Abstract
We present the case of a diver who experienced an uncontrolled ascent from 55 m and presented with a severe decompression illness. She was clinically shocked and in multi organ failure due to massive fluid shifts. She demonstrated bilateral lower limb loss of power and sensation and required multiple hyperbaric therapy sessions. With joint critical care, hyperbaric and physical therapy involvement, she was discharged some five weeks after her presentation with an independent level of function.
Keywords: Capillary leak, decompression illness, dive, hyperbaric oxygen therapy, rapid ascent
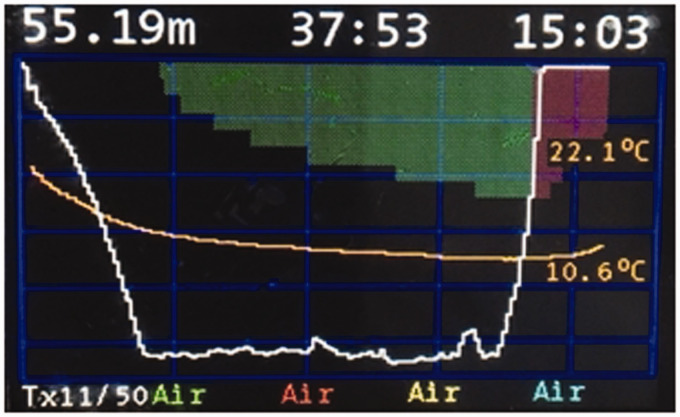
A 52-year-old female diver with decompression illness (DCI) arrived by helicopter at our district general hospital. She had dived to 55 m for 25 min when her buoyancy control device malfunctioned and she made a rapid uncontrolled ascent to the surface at 25 m per minute (Figure 1). She was breathing ‘trimix’ during the dive, a mixture containing 11% oxygen, 39% helium and 50% nitrogen.
Figure 1.
Depth-time profile of incident dive.
On the surface, she was confused and complained of chest discomfort, lower back pain and a ‘crunching’ sound in the back of her head. She was given high-flow oxygen by oronasal mask and remained clinically stable during the 1 h transfer by helicopter.
On arrival in the emergency department, she complained of nausea, fatigue, chest discomfort and lower back pain. Apart from the presence of an ocular prosthesis, implanted after enucleation following eye trauma, she had no past medical history and took no regular medications. On examination, she was found to be tachycardic with a heart rate of 160 beats per minute (bpm) and peripheries were cool; capillary refill time (CRT) was 4 s. Auscultation of the chest revealed reduced air entry and crackles in the left lower zone, but there were no signs of pneumothorax or subcutaneous emphysema present. She was confused with an Abbreviated Mental Test Score (AMTS) of 7/10 and power in all four limbs was reduced; grade 4/5 according to the Medical Research Council (MRC) scale.
Blood tests showed a markedly raised haemoglobin and haematocrit, with a raised white cell count, potassium, urea and creatinine (Table 1). Arterial blood analysis showed metabolic acidosis (Table 2). Electrocardiogram demonstrated sinus tachycardia and chest X-ray was unremarkable. She was treated with high-flow oxygen by facemask and received 2 L of intravenous crystalloid solution before transfer to the on site hyperbaric medicine unit.
Table 1.
Haematology and biochemistry results on admission.
| Value | Reference range | |
|---|---|---|
| Haemoglobin | 209 g.L−1 | 115–155 |
| Haematocrit | 63% | |
| White cell count | 23.2 × 109.L−1 | 4–11 |
| Platelets | 244 × 109.L−1 | 140–400 |
| Sodium | 138 mmol.L−1 | 133–146 |
| Potassium | 5.5 mmol.L−1 | 3.5–5.3 |
| Urea | 12.4 mmol.L−1 | 2.5–7.8 |
| Creatinine | 131 µmol.L−1 | 50–98 |
| Albumin | 22 g.L−1 | 35–50 |
| Creatine Kinase | 318 iu.L−1 | 25–200 |
Table 2.
