mortality using Akaike Information Criteria.
| OR | 95% | Confidence interval | p | |
|---|---|---|---|---|
| ICU mortality | ||||
| APACHE III | 1.039 | 1.026 | 1.053 | <0.001 |
| CRRT current day | 2.310 | 1.254 | 4.257 | 0.007 |
| Magnesium (meq/L) | 2.195 | 1.195 | 4.033 | 0.011 |
| Platelets (1000/µL) | 0.998 | 0.995 | 1.001 | 0.106 |
| Fluid balance prior day ≤−501 mL | 2.074 | 0.850 | 5.060 | 0.109 |
| Fluid balance prior day >671, ≤2560 mL | 1.177 | 0.491 | 2.821 | 0.716 |
| Fluid balance prior day >2560 mL | 0.818 | 0.335 | 1.998 | 0.659 |
| Fluid balance prior day missing | 0.460 | 0.469 | 1.252 | 0.128 |
| Intercept | 0.001 | 0.000 | 0.009 | <0.001 |
| Hospital mortality | ||||
| APACHE III | 1.038 | 1.025 | 1.050 | <0.001 |
| CRRT current day | 1.283 | 0.753 | 2.186 | 0.360 |
| Magnesium (meq/L) | 1.662 | 0.951 | 2.905 | 0.074 |
| Platelets (1000/µL) | 0.996 | 0.994 | 0.999 | 0.011 |
| Fluid balance prior day ≤−501 mL | 2.330 | 1.039 | 5.224 | 0.040 |
| Fluid balance prior day >671, ≤2560 mL | 1.608 | 0.727 | 3.557 | 0.241 |
| Fluid balance prior day >2560 mL | 1.297 | 0.587 | 2.866 | 0.520 |
| Fluid balance prior day missing | 0.626 | 0.260 | 1.503 | 0.294 |
| Intercept | 0.035 | 0.012 | 0.107 | <0.001 |
OR: odds ratio.
Table 3.
Multivariable logistic regression using LASSO.
| OR | 95% | Confidence interval | p | |
|---|---|---|---|---|
| ICU mortality | ||||
| APACHE III | 1.038 | 1.025 | 1.051 | <0.001 |
| CRRT current day | 2.253 | 1.251 | 4.058 | 0.007 |
| Intercept | 0.007 | 0.002 | 0.023 | <0.001 |
| Hospital mortality | ||||
| APACHE III | 1.036 | 1.024 | 1.048 | <0.001 |
| CRRT current day | 1.285 | 0.765 | 2.157 | 0.343 |
| Platelets (1000/µL) | 0.996 | 0.994 | 0.999 | 0.009 |
| Intercept | 0.035 | 0.012 | 0.107 | <0.001 |
OR: odds ratio.
Figure 3.
Cox plot showing time to mortality. Timing of RRT was not associated with mortality: hazard ratio = 0.934, 95% confidence interval = 0.529–1.340, p = 0.744.
Discussion
In our propensity-matched study, we found that EARLY initiation of RRT was not associated with improved in-hospital mortality. This is different from the ELAIN study that found improved mortality in the early initiation group.16 There are important differences between our and their study, particularly entry criteria, which was KDIGO stage 2 in ELAIN, while ours was based on clinical judgment, resulting in higher creatinine and BUN values in our patients. Many of our patients in our DEFERRED group would have received RRT based on achieving KDIGO stage 2 criteria, yet only a few eventually received RRT. The studies further differed in the occurrence of RRT in the DEFERRED groups
Our finding of no difference in mortality is similar to the observations of the AKIKI and STARRT-AKI studies.15,17 Findings in our study extend these results by including more patients and thus may be more clinically relevant. One interpretation of these findings is that avoiding RRT is associated with a better outcome than receiving RRT; at the same time, it appears that if RRT is needed, earlier initiation may be beneficial.
Our finding that only 11% of the DEFERRED group received RRT is less than the 51% in AKIKI and the 63% in STARRT-AKI and much less than the 91% in ELAIN. These differences may be related to different criteria for RRT in AKIKI and ELAIN: KDIGO stage 3 and stage 2, respectively, while STARRT-AKI, similar to our study, used clinical judgement to institute RRT in the deferred group.15–17
Our patients who received EARLY RRT had similar BUN (19 ± 10 vs. 19 ± 9 mmol/L [53 ± 24 mg/dL]) and creatinine (277 ± 272 vs. 288 ± 124 mmol/L [3.25 + 1.40 mg/dL]) to patients in AKIKI, similar BUN but slightly lower creatinine as STARRT-AKI patients (BUN 18 [12, 24] vs. 18 [12, 23] {50 [34,64] mg/dL}) and creatinine (221 [110, 327] vs. 286 [237, 355] mmol/L {3.24 [2.68–4.02] mg/dL}), and higher values than ELAIN (BUN = 14 ± 6 mmol/L [38.5 ± 15.5 mg/dL] and creatinine = 168 ± 51 mmol/L [1.9 + 0.64 mg/dL]).15,16 Both prospective studies also had rigid criteria for initiating RRT in the Deferred group. RRT was initiated in AKIKI in the Deferred group when electrolyte abnormalities occurred or oliguria or anuria persisted. ELAIN mandated RRT in the Deferred group when AKI had progressed to stage 3 or if electrolyte abnormalities, severe oliguria, or organ edema occurred.
Our findings differ from other retrospective studies in which we found increased ICU mortality and no difference in hospital mortality with early RRT.9,11–14 This most likely relates to differing patient populations and methods. We matched patients who received RRT to patients who did not receive RRT, thus greatly decreasing selection bias. A limitation of these other retrospective studies is that they only included patients who received dialysis. For the patients whose RRT was deferred, we do not know if they would have survived after receiving RRT or died because of RRT. This introduces a large bias in these retrospective studies by only including patients who received RRT. Our finding of worse mortality in the DEFERRED group who eventually received RRT is similar to a study by Wilson et al., who found that late initiation of RRT was associated with increased mortality, in a study that included all patients with AKI, not just those with RRT.27
Our study may be more generalizable than the prospective studies by not excluding patients based on the reasons for AKI.15–17 These studies also excluded patients for already receiving RRT or for the need for immediate RRT. By including patients at initiation of RRT and not when they achieved certain laboratory values, our study may be more generalizable.
The putative benefit of earlier RRT is correction of metabolic and uremic abnormalities which may have direct toxic effects on organs, and correction of fluid overload which is associated with worse organ function and death.28,29 RRT may also remove cytokines and thus ameliorate inflammation that exacerbates organ injury.30 Conversely, unnecessary initiation of RRT exposes patients who would recover without RRT to the risks of catheter insertion, infection, hypophosphatemia, and the removal of anti-inflammatory cytokines.31–33
There are several limitations to our study. We do not have reasons why RRT was or was not instituted on any given day. Increasing positive fluid balance is associated with poor outcomes, but not necessarily mortality.29,34 Even though we matched on urine output and not fluid balance, both the prior day’s and current’s fluid balance were similar between the two groups. We are limited by not knowing total fluid balance since hospital admission. Outside of the ICU, fluids inputs and outputs are not always recorded. There may be unmeasured confounders that are associated with both the decision to initiate RRT and mortality. Given that this study was conducted in a single SICU between 2011 and 2014, it may be subject to confounding, not be generalizable to other types of patients or institutions, and clinical practice may have changed since 2014. As it was a retrospective study, it should be considered hypothesis generating and not implying causality. Finally, while the DEFERRED group who eventually received RRT continued to worsen (increased APACHE III scores and continued high vasopressor use), we do not have information on mechanisms of death, which might help explain the higher mortality in the DEFFERED group who eventually received RRT.
There are several strengths of our study. We were able to use electronic medical records to include a large set of laboratory values, vital signs, fluids, and medicines that typically impact the decision to or not to institute RRT. We also included the trajectory of the illness by including values both for current and the prior day and we included fluid balance, acid-base status, electrolyte values, and diuretic use, in addition to BUN and creatinine values. This approach to instituting RRT more closely reflects standard clinical practice than rigid creatinine, urine or NGAL thresholds.35 By using propensity matching, we also found the clinical course (Figure 1, Table 1) at which patients have a 50% likelihood – equipoise – of receiving RRT. This may be useful in further studies.
In conclusion, we found that earlier initiation of RRT in SICU patients was associated with increased ICU mortality, but no difference in hospital mortality.
Supplemental Material
Supplemental material, INC892792 Supplemental Material for The effect of timing of initiation of renal replacement therapy on mortality: A retrospective case–control study by Milo Engoren, Michael D Maile, Michael Heung, James M Blum, Ross Blank, Lena M Napolitano, Pauline K Park, Krishnan Raghavendran, Elizabeth S Jewell and Craig Meldrum in Journal of the Intensive Care Society
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was performed at the University of Michigan and was supported entirely by departmental and institutional resources.