Arterial blood gas results on admission.
| pH | 7.31 | 7.35–7.45 |
| pCO2 | 4.46 kPa | 4.27–6.40 |
| pO2 (on 15 L O2) | 22.0 kPa | 11.0–14.4 |
| Lactate | 3.2 mmol.L−1 | 0.5–1.6 |
| Base excess | −8.3 mmol.L−1 | |
| Bicarbonate | 16 mmol.L−1 |
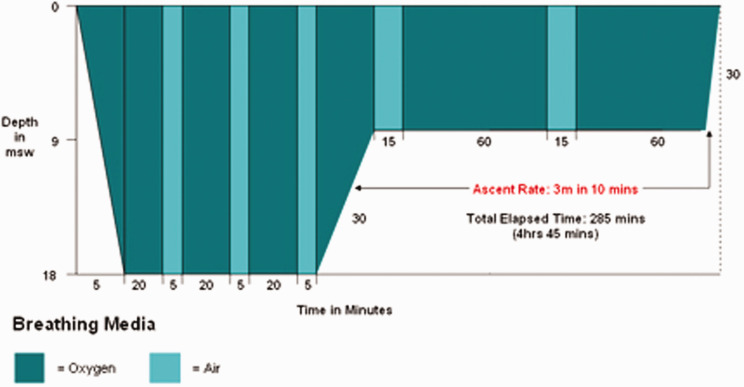
She received hyperbaric oxygen therapy (HBOT) within 90 min of arrival at the hospital. The chamber was pressurised to 284 kPa, the equivalent of immersion in 18 m of seawater (msw) following Royal Navy Table 62 (RN 62) (see Figure 2).
Figure 2.
A Royal Navy Table 62 without extensions.
Depending on the response to treatment, extensions can be added at 18 msw (20 min breathing oxygen and 5 min air) up to three times and at 9 msw (60 min oxygen and 15 min air) two more times.
A deeper treatment was considered but, as all abnormal neurology including higher mental function had resolved within the first hour at 18 msw, treatment continued on RN 62 with one extension at 18 msw. After HBOT and three more litres of intravenous crystalloid, her heart rate had settled to 95 bpm and AMTS was 10/10.
She was admitted to the acute medical unit. However, within 4 h, her lower limb weakness relapsed. Power was MRC grade 4/5 and 3/5 in right and left legs, respectively, and tone was increased in both legs with upgoing plantar reflexes. She also developed new neurological signs in the lower limbs, losing all modalities of sensation except vibration bilaterally up to the level of the umbilicus. Her skin was mottled, peripheries were cold, and CRT deteriorated to 8 s. She was oliguric with a urine output of 20 mL per hour and a blood lactate of 3.4 mmol.L−1. Urgent HBOT was indicated but, due to her haemodynamic instability, she was referred to the intensive care unit (ICU) for assessment and stabilisation first.
In order to monitor her cardiac output, an attempt to establish invasive arterial monitoring was undertaken. Initial attempts at a radial arterial line were unsuccessful therefore a brachial arterial line was sited with direct ultrasonographic visualisation. Her arterial trace and pressure were in keeping with a very poor cardiac output state. In the ICU, with some difficulty, a femoral arterial line and central venous pressure line were sited. A bedside Focused Intensive Care Echo (FICE) was performed to investigate her presumed poor cardiac output state, which demonstrated hyperdynamic ventricles and a moderate pericardial effusion which was not deemed to be significant following further review from a cardiology consultant.
Guided by invasive monitoring, she responded well to a further 1.5 L of intravenous crystalloid and subsequently underwent a second HBOT with three extensions at 18 msw and received a further 6 L of intravenous crystalloid during treatment. Her power and tone improved partially.
Computed tomography (CT) scan of her abdomen to investigate abdominal pain demonstrated widespread subcutaneous oedema and a 3 cm hypodensity in the liver, possibly a traumatic laceration caused by a reserve air cylinder forced against her abdomen during rescue. In a subsequent scan, the lesion had not enlarged and looked more like a benign structure such as a haemangioma, although a haematoma could still not be ruled out.