ORCID iD: Milo Engoren https://orcid.org/0000-0002-3367-1906 Michael D Maile https://orcid.org/0000-0001-8236-3371
References
- 1.Susantitaphong P, Cruz DN, Cerda J, et al. World Incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R, et al. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 3.Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis 2015; 65: 870–877. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery – 1995 to 2009. CMAJ 2012; 184: 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 2008; 36: S146–S151. [DOI] [PubMed] [Google Scholar]
- 6.Vaara ST, Reinikainen M, Kaukonen KM, et al. Association of ICU size and annual case volume of renal replacement therapy patients with mortality. Acta Anaesthesiol Scand 2012; 56: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 7.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011; 15: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eachempati SR, Wang JC, Hydo LJ, et al. Acute renal failure in critically ill surgical patients: persistent lethality despite new modes of renal replacement therapy. J Trauma 2007; 63: 987–993. [DOI] [PubMed] [Google Scholar]
- 9.Shiao CC, Ko WJ, Wu VC, et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS One 2012; 7: e42952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider AG, Eastwood GM, Seevanayagam S, et al. A risk, injury, failure, loss, and end-stage renal failure score-based trigger for renal replacement therapy and survival after cardiac surgery. J Crit Care 2012; 27: 488–495. [DOI] [PubMed] [Google Scholar]
- 11.Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med 1999; 25: 805–813. [DOI] [PubMed] [Google Scholar]
- 12.Demirkiliç U, Kuralay E, Yenicesu M, et al. Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg 2004; 19: 17–20. [DOI] [PubMed] [Google Scholar]
- 13.Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 2006; 1: 915–919. [DOI] [PubMed] [Google Scholar]
- 14.Elahi M, Asopa S, Pflueger A, et al. Acute kidney injury following cardiac surgery: impact of early versus late haemofiltration on morbidity and mortality. Eur J Cardiothorac Surg 2009; 35: 854–863. [DOI] [PubMed] [Google Scholar]
- 15.Gaudry S, Hajage D, Schortgen F, et al. initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122–133. [DOI] [PubMed] [Google Scholar]
- 16.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs. delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016; 315: 2190–2199. [DOI] [PubMed] [Google Scholar]
- 17.Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 2015; 88: 897–904. [DOI] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Lamontagne F, Joannidis M, et al. When to start renal replacement therapy in critically ill patients with acute kidney injury: comment on AKIKI and ELAIN. Crit Care 2016; 20: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DB, Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. J Am Stat Assoc 2000; 95: 573–585. [Google Scholar]
- 24.Engoren M. Does erythrocyte blood transfusion prevent acute kidney injury? Propensity-matched case control analysis. Anesthesiology 2010; 113: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 25.Choirat C, Honaker J, Imai K, et al. Zelig: everyone's statistical software. Version 5.1-2, http://zeligproject.org (accessed 21 November 2019).
- 26.Imai K, King G, Lau O. Toward a common framework for statistical analysis and development. J Comput Graph Stat 2008; 17: 892–913. [Google Scholar]
- 27.Wilson FP, Yang W, Machado CA. Dialysis versus non-dialysis in patients with AKI: a propensity matched cohort study. Clin J Am Soc Nephrol 2014; 9: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibney N, Hoste E, Burdmann EA, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol 2008; 3: 876–880. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Chen Z, Diao Y, et al. Associations of fluid overload with mortality and kidney recovery in patients with acute kidney injury: a systematic review and meta-analysis. J Crit Care 2015; 30: 860.e7–13. [DOI] [PubMed] [Google Scholar]
- 30.Gattas DJ, Rajbhandari D, Bradford C, et al. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med 2015; 43: 1622–1629. [DOI] [PubMed] [Google Scholar]
- 31.Yakupoglu U, Parmaksiz E, Ozdemir N. Two important factors in the follow-up of acute renal failure. Hypophosphatemia and respiratory alkalosis. Hemodial Int 2010; 14: 97–98. [Google Scholar]
- 32.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164: 1896–1903. [DOI] [PubMed] [Google Scholar]
- 33.Parienti J-J, Dugué AE, Daurel C, et al. Continuous renal replacement therapy may increase the risk of catheter infection. Clin J Am Soc Nephrol 2010; 5: 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival, and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 2009; 76: 422–427. [DOI] [PubMed] [Google Scholar]
- 35.Vaara ST, Reinikainen M, Wald R, et al. Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol 2014; 9: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INC892792 Supplemental Material for The effect of timing of initiation of renal replacement therapy on mortality: A retrospective case–control study by Milo Engoren, Michael D Maile, Michael Heung, James M Blum, Ross Blank, Lena M Napolitano, Pauline K Park, Krishnan Raghavendran, Elizabeth S Jewell and Craig Meldrum in Journal of the Intensive Care Society