Further HBOT was administered using Royal Navy Table 66 (pressurisation to 14 msw for 90 min) most days until, after 15 HBOT treatments in total, her function began to improve without HBOT. Renal function normalised and haemoglobin was within normal range by day 3. A repeat echo on day 4 showed normal biventricular size, good systolic function and resolution of the pericardial effusion. The abdominal discomfort improved during the sixth HBOT and was therefore assumed to be due to DCI rather than the liver lesion. Magnetic resonance imaging (MRI) of her spine revealed nothing untoward.
Four weeks later after intensive physiotherapy, further HBOT and transfer to our neuro-rehabilitation centre the patient was discharged home fully independent but with reduced exercise tolerance and impaired balance.
Discussion
The circumstances and clinical features are consistent with DCI due to evolved gas. This usually requires a dive of sufficient depth and duration for a substantial quantity of additional inert gas (in this case nitrogen and helium) to dissolve in body tissues. When a diver returns to the surface, bubbles often form as excess gas is released from tissues faster than it can be carried away in solution by venous blood which delivers dissolved gas and bubbles to the lungs. The bubbles are usually trapped in the alveolar capillaries and, in normal circumstances, inert gas diffuses from the capillaries into the alveolar spaces until the bubbles collapse and pulmonary venous blood is equilibrated with alveolar gas.
The main differential diagnosis was Arterial Gas Embolism (AGE) due to escaped gas which expands on ascent and ruptures lung tissue, forces itself into the pulmonary veins and reaches the left heart from where it is pumped into the systemic circulation as an AGE. This typically causes neurological signs very soon after surfacing but can manifest before arrival at the surface and can occur after shallow, short dives.
Most bubbles are probably intravascular with extravascular occurring only after extreme decompression. Bubbles have mechanical, embolic and biochemical effects on tissues and, if present in excess, cause clinical features most frequently attributed to the nervous system, joints and skin. Clinically they can present as neurological stroke-like symptoms, pain1 or skin discoloration. Intravascular bubbles also damage capillary endothelium and activate inflammatory cascade reactions leading to increased endothelial permeability, capillary leak, tissue oedema and haemoconcentration.2,3,4 Bubbles can also activate platelets and neutrophils, exacerbating clotting and ischaemia.5 Neurological DCI results from occlusion of capillary flow, intravascular coagulation, endothelial damage and leukocyte activation in the nervous system.6
The mainstay of DCI treatment is HBOT. Raising ambient pressure impedes release of new bubbles, shrinks existing bubbles and increases the partial pressure of the enclosed inert gas, encouraging it to diffuse back into solution, thus reducing mechanical damage to tissues. The absence of inert gas in the inhaled 100% oxygen maximises the diffusion gradient and promotes elimination of inert gas from bubbles and tissues. The elevated partial pressure of oxygen reduces tissue hypoxia, oedema, inflammatory responses and reperfusion injury. A study has shown that oxygen before recompression is also beneficial, making divers with DCI more responsive to HBOT.7
Increased endothelial permeability leads to extravasation of plasma.2,3 This has been reported for many years; however, there is insufficient evidence to identify the ideal resuscitation fluid. In 1966, Barnard et al.8 described resuscitation of post decompression shock with plasma. Gempp et al. advised treatment with plasma expanders such as albumin.9 Crystalloids are widely used in the treatment of DCI10–13 as in our patient who, on discharge from ITU, had a haemoglobin of 93 g/l and haematocrit 28% after receiving 12 L of fluid in total.
DCI patients of 20–30% have residual disability.14 Gempp et al. demonstrated a significant decrease in serum albumin in 52 divers presenting with neurological DCI compared with asymptomatic divers who had decompressed inadequately. They suggested using hypoalbuminaemia to predict neurological DCI.15 Other research has also shown a poorer outcome in divers with high haematocrit or requiring intravenous fluids.
In hospitals without a hyperbaric facility, initial treatment of DCI includes a high inspired concentration of oxygen and appropriate fluid resuscitation. Although the precise relationship between time to HBOT and outcome is not yet clear, best practice is to liaise with, and to transfer to, an appropriate hyperbaric chamber as early as possible.
A critically ill diver requires a Category 1 hyperbaric facility which can treat patients in any diagnostic category and administer Advanced Life Support during hyperbaric treatment. In the United Kingdom there are seven such units. If the closest appropriate facility is not known, advice is available 24 h a day from the British Hyperbaric Association National Diving Accident Helpline on 07831 151 523 in England and Wales or 0345 408 6008 in Scotland.
Conclusion
Extravasation of plasma is a recognised consequence of DCI but the potential severity of the effect is not widely appreciated. While the mainstay of DCI treatment is HBOT and physiotherapy is responsible for much functional recovery, timely intravascular fluid resuscitation can prevent avoidable long-term morbidity or even death.
A diver with capillary leak syndrome must be treated in a level 2 environment with full multi-disciplinary team involvement to ensure appropriate fluid management and cardiovascular support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michele Homsy https://orcid.org/0000-0003-3894-5408 Tim Martindale https://orcid.org/0000-0002-2021-2014
References
- 1.Vann RD, Butler FK, Mitchell SJ. Decompression illness. Lancet 2011; 377: 153–164. [DOI] [PubMed] [Google Scholar]
- 2.Boussuges A, Blanc P, Molenat F. Haemoconcentration in neurological decompression illness. Int J Sports Med 1996; 17: 351–355. [DOI] [PubMed] [Google Scholar]
- 3.Pollock NW, Butead D. Updates in decompressio illness. Emerg Med Clin N Am 2017; 35: 301–319. [DOI] [PubMed] [Google Scholar]
- 4.Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int 2017; 92: 37–46. [DOI] [PubMed] [Google Scholar]
- 5.Bosco G, Yang ZJ, Savini F, et al. Environmental stress on diving-induced platelet activation. Undersea Hyperb Med 2001; 28: 207–211. [PubMed] [Google Scholar]
- 6.Barak M, Katz Y. Microbubble: pathophysiology and clinical implications. Chest 2005; 128: 2918–2932. [DOI] [PubMed] [Google Scholar]
- 7.North Sea Medical Centre. HSE. 2007. http://www.hse.gov.uk/research/rrpdf/rr550.pdf (accessed 23 November 2019).
- 8.Barnard EE, Hanson JM, Rowton-Lee MA, et al. Post-decompression shock due to extravasation of plasma. Br Med J 1966; 2: 154–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gempp E, Lacroix G, Cournac JM, et al. Severe capillary leak syndrome after inner ear decompression sickness in a recreational scuba diver. J Emerg Med 2013; 45: 70–73. [DOI] [PubMed] [Google Scholar]
- 10.Hibi A, Kasugai T, Kamiya K, et al. Acute kidney injury caused by decompression illness successfully treated with hyperbaric oxygen therapy and temporary dialysis. CEN Case Rep 2017; 6: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viecelli A, Jamboti J, Waring A, et al. Acute kidney injury due to decompression illness. Clin Kidney J 2014; 7: 380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleeson PJ, Kelly Y, Ni Sheaghdha E, et al. A SCUBA diver with acute kidney injury. BMJ Case Rep 2015; 2015: bcr2014206345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalpa S, Meyne J, Kahler W, et al. Decompression illness with hypovolemic shock and neurological failure symptoms after two risky dives: a case report. Physiol Rep 2017; 5: e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gempp E, Blatteau JE. Risk factors and treatment outcome in scuba divers with spinal cord decompression sickness. J Crit Care 2010; 25: 236–242. [DOI] [PubMed] [Google Scholar]
- 15.Gempp E, De Meistre S, Lounge P. Serum albumin as a biomarker of capillary leak in scuba divers with neurological decompression sickness. Aviat Space Environ Med 2014; 85: 1049–1052. [DOI] [PubMed] [Google Scholar]